Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.51 no.5 Pretoria 2021
http://dx.doi.org/10.4314/sajas.v49i1.16
ARTICLES
Effect of exogenous protease on growth performance and meat quality in broilers reared on fishmeal-based diets
Najam-Us-Sahar#; Muhammad Aslam Mirza; Shaukat Ali Bhatti; Muhammad Saif ur Rehman
Institute of Animal and Dairy Sciences, University of Agriculture, Faisalabad 38040, Pakistan
ABSTRACT
This study determined the effect of protease supplementation in fishmeal-based diets on growth performance and meat quality of commercial broiler birds. Four hundred and thirty-two (432) day-old broiler chicks were divided into 36 experimental units of 12 chicks each. Nine experimental diets were formulated using three levels (0%, 25%, and 50%) of the fishmeal as protein source, on protein equivalent basis, with or without alkaline protease (CIBENZA® DP100), and with overestimation of nutritional value based on the enhanced digestibility coefficient (EDC) concept because of the use of alkaline protease. Feed consumption and bodyweight were measured weekly. On the last day of the trial, two birds from each pen were picked and processed for carcass and meat quality parameters. Data were analysed by analysis of variance in a 3 x 3 factorial arrangement of completely randomized design. Weight gain and feed conversion ratio (FCR) were improved in the birds with 25% fishmeal as a replacement for soybean meal (SBM) and enzyme supplementation. Similarly, higher protein digestibility, dressing percentage and thigh meat yield were observed in birds fed diets with 25% fishmeal with added enzyme. In the blood biochemical profile, uric acid levels were lower, and cholesterol and triglyceride were higher in the group fed diets with 25% fishmeal with EDC and enzyme. The addition of protease enzyme to the diet with 25% fishmeal improved growth performance, crude protein digestibility and carcass characteristics.
Keywords: complete blood count, enzyme, enhanced digestibility coefficient, soybean meal, serum biochemistry
Introduction
Precise feeding refers to the practice of meeting the nutrient requirements of birds accurately and involves the provision of the right amount of feed with optimum nutrient balance at right time (also called phase feeding) (Banhazi et al., 2012). After phase feeding, nutritionists devised a new term called 'enhanced digestibility coefficient', in which overestimation of the nutrients of various ingredients is being used under the influence of supplemented protease (Bertechini et al., 2020). Thus, dietary exogenous mono-component protease was used in fishmeal-based diets and was also reported to enhance the digestibility coefficients of amino acid-based diets. In another work with the same protease, increased digestibility was reported (Carvalho et al., 2009) of some amino acids (AAs) in SBM, corn and corn and wheat-based diets.
The gastrointestinal tract (GIT) of birds is not fully developed in the early days of its life, particularly secretion with sub-optimal levels of enzymes (Yin et al., 2018). A faster digesta passage rate and high demand for AA by the birds cause inadequate exposure to endogenous enzymes (Dosković et al., 2013) and a considerable portion of protein can be excreted (Lemme et al., 2004). The high demand of AAs by birds can be met by increasing dietary crude protein (CP) contents or by adding exogenous protease, which would complement the endogenous proteolytic activity to digest the dietary proteins well. Digestion of proteins begins in the proventriculus, where the pH is 2.5 to 3 (highly acidic), followed by the duodenum, in which the pH is 6 to 6.8 (neutral). In the first few weeks, the amount of proteases in pancreatic juice and pepsin is insufficient (Uni & Sklan, 1999). This insufficiency can be mitigated with the supplementation of protease in broiler diet. Proteases are pH specific, but there is a wide variation in the pH of the GIT from highly acidic to moderately neutral. This refutes the one-size-fits-all approach and permits the use of proteases that can act efficiently on a wide range of pH. Proteases may help in the solubility of dietary proteins and also hydrolysis of certain proteins (Caine et al., 1998).
Several studies explained the positive effects of exogenous protease supplementation in poultry diets. Birds fed a low protein diet (20.5% CP) with protease at 7500 PROT units/kg showed improved FCR compared with control (22.5% CP) (Angel et al., 2011). Moreover, the addition of 0.075% protease increased bodyweight gain (BWG) (Yadav & Sah, 2005). However, the response of chicken broilers to the addition of protease was not always positive. Several studies reported that protease supplementation did not improve (P >0.05) feed intake and FCR (Flores et al., 2016; Walk et al., 2019). Therefore, the present study was envisaged to evaluate the effect of commercial protease supplementation to fishmeal-based diets on growth, protein digestibility, serum biochemistry and microbial count of commercial broiler chickens.
Materials and Methods
This study investigated the effects on chicken broilers of protease in a fishmeal-based diet. Four hundred and thirty-two (432) broiler chicks were distributed into thirty-six (36) experimental units of 12 chicks each. Isocaloric and isonitrogenous diets were prepared using 0%, 25%, and 50% of fishmeal with and without protease (CIBENZA® DP100) supplementation. Because supplemental protease may increase AA digestibility (Table 1), a third trio of diets was formulated using the EDC (Bertechini et al., 2020) with software that was developed by Novus International (2016). The exogenous protease (CIBENZA® DP100) in this study was supplied by Novus International (Bangkok, Thailand). CIBENZA DP100 is a broad-spectrum protease that works on all protein sources and is used as a feed additive because it is heat stable protease and optimizes digestibility in poultry. It consists of dried Baciííus íicheniformis produced by fermentation combined with ground limestone, mineral oil, and natural flavour. The enzyme activity of the protease is said to be 600000 U/g and it is fed at the rate of 500 g/ton of feed. The diets for the starter and finisher phases are shown in Tables 2 and 3.
Bodyweight and feed offered and refused were recorded weekly. Feed intake was calculated by subtracting feed refused from feed offered. Feed conversion ratio was calculated by dividing feed intake (g) by weight gain (g). Protein efficiency ratio (PER) was calculated by dividing weight gain (g) by protein intake (Kamran et al., 2008). European production efficiency factor (EPEF) was calculated multiplying liveability, live weight (kg) with 100 and divided by FCR and age (days) using this formula (Marcu et al., 2013).
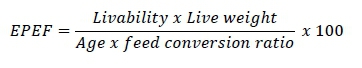
Digestibility of the dietary nutrients was measured with the indirect marker method. Acid insoluble ash (AIA) was used as an external marker. Celite® (a source of AIA) was added to feed at 1.0% of the ration on the 30th day of the experiment. An adaptation period of 48 hours was given to the birds before collection. After that fresh faecal samples were collected through sterilized spatula in zipper bags after every hour until sufficient amount was collected. Faecal samples were weighed and dried at 65 °C to preserve the samples and then contents of feed and faeces were analysed for nutrient composition (AOAC, 2000). This relationship was used to determine the digestibility coefficient for each nutrient.
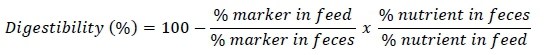
At the end of the trial, two birds were randomly chosen from each experimental unit, weighed and processed to determine the dressing percentage, breast meat yield, thigh meat yield and relative weights of the abdominal fat pad, heart, liver and gizzard. Meat portion from the breast was chilled at 4 °C before measuring the pH, water holding capacity and cooking loss. Following Jeacocke (1977), at three hours after slaughter approximately 1.5 g of breast muscle was homogenized in 10 mL water and pH was measured with a pH meter (MW102, Milwaukee Instruments, Inc., Rocky Mount, North Carolina, USA). To determine water-WHC, a 15 g sample of the breast muscle was centrifuged at 5000 rpm for 15 minutes, water released from the meat was drained off to avoid re-absorption, and the meat samples was reweighed to determine liquid loss (Pearson & Dutson, 1995). A meat sample weighing approximately 40 g was cooked to an internal temperature of 75 ± 1 °C in a water bath (80 ± 0.5 °C) for half hour. After the meat was cooled, cooking loss was calculated as the difference in weight of the sample before and after cooking (Ahmed et al., 2015).
At the end of the experiment, two birds from each unit were placed in a separate pen with a cleaned plastic sheet and fresh faecal samples were collected. Then, 1/10 serial dilutions of excreta were prepared in buffered peptone water. Nutrient and Rogosa (Rogosa et al., 1951) agars were used for total bacteria and lactobacilli counts, respectively. Rapid E. Coli 2 agar and E. Coli supplement was used to quantify E. coli. Blood samples were collected to separate serum for biochemical analyses through commercial assay kits that is, total protein, serum albumin, urea, uric acid, triglycerides, and cholesterol. Blood was collected in EDTA tubes for a complete blood count.
Data were subjected to analysis of variance appropriate to a 3 x 3 factorial arrangement of treatments to determine the effects of the diets, level of fishmeal and the interaction. If an effect was significant (P <0.05), then Tukey's HSD test was used to compare the means (Steel et al., 1997).
Results and Discussion
Growth performance data are given in Table 4. The interaction of fishmeal and diet formulation was highly significant for weight gain (P <0.01) and significant for feed intake (P <0.05). Thus, because the differences between the levels of fishmeal depended on the diet formulation and vice versa, means for the main effects are not shown. As a result, all of the efficiency ratios were significantly affected by the interaction between diet formulations and level of fishmeal that was included in it. When fishmeal was not included in the diet, feed intake was lower than when protease was provided in the conventional diet, but feed intake higher with the EDC formulated diet that included protease. Feed intake was similar across all diets that contained fishmeal.
Data describing carcass yields and meat quality from 35-day-old broilers are shown in Table 5. The interaction of diet formulation with the level at which fishmeal was included had an effect (P <0.05) on dressing percentage and the yield of thigh meat. The diet formulation had effects on breast yield and waterholding capacity, which approached statistical significance (P <0.10). The level of fishmeal in the diet affected the yields of breast and thigh and water-holding capacity. The yield of breast meat was higher for birds fed 0% fishmeal than those fed 25%. However, the birds that were fed 50% fishmeal had a breast yield that was intermediate between these extremes and were not significantly different from either. The birds fed 25% fishmeal yielded a greater percentage of thigh than birds fed 0% and 50%, which were similar. Waterholding capacity was greatest for the birds that were not fed fishmeal.
Data on total bacterial and coliform count are shown in Table 6. Differences in total bacterial count among the levels of fishmeal were significant in those diets to which fishmeal had been added, producing higher counts than the diet without fishmeal. However, these effects should be interpreted with caution because the interaction of the fishmeal effect with the effect of protease supplementation approached significance. The apparent interaction results from the high bacterial count for birds fed the 50% fishmeal diet, which was not supplemented with protease. No differences among the treatments were noted in coliform count. Odetallah et al. (2005) reported a reduction (P <0.05) of ileal Clostridium perfringens with the supplementation of protease.
An interaction between the level of fishmeal and diet formulation was observed for crude protein digestibility (P =0.04). Higher protein digestibility was found in diet where 25% of the SBM was replaced with fishmeal on protein equivalent basis with the addition of protease. However, diet formulation had no effect on CP digestibility in either the diet that did not contain fishmeal or the diet in which 50% of the SBM was replaced by fishmeal.
Data on serum biochemistry are presented in Table 7. The interaction between the level of fishmeal in the diet and protease supplementation was significant (P <0.01) for the levels of uric acid albumin, cholesterol, and triglycerides in the serum, and approached significance (P <0.10) for urea and total proteins. Serum uric acid levels were elevated in broilers fed 50% fishmeal supplemented with protease compared with those birds fed the same diet without protease. The birds fed the 50% fishmeal diet that was formulated with EDC had an intermediate level of serum uric acid. However, this pattern of effects was not observed when lower levels of fishmeal were fed. When feeding the diet without fishmeal or with 25% fishmeal, protease supplementation produced no significant differences. Serum albumin was higher in broilers that were fed 25% fishmeal and not supplemented with protease compared with those fed this level of fishmeal in a diet formulated using with EDC and supplemented with protease. The conventional diet with 25% fishmeal produced an intermediate response. Again, this pattern of effects was not observed with 0% or 50% fishmeal in which there were no differences that could be ascribed to protease supplementation. The interaction effects on cholesterol are perplexing. When the birds were fed the diet without fishmeal, there were no differences between the diet without protease, the diet with protease and the diet formulated using EDC and supplemented with protease. However, when feeding the 25% fishmeal diet the serum cholesterol was elevated in the broilers fed the diet formulated using EDC and supplemented with protease, whereas when feeding the 50% fishmeal diet, serum cholesterol was elevated in the birds fed the conventional diet supplemented with protease. These two diets that produced elevated levels of cholesterol produced the highest and lowest levels of triglycerides.
Protease supplementation was expected to increase nutrient digestibility and absorption, resulting in improved growth performance. The present results were in line with the findings of Mohammadigheisar and Kim (2018), who concluded that protease supplementation alleviated the adverse effects of low levels of dietary CP on weight gain and FCR. Similarly, reduced dietary CP decreased the growth of broiler chickens linearly, whereas protease supplementation improved BWG and FCR (Law et al., 2018). Broilers fed a wheat-based diet supplemented with protease (Cibenza® DP 100, Novus) also had improved (P <0.05) weight gain and FCR (Moss et al., 2017). Birds fed a low protein diet (CP 20.5%) supplemented with 7500 PROT units/kg protease (1 PROT unit is the amount of enzyme that releases 1 μmol of p-nitroaniline from 1 μΜ of substrate) showed improved FCR compared with a control diet that contained 22.5% CP (Angel et al., 2011). The addition of protease (serine) showed improvement (P <0.05) in weight gain and FCR at recommended CP level and lower in broiler diets (Fru-Nji et al., 2011). Addition of protease at 0.075% was reported to show improved (P <0.05) BWG in broiler chickens (Yadav & Sah, 2005). Broilers supplemented with protease in the starter phase, in either high or low CP diets, showed improved BWG and FCR (Odetallah et al., 2005). The results of the present study were not in line with the outcomes of some studies (Flores et al., 2016; Walk et al., 2019) that reported that protease supplementation did not improve (P >0.05) feed intake and FCR. These variations in results may have been because of the differences in the nature and source of protease used in the studies.
Dressing percentage and thigh meat yield were the highest in groups fed the diet in which 25% of dietary SBM was replaced with fishmeal and supplemented with protease. When 50% of dietary SBM was replaced with fishmeal, the carcass yield deteriorated (P <0.05). Breast meat yield and thigh meat yield were accordingly reduced (P <0.05), whereas there was no effect on breast meat yield. In accordance with this, Dessimoni et al. (2019) reported that diets containing the amino acid level recommended by Cobb-Vantress (2008) with supplementation of protease showed better slaughter weight than those with lower AA. Similarly, Dos-Santos et al. (2017) found that breast meat yield was reduced (P <0.10) with the inclusion of protease. Law et al. (2018) and Wang et al. (2006) reported that supplementation of protease (Versazyme) in low CP (17.2% in starter and 15.6% in finisher) diets improved (P <0.05) carcass yield and dressing percentage. In contrast, Freitas et al. (2011) observed that protease supplementation had no effect on carcass response. Park and Kim (2018) reported a lack of response in weight of breast muscle, abdominal fat, liver, bursa of Fabricius, spleen and gizzard with or without protease. Differences between the studies might be because of the protease product and level.
The highest water-holding capacity was observed in group fed SBM as a protein source. However, pH and cooking loss were unchanged for treatments. The results were similar to those of Park and Kim (2018), who reported that protease addition had no effect on water holding capacity, pH, drip loss and colour. On the other hand, other authors reported that the addition of protease had a beneficial effect. (Omojola & Adesehinwa, 2007; Symeon et al., 2009). Tabook et al. (2006) concluded that protease supplementation had no effect on broiler health. These changes were probably because of the variability in the weight and age of birds.
Protein digestibility was enhanced (P <0.05) in birds fed diet formulated from fishmeal and protease supplementation. This might be because of the enzyme supplementation that enhanced the digestibility of fishmeal proteins. The results of the study were in agreement with Cowieson et al. (2019), who stated that exogenous protease improved the protein and energy digestibility in GIT. Freitas et al. (2011) and Angel et al. (2011) reported that when protease was supplemented in high and low protein diets, an increase was noted in the digestibility of CP (P <0.05). Olukosi et al. (2007) showed that single protease improved (P <0.05) apparent ileal digestibility of nitrogen at a high enzyme dose. Results that agreed with the present study were reported by Yadav and Sah (2005), who stated that supplementation of protease in reduced CP (19% in starter and 17.5% in finisher) diets improved the CP digestibility in broiler diets. In contrast, Mahmood et al. (2017) observed no effect of protease supplementation on nitrogen retention with it being reduced with lower a CP diet.
Conclusion
Higher growth performance can be achieved using protein share from fishmeal up to 25% on protein equivalent basis in broiler diet. Further, the addition of protease to diets that included fishmeal had only minor effects on the performance of the broilers that were fed the relatively high CP diets that were used in this study.
Acknowledgements
The authors acknowledge the fellowship provided by the Higher Education Commission of Pakistan (HEC) to N.U. Sahar under the framework of HEC Indigenous PhD Fellowship Program.
Conflict of Interest Declaration
The authors declare there is no conflict of interest.
Author's contribution
NS: experimental work and manuscript writing; MAM: experimental designing and manuscript improvement; SAB: data analysis and manuscript improvement; MSR: manuscript improvement
References
Ahmed, H.O., Hassan, Z. & Abdul-Manap, M.N., 2015. Effect of slaughtering methods on meat quality indicators, chemical changes and microbiological quality of broiler chicken meat during refrigerated storage. J. Agri. Vet. Sci. 8, 12-17. DOI: 10.9790/2380-08911217 [ Links ]
Angel, C.R., Saylor, W., Vieira, S.L. & Ward, N., 2011. Metabolism and nutrition: Effects of a monocomponent protease on performance and protein utilization in 7- to 22-day-old broiler chickens. Poult. Sci. 90, 2281-2286. DOI: 10.3382/ps.2011-01482 [ Links ]
AOAC, 2000. Official methods of analysis. 17th edition. Association of Official Analytical Chemists. Maryland, USA. [ Links ]
Banhazi, T.M., Lehr, H., Black, J.L., Crabtree, H., Schofield, P., Tscharke, M. & Berckmans, D., 2012. Precision livestock farming: An international review of scientific and commercial aspects. Int. J. Agric. Biol. Eng. 5, 1-10. DOI: 10.3965/j.ijabe.20120503.00 [ Links ]
Bertechini, A.G., de Carvalho, J.C.C., Carvalho, A.C., Dalolio, F.S. & Sorbara, J.O.B., 2020. Amino acid digestibility coefficient values of animal protein meals with dietary protease for broiler chickens. Transl. Anim. Sci. 4, 1-11. DOI: 10.1093/tas/txaa187 [ Links ]
Caine, W.R., Verstegen, M.W.A., Sauer, C., Tamminga, S. & Schulze, H., 1998. Effect of protease treatment of soybean meal on content of total soluble matter and crude protein and level of soybean trypsin inhibitors. Anim. Feed Sci. Technol. 71, 177-183. https://doi.org/10.1016/S0377-8401(97)00139-9 [ Links ]
Carvalho, J.C.C., Bertehini, A.G., Rios, R.L., Mesquita, F.R., Lima, E.M.C. & Sorbara, J.O.B., 2009. Use of a protease to enhance the utilization of corn amino acids by broilers. Poult. Sci. 88, 69-70. https://www.researchgate.net/publication/301349366_Use_of_a_protease_to_enhance _the_utilization_of_corn_amino_acids_by_broilers [ Links ]
Cobb-Vantress, 2008. Broiler management guide. http://www.tt-trade.cz/docs/cobb-broiler-en.pdf [ Links ]
Cowieson, A.J., Sorbara, J.O.B., Pappenberger, G., Abdollahi, M.R. & Ravindran V., 2019. Toward standardized amino acid matrices for exogenous phytase and protease in corn-soybean meal-based diets for broilers. Poult. Sci. 99, 3196-3206. https://doi.org/10.1016/j.psj.2019.12.071 [ Links ]
Dessimoni, G.V., Dalólio, F.S., Moreira, J., Teixeira, L.V., Bertechini, A.G. & Hermes, R.G., 2019. Protease supplementation under amino acid reduction in diets formulated with different nutritional requirements for broilers. Braz. J. Poult. Sci. 21, 1-8. DOI: 10.1590/1806-9061-2017-0707 [ Links ]
Dos-Santos, T.T., Masey O'Neill, H.V., González-Ortiz, G., Camacho-Fernández, D. & López-Coello, C., 2017. Xylanase, protease and superdosing phytase interactions in broiler performance, carcass yield and digesta transit time. Anim. Nutr. 3, 121-126. DOI: 10.1016/j.aninu.2017.02.001 [ Links ]
Dosković, V., Bogosavljević-Bosković, S., Pavlovski, Z., Milošević, B., Škrbić, Z., Rakonjac, S. & Petričević, V.,, 2013. Enzymes in broiler diets with special reference to protease. World Poultry Sci. J. 69, 343-360. DOI:10.1017/S0043933913000342 [ Links ]
Flores, C., Williams, M., Pieniazek, J., Dersjant, Y., Awati, A. & Lee, J.T., 2016. Direct-fed microbial and its combination with xylanase, amylase, and protease enzymes in comparison with AGPs on broiler growth performance and footpad lesion development. J. Appl. Poult. Res. 25, 328-337. https://doi.org/10.3382/japr/pfw016 [ Links ]
Freitas, D.M., Vieira, S.L., Angel, C.R., Favero, A. & Maiorka, A., 2011. Performance and nutrient utilization of broilers fed diets supplemented with a novel mono-component protease. J. Appl. Poult. Res. 20, 322-334. https://doi.org/10.3382/japr.2010-00295 [ Links ]
Fru-Nji, F., Kluenter, A.M., Fischer, M. & Pontoppidan, K., 2011. A feed serine protease improves broiler performance and increases protein and energy digestibility. J. Poult. Sci. 48, 239-246. https://doi.org/10.2141/jpsa.011035 [ Links ]
Jeacocke, R.E., 1977. Continuous measurement of the pH of beef muscle in intact beef carcasses. J. Food Technol. 12, 375-386. https://www.researchgate.net/publication/229810183_Continuous_measurements_of _the_pH_of_beef_muscle_in_intact_beef_carcases [ Links ]
Kamran, Z., Sarwar, M., Nisa, M., Nadeem, M.A., Mahmood, S., Babar, M.E. & Ahmed, S., 2008. Effect of low-protein diets having constant energy-to-protein ratio on performance and carcass characteristics of broiler chickens from one to thirty-five days of age. Poult. Sci. 87:468-474. DOI: 10.3382/ps.2007-00180 [ Links ]
Law, F.L., Zulkifli, I., Soleimani, A.F., Liang, J.B. & Awad, E.A., 2018. The effects of low-protein diets and protease supplementation on broiler chickens in a hot and humid tropical environment. Asian-Aust. J. Anim. Sci. 31:12911300. DOI: 10.5713/ajas.17.0581 [ Links ]
Lemme, A., Ravindran, V. & Bryden, W.L., 2004. Ileal digestibility of amino acids in feed ingredients for broilers. World Poultry Sci. J. 60, 423-438. DOI: 10.1079/wps200426 [ Links ]
Mahmood, T., Mirzaa, M.A., Nawaz, H., Shahid, M., Athar, M. & Hussain, M., 2017. Effect of supplementing exogenous protease in low protein poultry by-product meal based diets on growth performance and nutrient digestibility in broilers. Anim. Feed Sci. Technol. 228:23-31. DOI: 10.1016/j.anifeedsci.2017.01.012 [ Links ]
Marcu, A., Vacaru, I., Gabi D., Liliana, P.C., Marcu, A., Marioara, N., Ioan, P., Dorel, D., Bartolomeu, K. & Cosmin, M., 2013. The influence of genetics on economic efficiency of broiler chickens growth. Anim. Sci. Biotech. 46, 339346. https://www.semanticscholar.org/paper/The-Influence-of-Genetics-on-Economic-Effciency-of-Marcu-Văcaru-Opriş/fe675a970be3fd76a1ae56164755261792ab142d [ Links ]
Mohammadigheisar, M. & Kim, I.H., 2018. Addition of a protease to low crude protein density diets of broiler chickens. J. Appl. Anim. Res. 46,1377-1381. DOI: 10.1080/09712119.2018.1512862 [ Links ]
Moss, A.F., Chrystal, P.V., Truonga, H.H., Liu, S.Y. & Selle, P.H., 2017. Effects of phytase inclusions in diets containing ground wheat or 12.5% whole wheat (pre- and post-pellet) and phytase and protease additions, individually and in combination, to diets containing 12.5% pre-pellet whole wheat on the performance of broiler chickens. Anim. Feed Sci. Technol. 234, 139-150. https://agris.fao.org/agris-search/search.do?recordID=US201800196786 [ Links ]
Novus International, 2016. CIBENZA®. https://www.novusint.com/th-th/Products/cibenza [ Links ]
NRC, 1994. Nutrient requirements of poultry. National Research Council. 9th revised edition. National Academy of Sciences, National Academy Press, 2101 Constitution Ave, Washington, DC 20418. [ Links ]
Odetallah, N.H., Wang, J.J., Garlich, J.D. & Shih, J.C.H., 2005. Versazyme supplementation of broiler diets improves market growth performance. Poult. Sci. 84, 858-864. https://doi.org/10.1093/ps/84.6.858 [ Links ]
Olukosi, O.A., Cowieson, A.J. & Adeola, O., 2007. Age-related influence of a cocktail of xylanase, amylase, and protease or phytase individually or in combination in broilers. Poult. Sci. 86, 77-86. DOI: 10.1093/ps/86.1.77 [ Links ]
Omojola, A.B. & Adesehinwa, A.O.K., 2007. Performance and carcass characteristics of broiler chickens fed diets supplemented with graded levels of Roxazyme G®. Int. J. Poult. Sci. 6, 335-339. DOI: 10.3923/ijps.2007.335.339 [ Links ]
Park, J.H. & Kim, I.H., 2018. Effects of a protease and essential oils on growth performance, blood cell profiles, nutrient retention, ileal microbiota, excreta gas emission, and breast meat quality in broiler chicks. Poult. Sci. 97, 2854-2860. DOI: 10.3382/ps/pey151 [ Links ]
Pearson, A.M. & Dutson, T.R., 1995. Quality attributes and their measurement in meat, poultry and fish products. Springer: Berlin, Germany. DOI: 10.1016/0956-7135(95)90004-7 [ Links ]
Rogosa, M., Mitchell, J.A. & Wiseman, R.F., 1951. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J. Bacteriol. 62(1), 132-133. doi: 10.1128/jb.62.1.132-133. [ Links ]
Steel, R.G.D., Torrie, J.H. & Dickie, D.A., 1997. Principles and procedures of statistics. A biometric approach. 3rd edition. McGraw-Hill, Toronto, Canada. [ Links ]
Symeon, G.K., Zintilas, C., Ayoutanti, A., Bizelis, J.A. & Deligeorgis, S.G., 2009. Effect of dietary oregano essential oil supplementation for an extensive fattening period on growth performance and breast meat quality of female medium-growing broilers. Can. J. Anim. Sci. 89, 331-334. https://cdnsciencepub.com/doi/pdf/10.4141/CJAS09027 [ Links ]
Tabook, N.M., Kadim, I.T., Mahgoub, O. & Al-Marzooqi, W., 2006. The effect of date fibre supplemented with an exogenous enzyme on the performance and meat quality of broiler chickens. Br. Poult. Sci. 47, 73-82. https://doi.org/10.1080/00071660500475160 [ Links ]
Uni, Z., Noy Y. & Sklan, D., 1999. Posthatch development of small intestinal function in the poult. Poult. Sci. 78, 215-222. https://pubmed.ncbi.nlm.nih.gov/10051034/ [ Links ]
Walk, C.L., Juntunen, K., Paloheimo, M. & Ledoux, D.R., 2019. Evaluation of novel protease enzymes on growth performance and nutrient digestibility of poultry: Enzyme dose response. Poult. Sci. 98, 5525-5532. http://dx.doi.org/10.3382/ps/pez299 [ Links ]
Wang, J.J., Garlich, J.D. & Shih, J.C.H., 2006. Beneficial effects of Versazyme, a keratinase feed additive, on body weight, feed conversion, and breast yield of broiler chickens. J. Appl. Poult. Res. 15, 544-550. https://doi.org/10.1093/japr/15.4.544 [ Links ]
Yadav, J.L. & Sah, R.A., 2005. Supplementation of corn-soybean based broiler's diets with different levels of acid protease. J. Inst. Agric. Anim. Sci. 26, 65-70. https://doi.org/10.3126/jiaas.v26i0.613 [ Links ]
Yin, D., Yin X., Wang, X., Lei, Z., Wang, M., Guo, Y., Aggrey, S.E., Nie W. & Yuan, J., 2018. Supplementation of amylase combined with glucoamylase or protease changes intestinal microbiota diversity and benefits for broilers fed a diet of newly harvested corn. J. Anim. Sci. Biotechnol. 9, 24-37. DOI: 10.1186/s40104-018-0238-0 [ Links ]
Submitted 11 February 2021
Accepted 24 July 2021
Published 25 October 2021
# Corresponding author: najamussaharsahar@gmail.com














