Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.52 n.2 Pretoria 2022
http://dx.doi.org/10.4314/sajas.v52i2.1
ARTICLES
An exogenous fibrolytic enzyme mixture enhances in vitro ruminai degradability of red grape pomace by-product
G. MhlongoI, III, #; C.M. MnisiI, III; V. MlamboII; B. DlaminiI
IDepartment of Animal Science, Faculty of Natural and Agricultural Science, North-West University, Mafikeng, South Africa
IISchool of Agricultural Sciences, Faculty of Agriculture and Natural Sciences, University of Mpumalanga, Nelspruit, South Africa
IIIFood Security and Safety Niche area, Faculty of Natural and Agricultural Science, North-West University, Mafikeng, South Africa
ABSTRACT
Usefulness of red grape pomace (GP) by-product in ruminant rations is restricted by its high fibre content. Pre-treatment with exogenous carbohydrases may partly resolve this problem. Therefore, the effects of various levels of an exogenous fibrolytic enzyme mixture (Viscozyme® L) on chemical nutrient content and in vitro ruminal fermentation of GP were studied. This product, with an enzyme activity of >100 fungal beta-glucanase per gram (FBG/g) and a density of 1.2 g/mL, was applied to GP at 0 (ENZ0), 20 (ENZ20), 30 (ENZ30), 35 (ENZ35), and 45 g/kg DM (ENZ45). Neutral detergent fibre (NDF), acid detergent fibre (ADF) and insoluble condensed tannins (ICT) declined linearly as enzyme levels increased. Significant negative linear trends were observed for the rate of gas production at 36 and 48 hours, whereas quadratic trends were observed at 12- and 24-hours incubation in response to enzyme levels. Cumulative gas production at 12 hours and 24 hours and in vitro organic matter degradability at 96 hours post incubation increased linearly as treatment levels increased. Effective gas production declined linearly (P =0.041; R2 =0.085), whereas the slowly degradable fraction (P =0.042, R2 =0.084), the fractional degradation rate (P =0.003, R2 =0.175) and potential gas production (P =0.043, R2 =0.083) showed quadratic trends as enzyme levels increased. Pre-treatment with Viscozyme reduced the fibre levels of GP and increased in vitro ruminal organic matter degradability. The optimum level for inclusion of Viscozyme was 5 g/kg.
Keywords: agricultural by-product, enzymes, fibre, fermentation, proximate constituents
Introduction
Red grape pomace (GP), a by-product of the vinification process of grape wine and juice, poses disposal challenges because it is bulky and is not easily biodegradable (Dwyer et al., 2014). Despite its potential value as a source of bioactive compounds, GP is frequently disposed of by dumping in landfills and being incinerated, causing air and soil pollution (Kumanda et al., 2019). It is important to identify alternative uses for GP to increase its value while protecting the environment. As part of ruminant diets, GP is a potential source of water-soluble monosaccharides such as fructose and glucose, water-insoluble polysaccharides (pectin, cellulose, and xyloglucan), and beneficial bioactive compounds (Corbin et al., 2015). Hemicellulose, cellulose, and lignin are the major components of GP cell walls (Han et al., 2003. Several hydrolytic enzymes, including hemicellulase, cellulase, pectinase, and xylanase, are required to degrade these cell-wall polymers (Beg et al., 2001). Red grape pomace cell walls also contain a pectin-rich layer that covers the cellulose-hemicellulose inner layer (Gao et al., 2016). A high concentration of lignin in GP causes low digestibility and adverse effects on animal performance (Kholif et al., 2017). Indeed, Molina-Alcaide et al. (2008) and Abarghuei et al. (2015) reported that the use of GP on ruminant diets resulted in low effective dry matter degradability ranging between 25% and 40%.
The use of exogenous enzymes in diets of ruminants has received substantial interest (Beauchemin et al., 2003). Viscozyme L is a mixture of hemicellulase, cellulase, xylanase, arabanase, and ß-glucanase, which are all capable of hydrolysing non-starch polysaccharides effectively (Yu et al., 2003; Guan & Yao, 2008). When this enzyme mixture was used to hydrolyse carbohydrates in rapeseed meal at the rate of 0, 12, 48, 96 and 192 FBG, according to the carbohydrate treatment method described by Shahidi (1990), an increase in protein digestibility from 41% to 68% was observed (Rodrigues et al., 2014). Pre-treatment of unripe apples with Viscozyme L was also demonstrated to be effective in extracting polyphenols (Zheng et al., 2009). Elghandour et al. (2016) postulated that exogenous enzyme application levels depend on the microbial strain, enzyme activity, and nature of the substrate. It is important to use the correct application rates because higher enzyme application rates may have an antagonistic effect on feed utilization and increase feed costs (Togtokhbayar et al., 2015) whereas lower rates may reduce enzyme effectiveness. The effectiveness and optimum rates of application of Viscozyme L to valorize GP by-product for ruminants have not been determined. Thus, this study investigated the chemical nutrient and in vitro ruminal fermentation responses of GP when pre-treated with incremental levels of a Viscozyme L mixture. It was assumed that pre-treatment with a Viscozyme L mixture would enhance the nutritive value of red GP for ruminants.
Materials and Methods
The experimental work was carried out at Northwest University Experimental Farm (Molelwane), South Africa. The GP (Vitis vinifera L. var. Shiraz) was procured from Blaauwklippen Wine Estate (Western Cape, South Africa) and sun-dried until constant weight. In preparation for enzyme treatment, the sun-dried GP was ground to pass through a 1 mm screen (Polymix-MFC 90D, Switzerland). Viscozyme L (Sigma-Aldrich, Modderfontein, South Africa), with an enzyme activity of > 100 FBG/g and 1.2 g/mL density, was applied at 0, 20, 30, 35 and 45 g/kg DM. Each of the five enzyme treatments was replicated five times, with each replicate GP sample being treated independently. The prescribed quantity of enzyme was mixed with 100 mL of distilled water, which was then sprayed onto 100 g of GP substrate in a partially closed foil plate to prevent water runoff. The mixture of the enzyme and GP was then left to react overnight at room temperature (~25 °C) before being oven-dried for 48 hours at 60 °C (Kumanda et al., 2019). The untreated replicate GP samples were processed in a similar way but without using the enzyme. The treated and dried GP samples were subsequently reground (1 mm sieve) and stored at room temperature in labelled sample bottles pending nutritional evaluation.
Dry matter (DM), organic matter (OM), and crude protein (CP) were determined according to methods of the Association of Official Analytical Chemists (AOAC, 2005). The samples were oven-dried for 12 hours at 105 °C to assess the DM content (method no. 930.15). ANKOM2000 fibre analyser (ANKOM Technology, New York) was used to determine NDF and ADF according to Van Soest et al. (1991), where samples (0.45 - 0.5 g) were refluxed with neutral and acid detergent solutions for 1 hour and 1 hour 15 minutes, respectively. A heat-stable bacterial α-amylase was included in the determination of NDF. Acid detergent lignin was evaluated by dissolving the cellulose in the ADF residue with 72% sulphuric acid. The NDF, ADF and acid detergent fibre (ADL) were expressed inclusive of the residual ash.
The concentrations of soluble total phenolics (SPh) and insoluble total phenolics (iPh) of the GP samples were analysed using the Folin-Ciocalteau method and expressed as tannic acid equivalents (Makkar, 2003), with absorbance measured at 750 nm wavelength. The modified butanol-HCl method was used to determine the soluble (SCT) and insoluble condensed tannins (iCT) of the GP samples (Porter et al., 1986), and absorbance was measured at 550 nm wavelength.
The procedures used to care for the rumen-cannulated donor cow were authorized by the Animal Care Research Ethics Committee of North-West University, South Africa (Approval no. NWU-00699-18-A5). The rumen liquor was collected before morning feeding from a Bonsmara cow fitted with a permanent cannula (~600 kg live weight). The cow had unlimited access to a mixture of lucerne hay and blue buffalo grass (Cenchrus ciíiaris), and fresh, clean water before the collection of the rumen liquor. Rumen liquor was collected from various locations in the rumen and hand-squeezed into pre-warmed (39 °C) thermos flasks. The rumen liquor was then blended and strained through a two-layered muslin cloth while being purged with deoxygenated CO2 to mimic the anaerobic environment of the rumen. About 1 g of enzyme-treated and untreated GP substrates were each weighed into serum bottles (125 mL) to which an ANKOM buffer solution (90 mL) was added, followed by inoculation with 10 mL of the processed rumen liquor. The serum bottles were then closed with airtight rubber stoppers. Blank serum bottles with buffered rumen fluid but without GP substrates were also incubated at 39 °C in an incubator (ECR Manufacturing; model no: SU131H; serial no: 128538; fans: 15 amps; elementary 0.0k). Gas pressure readings (RPT) were taken at 2, 4, 6, 8, 12, 24, 36, 48, 72 and 96 hours post inoculation by inserting a 23 g needle fitted to a pressure transducer (PX4200-015GI, Omega Engineering Inc., Canada) with a microprocessor that recorded pressure (psi). The following equation set for the RPT (Mhlongo et al., 2021) was used to convert the gas pressure readings (psi) to the gas volume (mL): y = 0.034x2 + 6.2325x + 1.8143, where y is the gas volume (mL) and x is the measured gas pressure (psi). Readings from two blank serum bottles were used to correct the gas readings. At the end of the incubation period (96 hours), bottles were placed in a cold room at 5 °C for 1 hour to restrict microbial fermentation of the substrate.
Pre-weighed sintered glass crucibles (100-160 μm porosity) were used under vacuum to retrieve the undegraded residues, which were then dried in an oven set at 105 °C and ashed at 600 °C to estimate OM degradability. Partitioning factors (mL/mg) were calculated as a ratio of in vitro organic matter degradability to cumulative gas production at 96 hours of the incubation period. Estimates of the cumulative gas production parameters to describe the dynamics of ruminal fermentation were obtained by fitting data to the 0rskov & McDonald (1979) non-linear model using SAS (SAS Institute, Cary, North Carolina, USA).
Data for linear and quadratic effects of incremental levels of enzyme treatment were evaluated with response surface regression analysis using PROC RSREG of SAS (SAS Institute, Cary, North Carolina, USA) to fit the following quadratic equation:
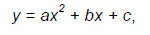
where: y is the dependent variable;
a and b are the coefficients of the equation;
c is the intercept; and
x is the enzyme treatment level (g/kg).
The quantity  is the x value for optimal response. The data were further analysed with one-way analysis of variance in which enzyme treatment was the only independent variable. Effects detected with P <0.05 were considered significant, and the least square means were separated using the probability of difference option in SAS.
is the x value for optimal response. The data were further analysed with one-way analysis of variance in which enzyme treatment was the only independent variable. Effects detected with P <0.05 were considered significant, and the least square means were separated using the probability of difference option in SAS.
Results and Discussion
Neither linear nor quadratic trends were observed in any of the response variables with increasing enzyme levels except for CP, NDF, ADF, SCT and iCT (Table 1). Significant negative linear trends were observed for CP (y = 11.7 (±0.157) - 0.039 (±0.015) x; P = 0.028; R2 = 0.308)), NDF (y = 369.7 (±9.33) - 9.20 (±3.90) x; P = 0.001; R2 = 0.210), ADF (y = 297.5 (±13.07) - 4.70 (±5.46) x; P = 0.016; R2 = 0.120) and iSCT (y = 0.31 (±0.062) - 0.03 (±0.026) x; P = 0.029; R2 = 0.10) as enzyme levels increased. A significant quadratic response (y = 0.36 (±0.047) + 0.07 (±0.020) x - 0.006 (±0.002) x2; P = 0.001; R2 = 0.20) was observed for SCT as enzyme treatment levels increased. There were no significant differences on the chemical components amongst the treatments.
Table 2 shows the effect of enzyme treatment of GP on cumulative gas production at 12, 24, 36 and 48 hours after incubation. Cumulative gas production at 12 hours (y = 2.97 (± 2.817) x + 25.08 (± 6.75); P = 0.024; R2 = 0.103) and 24 hours (y = 3.95 (± 3.459) x +38.5 (± 8.28); P = 0.036; R2 = 0.089) increased linearly as fibrolytic enzyme levels rose. However, no linear and quadratic trends (P >0.05) were observed for cumulative gas production at 36 and 48 hours post incubation with increasing enzyme levels. No significant differences were observed on cumulative gas production among the treatments.
The effect of enzyme treatment of GP on the rate of gas production at 24 hours post incubation is shown in Table 3. The rate of gas production at 36 hours (y = 0.802 (±0.080) - 0.034 (±0.332) x; P = 0.020; R2 = 0.109) and 48 hours (y = 0.66 (±0.068) - 0.016 (±0.028) x; P = 0.036; R2 = 0.09) linearly declined with incremental levels of enzyme treatments. However, quadratic trends were observed at 12 hours (y = 0.58 (±0.23) + 0.25 (±0.094) x - 0.019 (±0.008) x2; P = 0.026; R2 = 0.095) and 24 hours (y = 0.625 (±0.123) + 0.088 (±0.0513) x - 0.009 (±0.005) x2; P = 0.046; R2 = 0.079) incubation times. ENZ45 had lower rate of gas produced at 24 hours post incubation (0.424 mL/h) than ENZ30 and ENZ35, which were significantly similar.
Neither linear nor quadratic trends (P >0.05) were observed for fraction a and lag time in response to incremental levels of enzyme (Table 4). However, effective gas production decreased linearly (y = 74.0 (±11.0) - 0.18 (±4.60) x; P = 0.041 ; R2 = 0.085) as the enzyme treatment level increased. Treating GP with incremental levels of a fibrolytic enzyme mixture resulted in a quadratic response for b (y = 2.9 (±1.39) x2 -29.0 (± 15.7) x + 242.1 (± 37.5); P = 0.042; R2 = 0.084), c (y = 0.007 (± 0.0022) + 0.003 (± .0009) x - 0.0003 (± 0.0001) X2; P = 0.003; R2 = 0.175) and Pgas (y = 3.0 (± .44) x2 - 29.6 (± 16.23) x + 253.6 (± 38.9); P = 0.043; R2 = 0.083). Only fraction c was affected significantly by enzyme treatment, where ENZ35 had the fastest rate of gas production (0.02 %/h), whereas ENZ0 and ENZ45 had the slowest and similar (P >0.05) rates of gas production.
Table 5 shows that cumulative gas production and PF at 96 hours post incubation were not affected (P <0.05) by fibrolytic enzyme treatment whereas a significant treatment effect was observed for the in vitro organic matter degradability (OMD) at 96 hours post incubation. Untreated GP substrate had the lowest OMD (392.0 g/kg OM) while ENZ30, ENZ35 and ENZ45 substrates had the highest OMD values, which did not statistically differ. Substrate ENZ30 had similar (P >0.05) OMD to all the other treatments. As enzyme pre-treatment levels increased, OMD increased linearly (y = 389.7 (± 26.52) - 5.07 (± 2.48) x; P = 0.0001; R2 = 0.526).
In ruminant production, the extent to which forages are utilized depends on their chemical composition (Kholif et al., 2016). In the current study, GP substrates treated with Viscozyme L had lower NDF and ADF content than the untreated GP, indicating the efficacy of the fibrolytic enzyme treatment in breaking down the GP cell wall. These could be attributed to Viscozyme L being a mixture of carbohydrases with the ability to degrade non-starch polysaccharides and liberate bound nutrients. It is not clear why crude protein levels of GP substrates declined as the levels of exogenous enzyme pre-treatment increased. The quadratic response observed for SCT as enzyme treatment levels increased could be because as the fibre matrix is degraded by the enzyme, more SCT can be extracted from the substrate, whereas iSCT levels decline because they are bound to the fibre. Increased solubility of condensed tannins points to increased availability of these phenolics to the animal, but may not translate to beneficial effects because it depends on the nature of the condensed tannins and pH of the digestive tract.
The rate and extent of ruminal gas production can be good indicators of the digestibility of feeds and synthesis of microbial protein (Elahi et al., 2014; Elghandour et al., 2016). Increasing enzyme levels resulted in higher cumulative gas production at 24 and 36 hours post inoculation, which could be because of the reduction in fibre levels of the treated GP substrate. Similar findings were reported by Badhan et al. (2018), who observed an increase in gas production in ruminal batch cultures when barley straw substrate was treated with an exogenous fibrolytic enzyme. The increased ruminal gas production with enzyme treatment indicated enhanced ruminal fermentation because gas production is closely correlated with the amount of OM fermented (Díaz et al., 2013). According to Menke and Steingass (1988), gas volume at 24 hours after incubation has a link with the metabolizable energy in feedstuffs, suggesting that treating GP with Viscozyme L can increase the metabolizable energy content of the diet. Substrates treated with enzymes at 30 and 35 g/kg DM had the highest rates of gas production. However, the highest treatment rate (45 g/kg DM) produced substrate with the slowest rate of gas production at 24 hours after incubation. High exogenous enzyme pre-treatment levels can prevent binding of endogenous enzymes to substrate receptors and thus negatively affect rate substrate degradation in the rumen (Togtokhbayar et al., 2015). Togtokhbayar et al. (2015) also found that the application of low to moderate levels of an exogenous enzyme product with xylanase activity on wheat straw increased in vitro ruminal gas production.
Fibrolytic enzyme pre-treatment of GP influenced fraction c, with GP treated with the enzyme at 35 g/kg resulting in the fastest rate of gas production (c). The fraction b showed a quadratic response to incremental levels of Viscozyme L treatment, which was expected given that an increase in enzyme levels would also increase the degradable portion of fraction b to a point where further increases in the enzyme would not release any significant substrate for fermentation. In this study, the fitted gas production data showed no effect on lag time for ENZ0 (4.145), ENZ20 (2.514), ENZ30 (2.630), ENZ35 (4.612), and ENZ45 (2.743), suggesting that the inclusion of Viscozyme L did not enhance the rapid proliferation of rumen microbes to colonize substrates and initiate fermentation. According to Ribeiro et al. (2016), this could be because the types of microbes and fibrolytic enzymes found in the rumen were not utilized to create enzyme products that worked synergistically with the rumen natural enzymes produced by ruminal microbes. Moreover, Adesogan et al. (2014) and Meale et al. (2014) reported that fibrolytic enzymes products were not formulated to mimic the optimal activity of the rumen. Partitioning factors, which indicate fermentation efficiency, were not affected by the enzyme pre-treatment. Contrastingly, Arhab et al. (2009) reported that fermentation efficiency increased with graded levels of the supplemental enzyme owing to increased substrate degradability. Nonetheless, in vitro organic matter degradability was enhanced by fibrolytic enzyme treatment, suggesting that Viscozyme L was able to degrade complex substrates to simpler ones, thereby allowing enhanced ruminal fermentation and high nutrient bioavailability.
Conclusions
Pre-treatment of red grape pomace with Viscozyme L reduced the fibre levels and resulted in an increase in in vitro organic matter digestibility of the substrates. Based on the quadratic responses of the fermentation parameters b, c, and Pgas, the optimum Viscozyme L pre-treatment rate for GP waste was 5 g/kg, suggesting that higher enzyme levels had no beneficial effect on its nutritive value.
Authors' contributions
CMM, VM and BD conceptualized the study; BD collected the data; CMM and VM conducted the statistical analyses and interpreted the results; GM and CMM wrote the initial draft; GM, CMM, VM and BD finalized the manuscript. All the authors have read and approved the finalized manuscript.
Conflict of Interest Declaration
The authors have no conflict of interest to declare.
References
Abarghuei, M.J., Rouzbehan, Y. & Alipour, D., 2015. The effect of tannins in grape pomace and oak leaf on the in vitro organic matter digestibility and in situ disappearance of sheep. Iran. J. Appl. Anim. Sci. 5, 95-103. [ Links ]
Adesogan, A.T., Ma, Z.X., Romero, J.J. & Arriola, K.G., 2014. Ruminant nutrition symposium: Improving cell wall digestion and animal performance with fibrolytic enzymes. J. Anim. Sci. 92, 1317-330. DOI: 10.2527/jas.2013-7273 [ Links ]
AOAC, 2005. Method number 984.13. In: Official methods of analysis of AOAC International, 16th ed. AOAC, Arlington, VA, USA. [ Links ]
Arhab, R., Macheboeuf, D., Aggoun, M., Bousseboua, H., Viala, D. & Besle, J.M., 2009. Effect of polyethylene glycol on in vitro gas production and digestibility of tannin-containing feedstuffs from North African arid zone. Trop. Subtrop. Agroecosyst. 10(3), 475-486. http://www.revista.ccba.uady.mx/urn:ISSN:1870-0462-tsaes.v10i3.200 [ Links ]
Badhan, A., Ribeiro Jr, G.O., Jones, D.R., Wang, Y., Abbott, D.W., Di Falco, M., Tsang, A. & McAllister, T.A., 2018. Identification of novel enzymes to enhance the ruminal digestion of barley straw. Bioresour. Technol. 260, 76-84. DOI: 10.1016/j.biortech.2018.03.086 [ Links ]
Beauchemin, K.A., Colombatto, D., Morgavi, D.P. & Yang, W.Z., 2003. Use of exogenous fibrolytic enzymes to improve feed utilization by ruminants. J. Anim. Sci. 81(14)suppl_2, E37-E47. https://doi.org/10.2527/2003.8114_suppl_2E37x [ Links ]
Beg, O.A., Takhar, H.S., Soundalgekar, V.M. & Woo, G., 2001. Hydrodynamic and heat transfer modelling of a non- Newtonian fluid flowing through geomaterial with boundary effects. Numerical simulation. 2nd International Conference of Computational Heat and Mass Transfer, Rio de Janeiro, Brazil. 22-26 October 2001. [ Links ]
Corbin, K.R., Hsieh, Y.S.Y., Bett, N.S., Byrt, C.S., Henderson, M., Stork, J. & Burton, R., 2015. Grape marc as a source of carbohydrates for bioethanol: Chemical composition, pre-treatment and saccharification. Bioresour. Technol. 76-83. https://doi.org/10.1016/j.biortech.2015.06.030 [ Links ]
Díaz, A., Carro, M.D., Saro, C., Mateos, I., Odongo, E. & Ranilla, M.J., 2013. In vitro evaluation of commercial fibrolytic enzymes for improving the nutritive value of low-quality forages. Anim. Nutr. Feed Technol. 13, 461 -476 https://www.researchgate.net/publication/287307106_In_Vitro_Evaluation_of_Commercial _Fibrolytic_Enzymes_for_Improving_the_Nutritive_Value_of_Low-Quality_Forages [ Links ]
Dwyer, K., Hosseinian, F. & Rod, M., 2014. The market potential of grape waste alternatives. J. Food Res. 3(2), 91-106. [ Links ]
Elahi, M.Y., Nia, M.M., Salem, A.Z.M., Mansouri, H., Olivares-Perez. J. & Kholif, A.E., 2014. Effect of polyethylene glycol on in vitro gas production kinetics of Prosopis cineraria leaves at different growth stages. Ital. J. Anim. Sci. 13, 363-368. https://doi.org/10.4081/ijas.2014.3175 [ Links ]
Elghandour, M.M.Y., Kholif, A.E., Salem, A.Z.M., De Oca, R.M., Barbabosa, A., Mariezcurrena, M. & Olafadehan, O.A., 2016. Addressing sustainable ruminal methane and carbon dioxide emissions of soybean hulls by organic acid salts. J. Clean. Prod. 135, 194-200. https://doi.org/10.1016/j.jclepro.2016.06.081 [ Links ]
Gao, Y., 2016. Parameters involved in the enzymatic deconstruction of the wine grape cell wall matrix during winemaking. PhD (Agri) thesis, Stellenbosch University, Western Cape, South Africa. [ Links ]
Guan, X. & Yao, H., 2008. Optimization of Viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chem. 106(1), 345-351. https://doi.org/10.1016/j.foodchem.2007.05.041 [ Links ]
Han, S.O., Yukawa, H., Inui, M. & Doi, R.H., 2003. Regulation of expression of cellulosomal cellulase and hemicellulase genes in Clostridium cellulovorans. J. Bacteriol. 185, 6067-6075. DOI: 10.1128/JB.185.20.6067-6075.2003 [ Links ]
Kholif, A.E., 2017. Anaerobic ensiling of raw agricultural waste with a fibrolytic enzyme cocktail as a cleaner and sustainable biological product. J. Clean. Prod. 142, 2649-2655. DOI:10.1016/j.jclepro.2016.11.012 [ Links ]
Kholif, A.E., Morsy, T.A., Gouda, G.A., Anele, U.Y. & Galyean, M., 2016. Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Anim. Feed Sci. Technol. 217, 45-55. https://doi.org/10.1016/j.anifeedsci.2016.04.012 [ Links ]
Kumanda, C., Mlambo, V. & Mnisi, C.M., 2019. From landfills to the dinner table: Red grape pomace waste as a nutraceutical for broiler chickens. Sustainability 11(7), 1931. DOI:10.3390/su11071931 [ Links ]
Makkar, H.P.S., 2003. Quantification of tannins in tree and shrub foliage. A laboratory manual. Springer, Dordrecht. [ Links ]
Meale, S.J., Beauchemin, K.A., Hristov, A.N., Chaves, A.V. & McAllister T.A., 2014. Board-Invited review: Opportunities and challenges in using exogenous enzymes to improve ruminant production. J. Anim. Sci. 92, 427-442. DOI: 10.2527/jas.2013-6869 [ Links ]
Menke, K.H. & Steingass, H., 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28, 7-55. [ Links ]
Mhlongo, G., Mnisi, C.M. & Mlambo, V., 2021. Cultivating oyster mushrooms on red grape pomace waste enhances potential nutritional value of the spent substrate for ruminants. PLoS ONE 16(2), e0246992. https://doi.org/10.1371/journal.pone.0246992 [ Links ]
Molina-Alcaide, E., Moumen, A. & Martín-García, A.I., 2008. By-products from viticulture and the wine industry: Potential as sources of nutrients for ruminants. J. Sci. Food Agric. 88(4), 597-604. DOI:10.1002/jsfa.3123 [ Links ]
Ørskov, E.R. & McDonald, J., 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to the rate of passage. J. Agric. Sci. 92, 499-503. DOI: https://doi.org/10.1017/S0021859600063048 [ Links ]
Porter, L.J., Hrstich, L.N. & Chan, B.G., 1986. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1, 223-230. https://doi.org/10.1016/S0031-9422(00)94533-3 [ Links ]
Ribeiro, G.O., Gruninger, R.J., Badhan, A. & McAllister, T.A., 2016. Mining the rumen for fibrolytic feed enzymes. Anim. Front. 6, 20-26. https://doi.org/10.2527/af.2016-0019 [ Links ]
Rodrigues, I.M.M.A., Carvalho, M.G.V.S. & Rocha, J.M.S., 2014. Increasing the protein content of rapeseed meal by enzymatic hydrolysis of carbohydrates. Bioresources 9, 2010-2025. DOI:10.15376/biores.9.2.2010-2025 [ Links ]
Shahidi, F., 1990. Canola and rapeseed: Production, chemistry, nutrition and processing technology. Van Nostrand Reinhold, New York, USA. [ Links ]
Togtokhbayar, N., Cerrillo, M.A., Rodriguez, G.B., Elghandour, M.M., Salem, A.Z., Urankhaich, C., Jigjidpurev, S., Odongo, N.E. & Kholif, A.E., 2015. Effect of exogenous xylanase on rumen in vitro gas production and degradability of wheat straw. Anim. Sci. J. 86, 765-771. DOI: 10.1111/asj.12364 [ Links ]
Van Soest, P.V., Robertson, J.B. & Lewis, B.A., 1991. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74(10), 3583-3597. DOI: 10.3168/jds.S0022-0302(91)78551-2 [ Links ]
Yu, L., Perret, J., Parker, T. & Allen, K.G., 2003. Enzymatic modification to improve the water-absorbing and gelling properties of psyllium. Food Chem. 82(2), 243-248. https://doi.org/10.1016/S0308-8146(02)00520-4 [ Links ]
Zheng, H.Z., Hwang, I.W. & Chung, S.K., 2009. Enhancing polyphenol extraction from unripe apples by carbohydrate- hydrolysing enzymes. J. Zhejiang Univ. Sci. B. 10(12), 912-919. DOI: 10.1631/jzus.B0920186 [ Links ]
Submitted 18 June 2021
Accepted 2 October 2021
Published 1 April 2022
# Corresponding author: godfreymhlongo3@gmail.com














