Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.51 no.5 Pretoria 2021
http://dx.doi.org/10.4314/sajas.v51i51.5
ARTICLES
Effect of dietary supplementation with L-Carnitine and fenofibrate on broiler chickens
M. Azizi-ChekosariI; M. BouyehI, #; A. SeidaviI; M.R. VenturaII
IDepartment of Animal Science, Rasht Branch, Islamic Azad University, Rasht, Iran
IIDepartamento de Patología Animal, Producción Animal, Bromatología y Tecnología de los Alimentos, Universidad de Las Palmas de Gran Canaria, 35413 Arucas, Las Palmas, Canary Islands, Spain
ABSTRACT
A factorial experiment was conducted to investigate the effects of L-Carnitine and fenofibrate on broilers. There were four replicates of four treatments, with each replicate including ten male Ross 308
broiler chickens. The experiment was conducted over a 42-day period. The treatments consisted of two levels of L-Carnitine (200 and 400 mg/kg) and two levels of fenofibrate (50 and 100 mg/kg) as supplements to a basal diet. Growth, carcass characteristics, serum constituents, immune system responsiveness, cecal microflora, sensory attributes and fatty acid profiles of breast meat, and small intestine histology were characterized. During the finishing period, the chickens fed a diet containing 400 mg/kg L-Carnitine and 50 or 100 mg/kg fenofibrate had lower feed intake andfeed conversion ratio (FCR) and higher weight than the other treatments. Application of 400 mg/kg L-Carnitine in combination with two levels of fenofibrate reduced ventricular fat (P <0.05), cholesterol (P <0.01), triglycerides (P <0.05), and very low density lipoprotein (VLDL) (P <0.05). These treatments increased antibody titers against Newcastle disease (ND)and avian influenza virus (AIV). Among the sensory properties of breast meat, experimental treatments had a significant effect only on its aroma (P <0.05). In general, supplementing the diet of Ross 308 broilers with 400 mg/kg L-Carnitine and 50 or 100 mg/kg of fenofibrate is recommended.
Keywords: fat burning, growth, intestinal microflora, meat quality
Introduction
Fat accumulation in chickens is regarded as slaughterhouse waste, and producers are looking for ways to eliminate ventricular and blood fats to reduce production costs and to produce healthy chickens (Cartwright, 1986; Lien & Horng, 2001). In the meantime, changes in the amount and levels of dietary nutrients and the use of plant and chemical dietary fat-burning supplements that control fat in humans have been introduced as effective solutions to the problem. L-Carnitine and fenofibrate are chemical medicines that control blood lipids in humans (Yang & Keating, 2009; Golzar Adabi et al., 2011).
L-Carnitine is a plasma lipid-lowering drug that decreases cholesterol, triglycerides, free fatty acids, phospholipids, and low-density lipoproteins (LDL) and increases high-density lipoproteins (HDL) (Diaz et al., 2000). In addition to oxidation of fatty acids, L-Carnitine plays a role in carbohydrate metabolism (Mingrone et al., 1999). Currently, the use of L-Carnitine supplementation is increasing in the food sciences (Lien & Horng, 2001; Hrncar et al., 2015; Khatibjoo et al., 2016) to raise energy efficiency and dietary fat and to reduce the accumulation of ventricular and plasma fats
Previous studies reported the positive effect of using L-Carnitine in poultry and broilers diets to control blood lipids, ventricular fat, and overall poultry health (Golzar Adabi et al., 2011; Lien & Horng, 2001). L-Carnitine helped increase energy efficiency so that poultry could process dietary lipids more quickly and easily. L-Carnitine also reduced feed intake in chickens significantly (Khatibjoo et al., 2016; Mirzapor Sarab et al., 2016). Its effects on live weight gain, final weight, carcass characteristics and abdominal fat deposition were reported in various studies (Kidd et al., 2009; Rabie et al., 1997; Hrncar et al., 2015; Babazadeh Aghdam et al., 2015; Xu et al., 2003). Mast et al. (2000) found that L-Carnitine in the diet of broilers affected the function of the immune system. However, Mirzapor Sarab et al. (2016) found that L-Carnitine in the diet of broilers had no effect on antibody production against sheep red blood cells (SRBC) and Newcastle disease (ND). Lien and Horng (2001) showed decreased levels of serum triglyceride, but not those of cholesterol, phospholipids and lipoproteins in chickens fed L-carnitine. Zhang et al. (2010) attributed the decrease in serum triglyceride levels to increased catabolism of fatty acids.
Fenofibrate is also a chemical drug that is used to lower cholesterol levels in cardiovascular patients. Fenofibrate and fibric acid derivatives reduce fat, VLDL, LDL and triglycerides, and increase HDL (Yang & Keating, 2009; Ruotolo et al., 1998). Fenofibrate is readily available and is one of the most widely used fibric acid derivatives (Packard et al., 2002; Yang & Keating, 2009). Flores-Castillo et al. (2019) reported that fenofibrate also reduced cholesterol and triglycerides and increased HDL cholesterol in white New Zealand rabbits. In a study by Montanaro et al. (2005), fenofibrate increased the level of palmitic acid and decreased stearic acid in diabetic mice. But so far, the effect of fenofibrate has not been tested in poultry.
Thus, the goal of this study was to determine effects of L-Carnitine and fenofibrate levels and their interaction on growth, carcass traits, serum constituents, immune system, cecal microbial flora, sensory properties of breast meat, fatty acid profile of breast meat and small intestine histology in Ross 308 broilers.
Materials and Methods
The experimental protocol was approved by the Animal Ethics Committee of Rasht Branch, Islamic Azad University, and the experiment was conducted in accordance with the International Guidelines for Research involving animals (Directive No. 2010/63/EU).
To evaluate the effects of L-Carnitine and fenofibrate in the diet of broilers, a (2 χ 2) factorial experiment was performed based on a completely random design in four replicates and 10 chickens per replicate over 42 days. The basal diets (Table 1) were formulated according to the nutritional needs of Ross 308 broilers.
The four experimental diets consisted of the basal diet + 200 mg/kg L-Carnitine+ 50 mg/kg fenofibrate (C 200 mg/kg + F 50 mg/kg); the basal diet + 200 mg/kg L-Carnitine + 100 mg/kg fenofibrate (C 200 mg/kg + F 100 mg/kg); the basal diet + 400 mg/kg L-Carnitine+ 50 mg/kg fenofibrate (C 400 mg/kg + F 50 mg/kg); and the basal diet + 400 mg/kg L-Carnitine+ 100 mg/kg fenofibrate (C 400 mg/kg + F 100 mg/kg). The L-Carnitine and fenofibrate were purchased from Poursina Pharmaceutical Company (Karaj, Iran) and Razavi Pharmaceutical Services (Mashhad, Iran).
Environmental conditions were the same for all groups (16 pens) and included 23 hours of light and one hour of darkness, humidity of 50 - 60% and a temperature of 32 °C, which decreased 3 °C with each week of age. All chickens had free access to water and food throughout the experiment. The vaccination programme was performed according to recommendations of the veterinary administration to prevent bronchitis, ND and Gumboro (infectious bursal disease (IBD)) disease.
At the end of each week, the chickens were weighed, feed intake was determined, and the FCR was calculated. At the end of the experiment, the weights of the carcass organs were measured (Farrokhyan et al., 2014). Production efficiency index (PEI) was calculated using the following relationship (Aviagen, 2018):
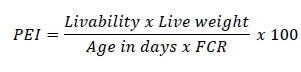
After 42 days, one chicken was randomly selected from each pen and blood was taken from a wing vein to measure serum constituents. Blood samples were stored at 30 °C until they clotted. The clear serum was then isolated from the blood samples by centrifugation at 3000 rpm (Eppendorf, 5702, Germany). The resulting serum samples were stored in 0.5 cc micro-tubes at -20 °C until analysed. Measurements of glucose, triglycerides, total cholesterol, protein, albumin, HDL, LDL and VLDL of blood samples were performed with an auto-analyser (Hitachi 917, Chiyoda, Tokyo, Japan) with commercial assay kits (Pars Azmoun, Iran, licensed from Diagnostic Systems Company, Germany).
To determine the effects of the levels of L-Carnitine and fenofibrate on the immune system, the SRBC test was performed and antibody titers against ND and AIV were ascertained. For this purpose, on days 28 and 35 of the experiment, 0.1 cc of diluted SRBC solution was injected into the wing vein of two identified chickens from each replicate. Seven days later, blood samples were taken from these chickens, and the serum was isolated 16 hours later (as described above). Hemagglutination was used to determine the antibody titer against SRBC (at 35 and 42 days old), ND, and AIV (42 days old) (Isakov et al., 2005). 2-Mercaptoethanol was used to measure the immunoglobulin M (IgM) titer (Arshami et al., 2010; Senaldi et al., 2002).
To examine the microbial flora of the digestive tract, a cecum sample was taken from a randomly selected 42-day-old chicken in each replicate. Its gastrointestinal tract was removed immediately after slaughter and 1 ml of the contents of the cecum was removed. The prepared sample was transferred to a container containing phosphate buffer and mixed thoroughly. MacConkey agar, blood agar, MRS, and AMB mediums were used to determine the overall frequency of Coíiforms, Clostridium, Lactobacillus and Escherichia coli, respectively (Miller & Wolin, 1974).
The breasts of two chickens were cooked for 45 minutes at 180 °C without spices or oil to assess the sensory characteristics of the meat. The cooked samples were then numbered and evaluated by trained people (six-person panel) and scores were assigned to colour, aroma, oral sensation, and general acceptance (scale 0 to 100) (Khajavi et al., 2014).
To investigate the profile of fatty acids in the breast, 20 g chopped breast meat was mixed with 50 ml methanol for 30 minutes, then 40 ml hexane was added and stirred for 20 minutes. After complete digestion, the mixed sample was rested until two phases had formed. Then, the top layer, containing the methyl esters and lipid fraction, was analysed with gas chromatography (Agilent 7890 Series Gas Chromatograph, USA) and the composition of its fatty acids was determined (Folch et al., 1957).
About 2 cm of the intestinal cecum of the slaughtered chickens was collected to examine the jejunum tissue. The samples were immediately transferred to plastic containers containing 10% formalin to prevent autolysis of the tissues and to maintain its physical structure. The samples were then dehydrated and made transparent with a tissue processor (LSC 1512, Germany). After 12 hours, the samples were removed from the device and 5 micrometer slices were cut from the tissue with a microtome. The samples were then stained with haematoxylin-eosin and laminated. Then histological parameters of the intestine, including villus height, crypt depth, and muscle thickness, were evaluated with a microscope (CX23 Olympus, Japan) with 10x and 40 x magnification (Sakamoto et al., 2000).
The data were analysed with SAS statistical software (SAS Institute Inc., Cary, North Carolina, USA). The design was a 2 χ 2 factorial, the model of which is as follows:
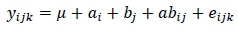
where: yijk= is an observed value,
μ is the mean common to all observations,
= the effect of the ith level of L-Carnitine in the diet,
bj = the effect of the jth fenofibrate in the diet,
abij= the interaction of the levels of L-Carnitine and fenofibrate, and
eijk= the experimental error.
Duncan's multiple range test was performed at 5% probability level to compare the treatment means
Results and Discussion
The effects of supplementation in broiler diets with various levels of L-Carnitine and of fenofibrate on feed intake, weight gain and at different ages are shown in Tables 2 and 3. Increasing the level of L-Carnitine reduced feed intake and FCR and weight gain in the finisher period (P <0.01). Concomitant use of L-Carnitine and fenofibrate in the diet had a significant effect on feed intake, weight gain and FCR of the entire period (P <0.01). The highest weight gain, the lowest feed intake, and the lowest feed conversion in the whole period belonged to the C 400 mg/kg + F 100 mg/kg treatment (Table 3). Feed intake in the periods of 1 - 14 days (P <0.01) and 15 - 28 days] (P <0.05), 15 - 28 days (P <0.01) and 29 - 42 days (P <0.01) and weight gain over 29 - 42 days (P <0.01) were affected significantly by the addition of L-Carnitine and fenofibrate to the diet (Tables 2 and 3).
The European production efficiency factor (EPEF) also increased significantly (P <0.01) with level of L-Carnitine and fenofibrate, so that the highest EPEF belonged to the C 400 mg/kg + F 100 mg/kg treatment (Table 3).
Researchers believed that the use of L-Carnitine in the diet increased energy efficiency and reduced feed intake (Khatibjoo et al., 2016; Mirzapor Sarab et al., 2016). Feed intake throughout the period decreased in the present study with increasing L-Carnitine level. Final weight gain and improved feed conversion were evident throughout with L-Carnitine and fenofibrate in the diet of broilers. L-Carnitine led to a reduction in feed intake by increasing the efficiency of energy consumption from dietary fats (Rabie & Szilagyi, 1998; Khatibjoo et al., 2016). Panahi et al. (2019) showed that L-Carnitine in the diet of broilers increased the weight at the end of the period. The weight gain in chickens fed with L-Carnitine may be because it increases insulin growth factor-I, which has 70 types of amino acids (Kita et al., 2002). Rabie and Szilagyi (1998) believed that L-Carnitine improved FCR because this fat-burning compound improved nitrogen metabolism. Parsaeimehr et al. (2013) stated that the addition of L-Carnitine to the diet of Ross 308 broilers improved weight, feed intake and feed conversion at the end of the period. Similar results are reported in Babazadeh Aghdam et al. (2015) and Hrncar et al. (2015). Akbari Azad et al. (2010) showed that 375 mg/kg of L-Carnitine in the diet reduced feed intake and weight gain and increased EPEF, which is consistent with the results of the present study.
In the present study, L-Carnitine and fenofibrate did not have significant effects on most carcass characteristics (Table 4). However, they reduced abdominal fat significantly, which is consistent with the results of several studies (Shirali et al., 2015; Xu et al., 2003; Hrncar et al., 2015). The effect of L-Carnitine on abdominal fat might be due to it reducing the activity of the enzymes that are involved in the synthesis of fatty acids glucose-6-phosphate dehydrogenase, malic dehydrogenase, and iso-citrate dehydrogenase (Xu et al., 2003; Rajabzadeh Nesvanet al., 2013). Some researchers believed that L-Carnitine reduced the accumulation of fat in tissues by altering fat metabolism (Burtle & Liu, 1994). In addition, the main effects of the levels of L-Carnitine and fenofibrate both increased breast percentage (P <0.01).
The interaction of levels of L-Carnitine and fenofibrate affected the percentages of jejunum and duodenum significantly (Table 5). Significant reductions in the percentage of each organ were observed due to the level of fenofibrate at the low level of L-Carnitine, but not at the high level.
Examination of the main effects showed that increasing the level of L-Carnitine decreased the amount of triglycerides, cholesterol, glucose and VLDL, and increased the amount of albumin, protein, HDL and HDL/LDL (Table 6). Likewise, increasing the level of fenofibrate significantly reduced triglycerides, cholesterol, and VLDL. The interaction effects were significant for cholesterol (P <0.01), triglycerides (P <0.01), LDL, HDL / LDL, and VLDL (P <0.05) of broiler blood. Cholesterol, triglycerides, and VLDL in the broilers fed with C 400 mg/kg + F 100 mg/kg were significantly lower than other treatments. Feeding C 400 mg/kg + F 100 mg/kg produced the lowest level of HDL/LDL and the two treatments of C 200 mg/kg + F 100 mg/kg and C 400 mg/kg + F 100 mg/kg had the lowest LDL levels
Rezaei et al. (2007) reported that L-Carnitine in the diet of broiler chickens reduced triglycerides, cholesterol and VLDL significantly, and the results of the present study are consistent with this observation. Hassan et al. (2011) also found that increasing the level of L-Carnitine in the diet reduced cholesterol significantly. Lien and Horng (2001) showed that L-Carnitine reduced triglycerides in broilers. Zhang et al. (2010) reported that L-Carnitine reduced blood triglycerides in broilers by increasing the catabolism of fatty acids. Cartwright (1986), on the other hand, believed that L-Carnitine reduced serum triglyceride by increasing the activity of the enzyme lipase. Decreases of triglycerides and VLDL in blood serum of broilers were reported in Xu et al. (2003), which is consistent with the results of the present study. Parsaeimehr et al. (2014) reported that L-Carnitine in the diet of broilers reduced the levels of triglycerides, cholesterol, LDL and VLDL in the blood, but had no significant effect on the amount of glucose, total protein, and HDL, which was consistent with the results of the present study.
The separate and combined effects of L-Carnitine and fenofibrate on the weight of immune organs and the function of the humoral immune system, responses to the SRBC antigen injection and antibody titers against ND and AIV are shown in Table 7. Increased concentrations of fenofibrate and L-Carnitine reduced the bursa of Fabricius significantly. Increasing the level of L-Carnitine increased IgG titer (Table 7). Norreh et al. (2015) reported that L-Carnitine increased the initial IgG titer in response to SRBC. In the present experiment, 400 mg/kg L-Carnitine plus 50 or 100 mg/kg fenofibrate in the diet of broilers significantly increased the antibody titer against ND and AIV compared with the other treatments. Famularo & De Simone (1995) stated that L-Carnitine prevented the death of B and T lymphocytes owing to cellular regression during the immune response of broiler chickens and led to increased antibody titers. It is believed that the body's immune cells use as much L-Carnitine as they need, and that high levels of the compound do not have a negative effect (Famularo & De Simone, 1995; Mast et al., 2000). It was also found that L-Carnitine improved humoral response to vaccination and boosted the immune system in poultry by producing monoclonal antibodies and increasing the ability of white blood cells to remove foreign agents (Mast et al., 2000; Deng et al., 2006).
The main effect of L-Carnitine on the population of Escherichia coli bacteria (P <0.05), coliform (P <0.05) and Lactobacilli (P <0.01) and of fenofibrate on the population of Lactobacilli bacteria (P <0.01) were significant (Table 8). As the concentration of L-Carnitine increased, the population of Lactobacillus bacteria rose, and the population of Escherichia coli and coliforms decreased. An increase in fenofibrate concentration enhanced the lactobacilli population (Table 8).
The interaction of L-Carnitine and fenofibrate had significant effects on the populations of Escherichia coli (P <0.05) and Lactobacilli (P <0.01). However, the populations of coliform bacteria and clostridium were not affected by the treatments. A comparison of the means showed that the bacterial population of Escherichia coli was reduced significantly by 400 mg/kg L-Carnitine combined with either 50 or 100 mg/kg fenofibrate compared with other treatments and the lowest bacterial colony was observed in the intestines of chickens fed C 400 mg/kg + F 100 mg/kg. It also had the largest population of lactobacilli among the treatments (Table 8). However, Hosseintabar et al. (2013) investigated the effect of L-Carnitine, methionine, and lysine on the microbial flora of cecum and the results showed no significant difference between treatments in the total population of aerobic bacteria and production of lactic acid bacteria Escherichia coli and Lactobacilli.
The effects of the treatments on the sensory properties of meat are shown in Table 9. The separate use of L-Carnitine and fenofibrate improved meat properties. Based on the results, the separate use of L-Carnitine on aroma, taste, oral sensation, and general acceptance was significant at the 5% probability level. Fenofibrate also (P <0.05) improved the aroma of the breast meat. The combined use of L-Carnitine and fenofibrate was significant only in meat flavour (P <0.05). But in general C 400 mg/kg + F 100 mg/kg and C 400 mg/kg + F 100 mg/kg improved the sensory properties of meat (Table 9).
Khatibjoo et al. (2016), Zhang et al. (2010) and Corduk et al. (2007) found that L-Carnitine in the diet did not have a significant effect on the sensory characteristics of meat. Parizadian et al. (2011) reported that L-Carnitine supplementation increased the quality of Japanese quail meat. Meat colour is one of its most important quality factors. The yellowness of the meat is affected by the myoglobin pigment in the muscles. L-Carnitine had an inhibitory effect on the oxidation of myoglobin in meat muscle and thus improved meat colour (Sarica et al., 2007; Zhang et al., 2010).
The levels of fatty acids in the breast muscle tissue are shown in Figures 1, 2 and 3. The highest levels belonged to palmitic acid (16:0) and stearic acid (18:0). Among unsaturated fatty acids, oleic acid (18:1c) was the most abundant, and its highest level belonged to the treatment containing 200 mg/kg L-Carnitine with 50 and 100 mg/kg fenofibrate. The amount of linolelaidic acid (18:2t) was highest in the treatments with 200 mg/kg L-Carnitine and 50 and 100 mg/kg fenofibrate. Linoleic acid levels (18:2c) were higher in the treatments with 400 mg/kg L-Carnitine and 50 and 100 mg/kg fenofibrate compared with the other treatments. Researchers believed that L-Carnitine was involved in energy production by transferring active fatty acids into the mitochondrial matrix and was essential to the entry of long-chain fatty acids into mitochondrion. L-Carnitine increased the animal's performance by improving the efficiency of use of energy from oxidation of fats (Dikelet al., 2010; Nogueira et al., 2011).
The effects of separate and combined uses of L-Carnitine and fenofibrate on the intestinal histology of broiler chickens are shown in Figures 4, 5 and 6. Villus height increased with levels of L-Carnitine and fenofibrate in the diet, and the longest villus height belonged to the treatment of C 400 mg/kg + F 100 mg/kg. This treatment also has the highest level of crypt depth and muscle thickness (Figures 4, 5 and 6).
Conclusion
Supplementation of the diet of broiler chickens with L-Carnitine and fenofibrate improved feed intake, weight gain, FCR for the entire period, and EPEF. The most effective treatments for improving blood, immune and sensory parameters, reducing ventricular fat and reducing intestinal microflora were C 400 mg/kg + F 50 mg/kg and C 400 mg/kg + F 100 mg/kg. These treatments were appropriate for maintaining chicken health, improving the qualitative and sensory properties of meat, increasing performance, and reducing feed intake and are therefore recommended.,
Authors' Contributions
MB, AS and MRV conceived the study, were responsible for its administration and finalized the article; MAC, MB, AS and MRV participated in curating the data, acquired funding for the research, and conducted the investigation; MAC and MB analysed the data; MAC, MB and AS identified the methodology, contributed resources to the project, and wrote the original draft of the article. MB and AS supervised the investigations.
Conflict of Interest Declaration
The authors confirm that they have no conflicts of interest relative to the work that is reported in this article.
References
Akbari Azad, G., Haghighi-Khoshkhoo, P., Ila, N., Moayer, F. & Dehghan-Nayeri, H., 2010. The effects of dietary L-Carnitine supplementation on overall performance, carcass traits, blood components and immune response in broiler chickens. JVCR 1(1), 7-17. (Persian). [ Links ]
Arshami, J., Hosseini, S. & Torshizi, M.E., 2010. Immunomodulatory effects of graded copper and zinc on SRBC titer and lymphoid organs in broiler chicks. J. Anim. Vet. Adv. 9 (11), 1510-1514. DOI: 10.3923/javaa.2010.1510.1514 [ Links ]
Aviagen, 2018. Ross 308 broiler management guide. https://eu.aviagen.com/?persist_locale=1 [ Links ]
Babazadeh Aghdam, A., Ghazi Harsini, Sh. & Daneshyar, M., 2015. The effect of different levels of L-Carnitine on performance, blood parameters and carcass characteristics of broiler chickens fed with high fat diets under heat stress condition. J. Vet. Res. 70 (3), 341-348. (Persian). [ Links ]
Burtle, G.J. & Liu, Q.H., 1994. Dietary carnitine and lysine affect channel catfish lipid and protein composition. J. World. Aqua. Soc. 25, 169-174. https://doi.org/10.1111/j.1749-7345.1994.tb00178.x [ Links ]
Cartwright, A.L., 1986. Effect of carnitine and dietary energy concentration on body weight and body lipid of growing broilers. Poult. Sci. 65, 21-29. [ Links ]
Corduk, M., Ceylan, N. & Ildiz, F., 2007. Effects of dietary energy density and L-Carnitine supplementation on growth performance, carcass traits and blood parameters of broiler chickens. S. Afr. J. Anim. Sci. 37 (2), 65-73. https://www.sasas.co.za/wp-content/uploads/2012/09/cordukm37issue2_0.pdf [ Links ]
Deng, K., Wong, C.W. & Nolan, J.V., 2006. Long-term effect of early life dietary L-Carnitine on lymphoid organs and immune responses in leghorn type chickens. J. Anim. Physiol. Anim. Nutr. 90, 81-86. DOI: 10.1111/j.1439-0396.2005.00569.x [ Links ]
Diaz, M., Lopez, F., Hernandez, F. & Urbania, J.I., 2000. L-Carnitine effects of chemical composition of plasma lipoprotein of rabbit fed with normal and high cholesterol diet. Lipids 35, 627-632. DOI: 10.1007/s11745-000-0566-2. [ Links ]
Dikel, S., Ünalan, B., Eroldoğan, O.T. & Özlüer Hunt, A., 2010. Effects of dietary L-Carnitine supplementation on growth, muscle fatty acid composition and economic profit of rainbow trout (Oncorhynchus mykiss). Turk. J. Fish. Aquat. Sci. 10, 173-180. DOI: 10.4194/trjfas.2010.0203 [ Links ]
Famularo, G. & De Simone, C., 1995. A new era for carnitine? Immunol. Today 16, 211-213. https://doi.org/10.1016/0167-5699(95)80159-6 [ Links ]
Farrokhyan, P., Bouyeh, M., Lartey, F. & Seidavi, A.R., 2014. The effects of dietary L-Carnitine and gemfibrozil on performance, carcass characteristics, cholesterol and triglycerides in broiler chicks. Avian Biol. Res. 7(3), 160-166. https://doi.org/10.3184/175815514X14067215301247 [ Links ]
Flores-Castillo, C., Luna-Luna, M., Carreón-Torres, E., López-Olmos, V., Frías, S., Juárez-Oropeza, M.A., Franco, M., Fragoso, J.M., Vargas-Alarcón, G. & Pérez-Méndez, O., 2019. Atorvastatin and fenofibrate increase the content of unsaturated acyl chains in HDL and modify in vivo kinetics of HDL-cholesteryl esters in New Zealand White Rabbits. Intern. J. Mol. Sci. 20, 1-15. https://doi.org/10.3390/ijms20102521 [ Links ]
Folch, J., Lees, M. & Stanley, G.H.S., 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497-509. [ Links ]
Golzar Adabi, Sh., Cooper, R.G., Ceylan, N. &Corduk, M., 2011. L-Carnitine and its functional effects in poultry nutrition. World Poultry Sci J. 67, 277-296. https://doi.org/10.1017/S0043933911000304 [ Links ]
Golzar Adabi, Sh., Moghaddam, Gh., Taghizadeh, A., Ahmad, N. & Farahvash, T., 2006. Effect of L-Carnitine and vegetable fat on broiler breeder fertility, hatchability, egg yolk and serum cholesterol and triglyceride. Int. J. Poult. Sci. 5(10), 970-974. DOI: 10.3923/ijps.2006.970.974 [ Links ]
Hassan, M.S.H., Youssef, S.F. & El-bahy, N.M., 2011. Effects of L-Carnitine and ascorbic acid supplementation on productive, reproductive, physiological and immunological performance of golden montazah laying hens. Poult. Sci. 31 (2), 557-578. [ Links ]
Hosseintabar, B., Dadashbeiki, M., Bouyeh, M. &Seidavi, A., 2013. Is the amount of L-Carnitine and methionine-lysine effect on the microbial flora of broiler cecum? J. Pure Appl. Micro. 8(1), 353-360. [ Links ]
Hosseintabar, B., Dadashbeiki, M., Bouyeh, M., Seidavi, A. R., van den Hoven, R.& Gamboa, S., 2015. Effect of different levels of L-Carnitine and lysine-methionine on broiler blood parameters. Revista MVZ Cordoba. 20(3), 4698-4708. DOI: 10.21897/rmvz.40 [ Links ]
Hrncar, C., Verguliaková, S., Svorad, P., Weis, J., Arpášová,, H., Mindek, S., Fik, M. & Bujko, J., 2015. Effect of L-Carnitine supplementation on fattening and carcass parameters of broiler chickens. Acta Fytotechn. Zootechn. 18(1), 15-19. [ Links ]
Isakov, N., Feldmann, M. & Segel, S., 2005. The mechanism of modulation of humeral immune responses after injection of mice with SRBC. J. Immunology 128, 969-975. [ Links ]
Khajavi, H., Torshizi, M.& Ahmadi, H., 2014. Effect of feeding different levels of dietary vermi-humus on growth performance and meat quality in broiler chickens. J. Anim. Prod. 16(2), 113-122. [ Links ]
Khatibjoo, A., Poormalekshahi, A.A., Fattahnia, F., Jaefai, H. & Aelaei, M., 2016. Effects of supplementation time of L-Carnitine and garlic powder on performance and carcass characteristics of broiler chickens. Iran. J. Appl. Anim. Sci., 8 (1), 132-140. (Persian). [ Links ]
Kidd, M.T., Gilbert, J., Corzo, A., Page, C., Virden, W.S. & Woodworth, J.C., 2009. Dietary L-Carnitine influences broiler thigh yield. Asian Austral. J. Anim. Sci. 22 (5), 681-685. https://doi.org/10.5713/ajas.2009.60665 [ Links ]
Kita, K., Kato, S., Aman Yaman, M., Okumura, J. & Yokota, H., 2002. Dietary L-Carnitine increases plasma insulin-like growth factor-I concentration in chicks fed a diet with adequate dietary protein level. Brit. Poult. Sci. 43, 117-121. DOI: 10.1080/00071660120109980 [ Links ]
Lien, T.F. & Horng, Y.M., 2001. The effect of supplementary dietary L-Carnitine on the performance, serum components, carcass traits and enzyme activities in relation to fatty acid beta-oxidation of broiler chickens. Br. Poult. Sci. 42, 92-95. DOI: 10.1080/713655014 [ Links ]
Mast, J., Buyse, J. & Godderis, B.M., 2000. Dietary L-Carnitine supplementation increases antigen-specific immunoglobulin G in broiler chickens. Br. J. Nutr. 83,161-166. [ Links ]
Miller, T.L. & Wolin, M.J., 1974. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. J. Appl. Microbiol. 27, 985-987. DOI: 10.1128/am.27.5.985-987.1974 [ Links ]
Mingrone, G., Greco, A.V., Capristo, E., Benedetti, G., Giancaterini, A., Gaetano, A. & de Gasbarrini, G., 1999. L- Carnitine improves glucose disposal in type 2 diabetic patients. J Am. Coll. Nutr. 18(1), 77-82. DOI: 10.1080/07315724.1999.10718830 [ Links ]
Mirzapor Sarab, S., Salari, S., Mirzadeh, Kh. & Aghaei, A., 2016. Effect of different levels of vitamin C and L-Carnitine on performance and some blood and immune parameters of broilers under heat stress. Iran. J. Appl. Anim. Sci. 8 (1), 141-153. (Persian) [ Links ]
Montanaro, M.A., Bernasconi, A.M., Gonzalez, M.S., Rimoldi, O.J. & Brenner, R.R., 2005. Effects of fenofibrate and insulin on the biosynthesis of unsaturated fatty acids in streptozotocin diabetic rats. Prostaglandins Leukot. Essent. Fatty Acids 73(5), 369-378. DOI: 10.1016/j.plefa.2005.06.004. [ Links ]
Murali, P., George, S. K., Ally, K., Dipu, M.T. & Dominic, G., 2015. Effect of dietary L-Carnitine supplementation with animal fat on carcass characteristics of broiler chicken. J. Anim. Res. 5(4), 713-717. DOI: 10.5958/2277-940X.2015.00119.9 [ Links ]
Nogueira, N., Cordeiro, N., Canada, P., Cuze Silva, P. & Ozório, R.O.A., 2011. Separate and combined effects of cyclic fasting and L-Carnitine supplementation in red porgy (Pagrus pagrus, L.). Aquac. Res. 41, 795-806. https://doi.org/10.1111/j.1365-2109.2010.02596.x [ Links ]
Norreh, Z., Khatibjoo, A., Fattahnia, F. & Akbari-Gharaei, M., 2015. Investigation of performance and immune response of broiler chickens fed diet containing butyric acid and L-Carnitine supplement. Anim. Prod. 17 (2), 269-279. (Persian) DOI: 10.22059/JAP.2015.53376 [ Links ]
Packard, K.A., Backes, J.M., Lenz, T.L., Wurdeman, R.L., Destache, C. & Hilleman, D.E., 2002. Comparison of gemfibrozil and fenofibrate in patients with dyslipidemic coronary heart disease. Pharmacotherapy 22 (12), 15271532. DOI: 10.1592/phco.22.17.1527.34128 [ Links ]
Panahi, H., Bouyeh, M., Behzadpour, D., Seidavi, A. R., Simoes, J., Tufarelli, V., Staffa, V.N., Tinelli, A., Ayasan, T. & Laudadio, V., 2019. Effect of dietary simvastatin and L-Carnitine supplementation on blood biochemical parameters, carcass characteristics and growth of broiler chickens. J. Indonesian Trop. Anim. Agric. 44 (4), 372-381. DOI: 10.14710/jitaa.44.4.372-381 [ Links ]
Parizadian, B., Ahangari, Y.J., Shams Shargh, M. & Sardarzadeh, A., 2011. Effects of different levels of L-Carnitine supplementation on egg quality and blood parameters of laying Japanese quail. Poult. Sci. J. 10, 621-625. [ Links ]
Parsaeimehr, Kh.,Farhoomand, P., Afrouziyeh, M., Cheraghi, H. & Hoseinzadeh, S., 2014. The effects of different levels of L-Carnitine on performance, carcass characteristics and some blood parameters of broiler chickens. Anim. Sci. J. 24 (3), 43-51. (Persian). [ Links ]
Parsaeimehr, Kh.,Farhoomand, P., Afrouziyeh, M., Najafi, R. & Ahmadie Naghdehi, A.A., 2013. Effects of L-Carnitine with different dietary fat sources on performance and some blood metabolites of broiler chicken. Anim. Prod. Sci. 1(4), 27- 34. (Persian). [ Links ]
Rabie, M.H. & Szilagyi, M., 1998. Effects of L-Carnitine supplementation of diets differing in energy levels on performance, abdominal fat content, and yield and composition of edible meat of broilers. Br. J. Nutr. 80, 391-400. DOI: 10.1079/096582198388256 [ Links ]
Rabie, M.H., Szil Ágyi, M., Gippert, T., Votisky, E. & Gerendai, D., 1997. Influence of dietary L-Carnitine on performance and carcass quality of broiler chickens. Acta Biol. Hung. 48, 241-252. [ Links ]
Rajabzadeh Nesvan, M., Rezaee, M. & Ansari Pirsaraee, Z., 2013. Effect of L-Carnitine supplementation to finisher diets with different sources of fat on the performance, carcass characteristics and body composition in broiler chickens. J. Anim. Sci. Res.13, 51-68. (Persian). [ Links ]
Rezaei, M., Attar, A., Ghodratnama, A. & Kermanshahi, H., 2007. Study the effects of different levels of fat and L- Carnitine on performance and carcass characteristics of broiler chicks. Pak. J. Bio. Sci. 10(12),1970-1976. DOI: 10.3923/pjbs.2007.1970.1976 [ Links ]
Ruotolo, G., Ericsson, C.G., Tettamanti, C., Karpe, F., Grip, L., Svane, B., Nilsson, J., de Faire, U. & Hamsten, A., 1998. Treatment effects on serum lipoprotein lipids apolipoproteins and low density lipoprotein particle size and relationships of lipoprotein variables to progression of coronary artery disease in the bezafibrate coronary atherosclerosis intervention trial (BECAIT). J. Am. Coll. Cardiol. 32, 1648-1656. DOI: 10.1016/s0735-1097(98)00442-2 [ Links ]
Sakamoto, K., Hirose, H., Onizuka, A., Hayashi, M., Futamura, N., Kawamura, Y. & Ezaki, T., 2000. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 94, 99-106. DOI: 10.1006/jsre.2000.5937 [ Links ]
Sarica, S., Corduk, M., Ensoy, U., Basmacioglu, H. & Karatas, U., 2007. Effects of dietary supplementation of L-Carnitine on performance, carcass and meat characteristics of quails. S. Afr. J. Anim. Sci. 37, 189-201. https://www.sasas.co.za/wp-content/uploads/2012/09/sarica37issue3_0.pdf [ Links ]
Senaldi, G., Stolina, M., Guo, J., Faggioni, R., McCabe, S., Kaufman, S.A., Van, G., Xu, W., Fletcher, F.A., Boone, T. Chang, M-S. Sarmiento, U. & Cattley, R.C., 2002. Regulatory effects of novel neurotrophin-1/b cell-stimulating factor-3 (cardiotrophin-like cytokine) on B cell function. J. Immunology. 168(11), 5690-5698. DOI:10.4049/jimmunol.168.11.5690 [ Links ]
Shirali, M.A., Salari, S., TabatabayiVakili, S., Sari, M. & Jahanian, R., 2015. Effect of vitamin E and L-Carnitine on growth performance, blood parameters and immune response of broiler chickens under heat stress. Anim. Sci. J.110, 115-128. (Persian). DOI: 10.22092/ASJ.2016.106525 [ Links ]
Xu, Z.R., Wang, M.Q., Mao, H.X., Zhan, X.A. & Hu, C.H., 2003. Effect of L-carnitine on growth performance, carcass composition and metabolism of lipids in male broiler. Poult. Sci. 82, 408-413. DOI: 10.1093/ps/82.3.408 [ Links ]
Yang, L.P. & Keating, G.M., 2009. Fenofibric acid: In combination therapy in the treatment of mixed dyslipidemia. Am. J. Cardiovasc. Drugs 9 (6), 401-409. DOI: 10.2165/11203920-000000000-00000 [ Links ]
Zhang, Y.Q., Bai, M.X., Zhao, L., Wang, Q. & Ji, C. 2010. Effects of dietary acetyl-L-Carnitine on meat quality and lipid metabolism in Arbor Acres broilers. Asian Austral. J. Anim. Sci. 23, 1639-1644. https://www.animbiosci.org/journal/view.php?doi=10.5713/ajas.2010.10168 [ Links ]
Submitted 20 April 2020
Accepted 25 June 2020
Published 26 September 2021
# Corresponding author: mbouyeh@gmail.com














