Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.51 no.1 Pretoria 2021
http://dx.doi.org/10.4314/sajas.v51i1.12
ARTICLES
True phosphorus digestibility of cottonseed meal and rice husk supplemented with microbial phytase in broiler chickens
I.I. Ilaboya#; E.A. Iyayi
Department of Animal Science, University of Ibadan, Ibadan, Nigeria
ABSTRACT
This research aimed to determine effects of phytase in cottonseed meal (CM) and rice husk (RH) based diets on true phosphorus digestibility (TPD) by broiler chickens. Two studies were conducted with 576 one-day-old broiler chickens using regression analysis to determine the TPD in these diets and the response to phytase supplementation. Six semi-purified diets were formulated to contain 150 g, 300 g, and 450 g each of CM/kg (experiment 1) and RH/kg (experiment 2) with phytase supplied at 0 and 1000 units/kg. Titanium dioxide was added to the diets at the rate of 5 g/kg as an indigestible maker. A total of 288 broiler chickens in each study were weighed and allotted to the six diets with six replicates of eight birds in a randomized complete block design. The birds were fed the experimental diets until day 26 post hatch. The coefficients of true phosphorus retention (TPR) were 0.8 for CM and 0.78 for RH without phytase; 0.93 for CM and 0.92 for RH with phytase. True phosphorus digestibility was 0.82 for CM and 0.75 for RH without phytase; and 0.95 for CM and 0.92 for RH with phytase. Phytase supplementation resulted in 13.27 and 17.94 % increases in TPD; and 12.29 and 13.61 % increases in TPR by birds fed the CM and RH diets, respectively. Phytase supplementation of CM and RH based diets increased TPD and improved total TPR and true ileal phosphorus digestibility in broiler chickens.
Keywords: Arbor Acre, ileal phosphorus digestibility, regression technique, semi-purified diet, total tract phosphorus retention
Introduction
Plant-derived ingredients in poultry feed such as cottonseed meal (CM) and rice husk (RH) have limited available phosphorus owing to the phytate-phosphorus complex. This results in low true ileal phosphorus digestibility (TIPD) and high endogenous phosphorus loss (EPL) with negative environmental consequences. Exogenous phytase can break the phytate-phosphorus complex and facilitate phosphorus utilization (Humer et al., 2015). However, information on TIPD and EPL in broiler chickens fed diets supplemented with phytase is scanty. Therefore, the effects of phytase supplementation in CM- and RH-based diets on TIPD in broiler chickens were investigated.
Phosphorus (P) is an indispensable nutrient for plants and animals but has limited bioavailability in plant-derived feed ingredients for poultry. Most of the P in grains and oilseed meals is in the form of phytate (Ravindran et al., 2008). Phytate (C6H18O24P6) is the principal storage form of phosphorus in plants and it chelates nutrients - minerals such as potassium, magnesium, and calcium - which are necessary for phosphorus absorption (Adeola & Sands 2003). Phytate is poorly utilized by poultry because they have limited amounts of intestinal phytase. As a result, poultry diets are supplemented with inorganic P sources, resulting in large amounts of P in the diet, which are subsequently passed into the environment, causing eutrophication (Mutucumarana et al., 2014). The activities of intrinsic phytase in diets for poultry and endogenous phytase in the digestive tract are not enough for efficient hydrolysis of phytate (Adeola & Sands, 2003; Applegate et al., 2003). Dietary supplementation of phosphorus with exogenous phytase is effective in improving phosphorus digestion in poultry diets (Fan et al., 2001). But supplementing poultry diets with phosphorus based on feed formulation calculations may lead to a deficiency in phosphorus or excretion of excess dietary phosphorus into the environment (Iyayi et al., 2013). Rodehutscord (2009) asserted that in assessing P availability, the measurement of digestible P remains the preferred method for poultry. The shift from measuring apparent digestible P to TDP of ingredients used in feeding poultry took place because of varying proportions of phytate and the limitations of measurements of apparent digestibility. Use of TPD provides a more reliable template for measuring digestible P in broiler feeds.
Until recently, only apparent P digestibility estimates were reported in the literature. However, these estimates do not account for the confounding effect of endogenous P losses (EPL). The estimation of EPL of some feed ingredients by the regression technique is important to determine the TPD of ingredients in broiler feed (Fan et al., 2001). Endogenous phosphorus loss is reduced and hence TPD may increase with phytase supplementation owing to increased release of P from the phytate-phosphorus complex (Akinmusire & Adeola 2009; Iyayi et al., 2013). A plethora of research findings are available on TPD in feed ingredients used in pigs (Fan et al., 2001; Akinmusire & Adeola, 2009). Corresponding data for poultry are limited, however, with few research studies (Iyayi et al., 2013; Liu et al., 2013; Mutucumarana 2014). True digestible phosphorus in some feed ingredients was documented by these authors, but TDP results might differ across climactic regions based on differences in the ingredients, the method of oil extraction, agronomical practice during cultivation, and the composition of the assay diets. The objective of this study was to determine TPD in response to phytase supplementation of broiler chickens fed diets containing CM and RH.
Materials and Methods
The ethics committee on Research and Innovation of the University of Ibadan approved this research on 28 April 2016. This approval conforms to the ethical standards laid down in 1964 Declaration of Helsinki and its later amendment. All birds were healthy throughout the studies and no mortality was recorded.
The test ingredients used in the experiments were CM and RH. Rice husk is a by-product of rice grains that is obtained by mechanical milling in contrast to the solvent extraction technique that is used for CM extraction. A total of 576 one-day-old Abor Acre broiler chicks were raised in floor pens in a well-ventilated and illuminated standard poultry house. On arrival, the birds were fed a commercial starter diet, which met the nutrient requirements for broiler chickens for 14 days (NRC, 1994). On day 14, the birds were transferred to metabolic cages. At day 20 post hatch, two groups of 288 birds were tagged, weighed individually and randomly allotted to experimental diets in each of the two experiments, with six replicates in a randomized complete block design using the experimental animal allotment programme of Kim and Lindemann (2007). Birds had free access to water and the experimental diets for eight days, with the first two days being allowed for acclimatization to the experimental diets.
Six semi-purified diets were formulated containing 150 g, 300 g, and 450 g each of CM and RH/kg (Tables 1 and 2, respectively) with or without 1000 units of phytase/kg diet. The dietary inclusion levels of the test ingredients were obtained by the gradual replacement of cassava starch with the CM or RH for the two experiments, respectively. Titanium dioxide was included in the diets at the rate of 5g/kg as an indigestible marker. Phytase (3-phytase derived from Aspergillus niger) was used as the exogenous phytase.
The CM used in this current study contained more CP and total P, but less GE and Ca than RH (Table 3). Increases in the contents of CP, Ca and total P in the diets without or with phytase were because of the graded inclusion of CM and RH at the expense of cassava starch in the two experiments.
Nutrient densities for the CM and RH diets are presented in Tables 3 and 4, respectively. In general, the diets that were based on RH were less nutrient dense than those based on CM.
Feed intake was calculated as the difference between amounts offered and refused on a cage basis during the eight-day feeding trial. Birds were weighed at days 20 and 28 post hatch on a cage basis to calculate bodyweight gain. Between days 25 and 27 post hatch, samples of fresh excreta were collected once daily from pans placed beneath the cages. Daily collections were pooled on a replicate cage basis, bulked, and stored in freezer at -4 °C. Samples were taken and air-dried in a force-draught oven at 55 °C for five days. On day 28, the birds were euthanized by carbon (IV) oxide asphyxiation and dissected to obtain digesta from the distal two thirds of the ileum using the procedure of Rodehutscord et al. (2012). Digesta samples were obtained by flushing the ileal content with deionized water, being careful not to squeeze the dissected portion. Samples from each replicate cage were pooled. The digesta samples were frozen and freeze-dried. The excreta and ileal samples were milled and stored in air-tight plastic sample bags at -4 °C until needed for analysis.
Dry matter was determined by drying the excreta and ileal samples at 105 °C for 24 hours in a pre-weighed dried crucible in a conventional oven (method no 930.15) (AOAC International, 2005). Samples were ashed and phosphorus concentration was determined colorimetrically (UV) at 400 nm following digestion with nitric and perchloric acid. Titanium concentrations in the ashed samples of feed, excreta and ileal were determined by the colorimetric method following digestion with concentrated sulphuric acid and absorbance read at 410 nm (Short et al. (1996).
Apparent precaecal digestibility and retention coefficients of P in the two experiments were calculated by the index method (Iyayi et al., 2013), which is based on indigestible marker ratios according to the equation:
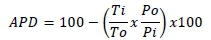
Where: APD is apparent digestibility of phosphorus expressed as a percentage,
Ti is the concentration of titanium in dietary intake,
To is the titanium concentration in ileal or excreta output,
Po is the phosphorus concentration in ileal or excreta output, and
Pi is the phosphorus concentration in dietary intake.
The dietary P intake was calculated as concentration in grams of P intake per kilogram of DM. The amount of digestible P was calculated from the intake of P and its digestibility, Apparent P retention (APR) was calculated as proportion of the P intake that was not voided in in the excreta.
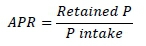
Data were analysed using the GLM procedure of SAS (SAS Institute Inc., Cary, North Carolina, USA). Each experiment was analysed separately. The model consisted of replications (5 df), the level of the basal ingredient (2 df), phytase level (1 df), and the interaction (2 df). Orthogonal polynomial contrasts were used to determine effects of graded levels of the basal ingredient on all response criteria. Levels of digested P (g/kg DMI) and retained P (g/kg of DMI) were regressed on P intake (g/kg DM) separately for the two levels of phytase supplementation. Estimates of the regression parameters were compared within sampling sites (ileal and excreta) with or without phytase using student's t-test.
Results and Discussion
When CM was the base of the diet, the interaction of its inclusion level with the level of phytase supplementation were significant for ileal output of P (P =0.002), excreted P (P =0.009), APD (P =0.034), but not for APR (P = 0.093) (Table 6). Without phytase, ileal output of P was greater for birds fed the 300 g/kg CM diet than for birds fed the diets with either a lower or higher level of CM. With phytase, no such effect was observed. Without phytase, the responses in excreted P were somewhat similar to those for ileal output of P, except that the levels of excreted P for birds fed 300 g/kg and 450 g/kg CM were similar. In contrast, when the diets were supplemented with phytase, the excreted P was reduced for birds fed the 300 g/kg CM diet than those fed either the 150 g/kg or 450 g/kg CM diets, which produced similar responses. As a consequence of these effects the APD was lower for birds fed the intermediate level of CM compared to either extreme when phytase was not provided. However, when phytase was provided there was a graded increase in APD with the level of CM that was fed. Either with or without phytase supplementation, there was a marked increase in APR from the low level of dietary CM to the higher levels which were more nearly similar to each other. The effect of phytase supplementation on APR was negligible (P =0.35).
Far fewer significant effects were observed with the RH based diets than were observed when graded levels of CM were fed (Table 7). Interactions between the level of dietary RH and supplemental phytase were not significant for ileal P output, excreted, APD or APR (P >0.5). Supplementation with phytase increased (P =0.04) APR across all levels of RH that were fed.
There were no linear and quadratic responses (P >0.05) in the ileal output and apparent ileal P digestibility of birds on the RH-based diet, irrespective of supplementation. A quadratic response was observed in excreta P output (P <0.01) at 300 g/kg RH inclusion for diets supplemented with phytase. Apparent ileal digestibility values ranged between 88.52% and 92.30% and 93.34% to 93.53% for diets without supplemental phytase and with supplemental phytase, respectively. Apparent ileal phosphorus digestibility for birds fed phytase-supplemented diets was 9.32% higher than the value calculated for birds fed unsupplemented diets. Apparent P retention values ranged from 88.26% to 98.25%. Without phytase, apparent P retention did not differ (P >0.05), but with the supplementation of phytase there was a quadratic response (P <0.05) at 300 g/kg RH inclusion level. The TPD of RH without phytase at 75% was lower (P <0.01) than the TPD at 92% with phytase supplementation. The true P retention at 91% with phytase supplementation was higher (P <0.01) than that at 78% without phytase (Table 4). Phytase supplementation effectively increased digestible P at the ileal site by 17.94 percentage points, and a corresponding increase in true P retention by 13.61 percentage points with the addition of phytase.
In the context of the current studies, the strong linear relationships that were observed between ileal digested P, retained P, and dietary P intake for all the test ingredients were the primary requirements for the use of the regression technique. Other authors reported a strong linear relationship between digested P outputs and dietary P intake (Akinmusire & Adeola, 2009; Iyayi et al., 2013; Mutucumarana, 2014). This relationship permits the theoretical estimation of dietary independent endogenous P loss (g/kg DMI) and the simultaneous measurement of true ileal P digestibility (Fan et al., 2001). Estimating the TPD of CM and RH is important because these feedstuffs are used as alternative protein and carbohydrate sources in broiler chicken feed. This result agrees with other studies, which used soybean meal (Fan et al., 2001; Ajakaiye et al., 2003; Diger & Adeola, 2006), canola meal and peanut flour (Iyayi et al., 2013) as the test ingredients. In the diet formulation for CM and RH studies, an increase of P in the diets was achieved by raising the amounts of CM and RH to ensure graded levels of P intake by the birds. This study also involved the development of a database of TPD values for a wide range of feedstuffs. Up-to date knowledge of the effect of microbial phytase on TPD would assist in formulating diets that would minimize P excretion. The range in phytase activity in the unsupplemented diets was less than 100 units/kg, whereas the diets with supplemental phytase activity ranged from 854 to 1247 units/kg. Weremko et al. (1997) reported phytase activity above 500 units/kg to increase P digestibility but less distinctly, but a further rise in phytase dosage (up to 1000 units) increased P digestibility.
In experiment 1, ileal P output accounted for 18% to 32% of P intake in diets without phytase and 8% to 10% in diets with phytase. In experiment 2, ileal output was 9% to 11% of the P intake without phytase and 6% to 7% in diets with phytase. Excreta P output accounted for between 28% and 50% P intake for the unsupplemented diets and 20% to 40% with supplemental phytase. Phytase supplementation and its interaction with P was responsible for the significant reduction of ileal and excreta P and increased apparent P digestibility, but the level of P resulted in an increase in apparent P retention in experiment 1, and in experiment 2 phytase supplementation significantly increased apparent P retention. As expected, the addition of phytase decreased P excretion. This result agrees with other authors that reported the efficacy of microbial phytase in hydrolysing phytate P, improving its utilization by poultry and swine, and consequently leading to a reduction in P excretion (Baxter et al., 2003; Akinmusire & Adeola, 2009).
The positive effect of phytase supplementation was evidenced in the TPD data. Phytase supplementation increased true ileal P digestibility of CM and RH with estimated TIPD values of 95.42% and 92.94%, respectively. Liu et al. (2013) reported estimated TIPD for soybean meal in the range of 46 to 71. These reported findings were based on the varying calcium to phosphorus ratios used in their study. Phytase supplementation resulted in 13.27% and 17.94% increases in TIPD for CM and RH, respectively.
Simultaneously, supplementation of CM and RH with phytase increased (TP), estimated values of 92.75% and 91.83%, respectively. This translated to 12.29% and 13.61% increases. Akinmusire and Adeola (2009) reported improvement in TPD of canola and soybean meal with the addition of phytase to the diets of growing pigs. Rutherfurd et al. (2004) reported a 10% to 12% increase in TPD at the terminal ileum with phytase supplementation of low P diets containing soybean meal, wheat bran, and rapeseed meal in broiler chickens with a corresponding 10.5% increase in phytate degradation at the terminal ileum. Phytase supplementation had also been reported to improve Ca and P retention in broiler chickens (Viveros et al., 2002) and digestible P in pigs, with a reduction in excreted P by 21.5% (Dilger & Adeola, 2006). The results obtained in these studies on improved ileal digestible P and a corresponding P retention in both experiments agreed with these earlier findings.
A comparison of the TIPD and TPR estimates obtained at the sites of sampling for diets with and without phytase indicated significant variation, which attests to the influence of hindgut microflora on phosphorus digestibility and retention. Ileal digestible P is not influenced by hindgut microflora, because it is the portion of dietary total P that is not found in the faeces, whereas P retention is the proportion of dietary total P that is retained in the body, which could be affected by hindgut microflora (Rodehutscord et al., 2012). Percentage increases at both sites of sampling were observed to be highest in the RH diet compared with values obtained for CM. The lower TPD in CM in relation to RH could be attributed to the antinutritional factors in CM, which is reported, apart from phytic acid, to contain antinutrients such as gossypol, which are known to reduce nutrient digestibility in non-ruminant animals (Dersjant-Li et al., 2014). It could also be attributed to the presence of adequate substrate for microbial phytase action (Dersjant-Li et al., 2014). Dersjant-Li et al. (2014) concluded that the efficacy of phytase depends on the concentration of phytate in feed ingredients, which was categorized under dietary-related factors. The difference in processing methods used to produce CM and RH probably resulted in varying concentrations of phytate in their by-products. Hence better substrate to enzyme concentration might have triggered the observed percentage point increase in true ileal P digestibility and TPR for RH.
The results from the two experiments show that irrespective of the assayed feedstuff, there was a similarity in the effect of phytase on P digestibility. Phytase had no effect on P retention in experiment 1 but had a significant effect in experiment 2 (Table 3). Similar effects on ileal P digestibility and retention were reported by Leytem et al. (2008), who recorded an increase in the hydrolysis of phytate P and a threefold increase in P retention in a study with corn, wheat, barley, and oats in broiler chickens as a result of the addition of 1000 phytase units to the diets. Phytase supplementation has also been reported to improve digestible P in pigs with a reduction in excreta P by 21.5% (Harper et al., 1997). These reports support the results in this study of improved ileal digestible P and a corresponding P retention in both experiments.
Estimates of EPL that were observed for broiler chickens in the two studies were not statistically different from zero. Phytase supplementation of CM and RH reduced ileal endogenous phosphorus loss and the total tract endogenous flow of P. Estimated ileal endogenous P loss for birds fed diets with and without phytase (g/kg DMI) were 0.171 and 0.211 for experiment 1, and 0.014 and 0.384 for experiment 2. This implied that supplementation of the test ingredients with phytase at 1000 units per kg of diets reduced estimated ileal endogenous phosphorus losses. Simultaneously, at the total tract section, estimated total tract endogenous P loss for birds fed diets with and without phytase were 0.581 and 0.620 g/kg DMI, and 0.338 and 0.766 g/kg DMI for experiment 1 and experiment 2, respectively. Negative estimate values were not observed in endogenous P flows at both sites of sampling for the RH diet, whereas negative values were seen in the CM diet. However, reported negative values reflect the anomaly associated with regression method, and show the drawback inherent in it. Research findings have documented negative endogenous P loss at ileal and total tract sections for pigs (Akinmusire & Adeola, 2009) and poultry (Iyayi et al., 2013; Mutucumarana, 2014) with more reports being documented for pigs than for broiler chickens.
Conclusions
Supplementation of cottonseed meal- and rice husk-based diets with phytase at 1000 units/kg of diet reduced endogenous phosphorus losses at both sites of sampling (ileal and total tract sections) in experiments 1 and 2. It also led to an increase in true ileal phosphorus digestibility and true total tract retention of phosphorus in both diets. Awareness of supplementing poultry diets with phytase should be intensified because the use of exogenous phytase would not only minimize eutrophication, but decrease excessive dependence on inorganic phosphates in poultry.
Acknowledgements
The authors are grateful to Adelani and Taofeeq, Department of Animal Science, University of Ibadan, for some of the analytical procedures
Authors' Contributions
Both authors contributed to all aspects of the research.
Conflict of Interest Declaration
The authors declares that there is no conflict of interest.
References
Adeola, O. & Sands, J., 2003. Does supplemental dietary microbial phytase improve amino acid utilization? A perspective that it does not. J. of Anim. Sci. 81, E78-E85. [ Links ]
Ajakaiye, A., Fan, M.Z., Archbold, T., Hacker, R.R., Fosberg, C.W. & Phillip, J.P., 2003. Determination of true digestive utilization of phosphorus and the endogenous phosphorus outputs associated with soybean meal for growing pigs. J. Anim. Sci. 81, 2766-2775 [ Links ]
Akinmusire, A.S. & Adeola, O., 2009. True digestibility of phosphorus in canola and soybean meal for growing pigs: Influence of microbial phytase. J. of Anim. Sci. 8, 977-983. [ Links ]
Applegate, T.J., Angel, R. & Classen, H.L. 2003. Effect of dietary calcium, 25-hydroxycholecalciferol, or bird strain on small intestinal phytase activity in broiler chickens. Poult. Sci. 82,1140-1148. [ Links ]
Ajakaiye, A., Fan, M.Z., Archbold, T., Hacker, R.R., Fosberg, C.W. & Phillip, J.P., 2003. Determination of true digestive utilization of phosphorus and the endogenous phosphorus outputs associated with soybean meal for growing pigs. J. Anim. Sci. 81, 2766-2775 [ Links ]
AOAC International, 2005. Official methods of analysis. 18th edition. AOAC International, Washington, DC. [ Links ]
Baxter, C.A., Joern, B.C., Ragland, D., Sands, J.S. & Adeola, O., 2003. Phytase, high available-phosphorus corn, and storage effects on phosphorus levels in pig excreta. J. Environ. Qual. 32, 1481-1489. [ Links ]
Dersjant-Li, Y., Awati, A., Schulze, H. & Partridge, G., 2014. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tracts and influencing factors. J. Sci. Food Agric. 95, 878-896. [ Links ]
Dilger, R. N. & Adeola, O. 2006. Estimation of true phosphorus digestibility and endogenous phosphorus loss in growing chicks fed conventional and low-phytate soybean meals. Poult. Sci. 85, 661-668. [ Links ]
Fan, M.Z, Archbold, T., Sauer, W.C., Lackeryram, D., Rideout, T.T., Gao, Y., De Lange, C.F. & Hacker, R.R., 2001. Novel methodology allows simultaneous measurement of true phosphorus digestibility and the gastrointestinal endogenous phosphorus outputs in studies with pigs. J. of Nutri. 131, 2388-2396. [ Links ]
Harper, A.F., Kornegay, E.T. & Schell, T.C. 1997. Phytase supplementation of low-phosphorus growing finishing pig diets improves performance, phosphorus digestibility and bone mineralization and reduces phosphorus excretion. J. Anim. Sci. 75, 3174-3186. [ Links ]
Humer, E., Schwarz, C. & Schedle, K., 2015. Phytate in pig and poultry nutrition. J. Anim. Phys. Anim. Nutr. 99, 605-625. [ Links ]
Iyayi, E.A., Fru-Nji, F. & Adeola, O., 2013. True phosphorus digestibility of black-eyed pea and peanut flour without or with phytase supplementation in broiler chickens. Poult. Sci. 92. 1595-1603. [ Links ]
Kim, B.G. & Lindermann, D.M., 2007. A new spreadsheet method for Experimental Animal Allotment Program (EAAP). J. of Anim. Sci. 82 (Suppl. 2), 112. https://afs.ca.uky.edu.swine/experimental-animal-allotment-program-eaap-version-11 [ Links ]
Leytem, A.B., Willing, B.P. & Thacker, P.A., 2008. Phytate utilization and phosphorus excretion by broiler chickens fed diets containing cereal grains varying in phytate and phytase content. Anim. Feed Sci. & Tech. 146, 160-168. [ Links ]
Liu, J.B., Chen, D.W. & Adeola, O., 2013. Phosphorus digestibility response of broiler chickens to dietary calcium to phosphorus ratios. Poult. Sci. 92, 1572-1578. [ Links ]
Mutucumarana, R.K., 2014. Measurement of true ileal digestibility of phosphorus in feed ingredients for poultry. PhD thesis, Massey University, New Zealand. pp 173. [ Links ]
Mutucumarana, R.K., Ravindran, V., Ravindran, G. & Cowieson, A.J., 2014. Measurement of true ileal digestibility of phosphorus in some feed ingredients for broiler chickens. J. of Anim. Sci. 92, 5520-5529. [ Links ]
NRC, 1994. Nutrient requirements of poultry. 9th revised edition. National Academy Press, Washington, DC. [ Links ]
Ravindran, V., Cowieson, A.J. & Selle, P.H., 2008. Influence of dietary electrolyte balance and microbial phytase on growth performance nutrient utilization and excreta quality of broiler chicks. Poult. Sci. 87, 677-688. [ Links ]
Rodehutscord, M., 2009. Approaches & challenges for evaluating phosphorus sources for poultry. In: Proceedings and Abstract of the 17th European Symposium on Poultry Nutrition, Edinburgh, UK. 23-27 August 2009. Pp 2-6. [ Links ]
Rodehutscord, M., Dieckmann, A., Witzig, M. & Shastak, Y., 2012. A note on sampling digesta from the ileum of broilers in phosphorus digestibility studies. Poult. Sci. 91, 4. [ Links ]
Rutherfurd, S.M., Chung, T.K., Morel, P.C.H. & Moughan, P.J., 2004. Effect of microbial phytase on ileal digestibility of phytate phosphorus, total phosphorus and amino acids in a low-phosphorus diet for broilers. Poult. Sci. 83, 61-68. [ Links ]
Short, F.J., Gorton, P., Wiseman, J. & Boorman, K.N., 1996. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. & Technol. 59, 215-221. [ Links ]
Viveros, A., Brenes, A., Arija, I. & Centeno, C. 2002. Effects of microbial phytase supplementation on mineral utilization and serum enzyme activities in broiler chicks fed different levels of phosphorus. Poult. Sci. 81, 1172-1183. [ Links ]
Weremko, D., Fandrejewski, H., Zebrowska, T., Hian, I.K., Kim, J.H. & Cho, W.T., 1997. Bioavailability of phosphorus in feeds of plant origin for pigs. Asian J. Anim. Sci. 10, 551-566. [ Links ]
Submitted 19 July 2019
Accepted 11 June 2020
Published 22 January 2021
# Corresponding author: bina.ilaboya@gmail.com














