Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.50 n.4 Pretoria 2020
http://dx.doi.org/10.4314/sajas.v50i4.8
ARTICLES
Performance of Rhode Island Red, Black Australorp, and Naked Neck crossbreds under alternative production systems
M. UsmanI, #; A. MahmudI; J. HussainI; A. JavidII
IDepartment of Poultry Production, Faculty of Animal Production and Technology, University of Veterinary and Animal Sciences, Lahore, 54000, Punjab, Pakistan
IIDepartment of Wildlife and Ecology, Faculty of Fisheries and Wildlife, University of Veterinary and Animal Sciences, Lahore, 54000, Punjab, Pakistan
ABSTRACT
The effects of the production system, breed cross, and their interaction on performance, egg quality, and hatching traits were evaluated. Rhode Island Red and Black Australorp were crossed with Naked Neck chickens (first generation RNN, and BNN, respectively). These crosses were mated among themselves and crossed to produce four crossbreds: RR (RNN x RNN), BB (BNN x BNN), RB (RNN x BNN), and BR (BNN x RNN). Thirty-six pullets and 9 cockerels from each crossbred were maintained in three production systems: the aviary system (AV), conventional cages (CC), and enriched cages (EC). Thus there were 48 pullets and 12 cockerels in each production system. Bodyweight, egg production percentage, and egg weight were highest in EC, followed by CC and AV. Higher egg weight, egg surface area, and egg volume were also observed in EC compared with CC and AV. Fertility and hatchability were higher and early embryonic mortality was lower in AV than in EC and CC. Bodyweight, egg production percentage, egg weight, egg volume, and surface area were higher for RB and BR than for BB and RR. Fertility and hatchability were similar for RB and BR. RR was similar to BR, but lower than RB. BB had the lowest fertility and hatchability. Thus, chickens in EC performed better than in the other systems, except that hatching traits were better in AV. RB and BR performed better than BB and RR.
Key words: breed crosses, chicken, egg quality, hatchability
Introduction
The poultry sector in developing countries understands the pivotal significance of fulfilling dietary requirements and alleviating poverty, serving as a major animal protein source and delivering essential nutrients. This sector is reliant on commercial exotic breeds and pays no attention to rural chicken breeds. Thus indigenous chicken breeds are being ignored, although they still contribute to the national demand for meat and eggs (Anonymous, 2019). This triggers unintentional loss of birds that have the genetic potential to endure harsh climatic conditions, better robustness against stressors, and superior adaptability to local climatic conditions. Naked Neck is one of the most important dual-purpose utility breeds among rural chicken breeds in Pakistan. It has promising traits such as better productivity and survivability in a hot climate, better feed efficiency, and a larger egg size (Garces et al., 2001; Nwachukwu et al., 2006). Its average egg production is 138 eggs in 52 weeks, and the bodyweight of female and male are 1.1 kg and 1.5 kg (Grobbelaar et al., 2010). In several countries, various strategies have been applied to develop a dualpurpose rural chicken breed with further improvement in its production traits (Mallia, 1999). This genetic improvement can be achieved through crossbreeding and selection. Cross breeding produces improvements in growth rate, reproductive traits and feed conversion efficiency without disturbing the potential of acclimatization, and ultimately reducing production costs (Adebambo et al., 2011). Better productive performance and adaptability traits of Naked Neck can be exploited through heterosis and complementarity in crosses between Rhode Island Red and Black Australorp. These crosses have genetic potential for high levels of egg production and meat yield and a possibility of higher economic returns. This would help to develop a cross breed with improved production and maintained acclimatizing abilities.
Better management and provision of a suitable environment are also necessary to exploit genetic potential (Menge et al., 2005). Alterations in housing for chickens are required to achieve the optimum performance of genetically improved chickens (Preisinger, 2005). The conventional cage system (CC), which was developed in the 1930s, has been used since the 1950s to maximize profit and production by allowing an increased number of hens in a small area (Sosnowka-Czajka et al., 2010; Jones et al., 2014). However, increases in stocking density resulted in increased welfare concerns in Europe during the 1960s and thereafter questions were raised about restricted movement and inhibited expression of natural behaviours in the constricted bare environment of CC (Mench et al., 2011). Continued amendments and improvements of CC led to development of the enriched cage system (EC) in Germany during the 1980s (Appleby, 1998). The EC featured more space per bird and tools such as perches, nests and scratching areas in which birds could express their natural behaviour (Lay et al., 2011; Mench and Blatchford, 2014). Additionally, banning of the CC system led to development of new housing systems (van Asselt et al., 2015). These newly developed systems included the aviaries, free range, barns ,and organic systems which have recently be studied for their effects on performance (Tactacan et al., 2009) and health (Rodenburg et al., 2008; Lay et al., 2011). Production systems may affect production performance, egg quality, and the welfare of chickens, but the relationships between genotype and production system should not be undervalued, especially with genetically improved chickens. Indigenous chicken breeds are good in terms of adaptability, but their performance in alternative production systems after crossing with exotic breeds has not been evaluated. Therefore, the present trial was conducted to evaluate the production performance, egg quality attributes, and hatching traits of Naked Neck, Black Australorp, and Rhode Island Red crossbreds under alternative production systems.
Materials and Methods
The care and use of the birds were in accordance with the laws and regulations of Pakistan. The experimental procedures were approved by the Committee of Ethical Handling of Experimental Birds, University of Veterinary and Animal Sciences, Lahore, Pakistan (No. DR/124).
The trial was conducted at the Indigenous Chickens Genetic Resource Centre (ICGRC), Department of Poultry Production, Ravi Campus, University of Veterinary and Animal Sciences, Pattoki. This city is located at 73°50'60 E and 31°1'0 N at an altitude of 186 m and has a tropical hot and humid climate. The temperature ranges between 12 °C and 45 °C. The present study was a continuation of an earlier project, in which the performance of progeny (F1) from Rhode Island Red x Naked Neck (RNN) and Black Australorp x Naked Neck (BNN) was evaluated (Ahmad et al., 2019). In the present study, reciprocal crosses of BNN and RNN were made to comprise a second generation (F2). For this purpose, 200 heterozygous partially feathered chickens comprising 50 birds (10 male x 40 female) from each crossbred of first generation were used to produce a two-breed diallel of BNN and RNN (Figure 1).
More than 1200 hatching eggs were acquired when the birds were 33 weeks old. A total of 720 day-old chicks in the second generation; 180 each from BB, BR, RR, and RB, which were hatched at Avian Research and Training Centre (ARTC), UVAS, Lahore, were transported to ICGRC, Ravi Campus, UVAS, Pattoki. The birds were fed a commercial breeder ration (Leeson & Summers, 2005) (Table 1). The chicks were brooded under standard managerial conditions up to six weeks of age. During the brooding phase, the birds were vaccinated against Infectious Bronchitis (IB) and Newcastle Disease (ND) according to the local area schedule. To evaluate the productive efficiency and hatching traits, 180 birds, comprising 144 pullets with 36 cockerels (36 pullets and 9 cockerels from each crossbred), were maintained in three production systems with 48 pullets and 12 cockerels in each during the production phase (27 - 46 weeks). Thus, the experiment had a 3 x 4 factorial arrangement of treatments in which housing systems and breed cross were main effects.
Chickens that were reared in ECs and AVs were provided with perches and dust bathing areas. They were kept in open-sided windowed enclosures that were ventilated with ceiling fans and galvanized round feeders and plastic manual drinkers were used. Birds reared in CC were maintained in environmentally controlled poultry sheds, equipped with galvanized three-tiered battery cage systems, automatic manure belts, automatic water nipple lines and feed trolleys (FACCO, Poultry Equipment-C3, San Martino, Italy). Fresh water was supplied ad libitum. The physical characteristics of each system are explained in Table 2.
The experiments lasted about five months (August to December), during which the minimum to maximum temperature and humidity were maintained in the range of 18 °C to 30 °C and 55% to 72%, respectively, in open-sided enclosures (AV and EC), whereas in environmentally controlled houses (CC), the minimum and maximum temperature and humidity ranged between 18 °C and 25 °C and 64% and 76%, respectively. The lighting schedule for the CC system was applied according to the HyLine W36 management guide (2018) (Table 3). Rice husk was used as litter in ECs and AVs. Approximately 10 cm depth of bedding material was maintained and was racked daily to retain the condition.
Egg collection records were used to calculate hen/day egg production percentage and egg weight (g) (Shafik et al., 2013). To evaluate internal and external egg quality parameters (egg weight, shell thickness, Haugh unit score and yolk index), a total of 60 eggs (five eggs per treatment group) were used at the start and the end of the experiment (adapted from Gikunju et al., 2018). Eggs were stored at 14 - 16 °C with 7080% relative humidity and transported to the hatchery (Victoria Inc.) at ARTC to evaluate fertility, hatchability, dead in shell, and dead germ percentage (adapted from Adeleke et al., 2012).
Effects of the production systems and breed crosses were evaluated for productive performance, egg quality and hatching traits. The GLM procedure of SAS (SAS Institute, Inc., Cary, North Carolina, USA) was used to test the main effects and their interaction. The model was:
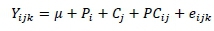
where: Yijk = an observation of a dependent variable from the kth experimental unit,
μ = population mean,
Pi= the effect of the ith production system,
Cj= the effect of the jth breed cross,
PCij= the interaction between the ith production system and jth breed cross, and
eijk = the residual effect after having accounted for the other effects in the model (NID ~ 0, σ2).
Tukey's HSD test (Tukey, 1953) was used to compare treatment means at a significance level of P =0.05. For initial and final egg quality traits, independent student's t-tests were applied.
Results and Discussion
Significant differences were observed between the production system and breed (Table 4). Chickens that were reared in EC had the highest (all P <0.0001) initial bodyweight, final bodyweight, egg production percentage, and egg weight, followed by those reared in CC and AV. Initial bodyweight final bodyweight, egg production percentage and egg weight were higher (all P <0.0001) in RB and BR than in RR and BB. Significant interactions were observed between production system and breed crosses. However, these interactions only resulted in changes in the magnitude of differences between the levels of the main effects and not in any changes in rank. Egg weight in RB and BR crosses reared in EC was higher than in the other treatment groups.
Significant differences were observed between production systems and breed crosses in egg morphometric traits and egg quality traits at 26 weeks old (Tables 5, 6, and 7). Higher (all P <0.005) egg weight, surface area, volume and Haugh unit score were observed in the eggs of chickens reared in ECs than those of CCs and AVs. Eggs from chickens reared in EC and CC had higher shape index (P =0.0002) than those of AVs. Non-significant differences were observed in yolk index (P =0.14) and shell thickness (P =0.14) between these systems. Higher egg weight (P =0.02) and volume (P <0.0001) were found in the eggs of RB and BR chickens than in those of RR and BB. Higher surface area (P =0.0074) was observed in the eggs of BR chickens, followed by RB, BB and RR. The shape index (P =0.05) remained the same in RR, BR, and RB, but was lower in BB. Non-significant differences were observed in Haugh unit score (P =0.22), yolk index (P =0.14) and shell thickness (P =0.58) among crosses. Significant (all P <0.01) interactions between production system and breed crosses were observed in egg weight, egg surface area, egg volume, egg shape index and Haugh unit score. Non-significant interactions were observed for the egg yolk index (P =0.12) and eggshell thickness (P =0.50).
Significant differences were observed in the hatching traits (Table 8). Chickens reared in AVs had higher fertility (P =0.0027) and hatchability percentage (P <0.0001) than those reared in ECs and CCs. Higher infertility (P =0.0012) and dead germ percentage (P =0.0397) were observed in the chickens reared in CCs and ECs. Late embryonic mortality did not differ (P =0.0951) between systems. RB chickens showed the highest fertility (P =0.0002) and hatchability (P <0.0001) percentages, followed by BR, RR, and BB. A higher infertility (P =0.0366) percentage was observed in chickens of BB genotype than in BR, RR, and RB.
The lowest embryonic mortality (P =0.0535) was observed in BR compared with RB, RR, and BB. Non-significant differences were observed in early embryonic morality (P =0.0870) among breed crosses. A higher fertility (P =0.0071) percentage was observed in chickens of RB genotype reared in AV, whereas a higher hatchability (P <0.0001) percentage was observed in RB and BR reared in AV. Non-significant interactions were observed in infertility (P =0.0576), early embryonic mortality (P =0.1855), and late embryonic mortality (P =0.0681.
Chickens in the EC system showed the highest egg production percentage and egg weight, whereas chickens in AVs had the lowest. The higher egg weight and greater number of eggs in EC could be attributed to the stress-free environment and efficient utilization of nutrients in the formation of eggs. In previous studies, non-significant differences were reported in the egg production of hens reared in AVs, CCs, barns and ECs (Neijat et al., 2011; Ahammed et al., 2014). However, a higher egg production percentage was reported in chickens reared in CCs compared with free range and AVs (Tauson et al., 1999; Leyendecker et al., 2001). A higher production percentage, egg weight, bodyweight and cumulative egg mass were reported by Ahmad et al. (2019) in an intensive system compared with semi-intensive and free-range systems. Poor feed conversion was observed in Lohmann LSL white layer and Lohmann LT brown layer hens in a free range system compared with AV, EC, and CC (Leyendecker et al., 2001). The influence of the production system on the feed conversion ratio of Hisex Brown layers (Englmaierova et al., 2014) and Lohmann LSL and Lohman Brown Classic (Onbasilar et al., 2015) was also observed. Higher feed intake and feed conversion ratio was observed in Lohmann Brown layers reared in a barn system compared with AV and CC (Ahammed et al., 2014). Rehman et al. (2016) reported improvement in the production performance of native Aseel chicken reared in semi-intensive and confined systems. The effect of rearing system on egg mass has also been observed (Hidalgo et al., 2008; Tactacan et al., 2009; Onbasilar et al., 2015). The bodyweight of hens reared on a floor system was higher compared with a cage system (Singh et al., 2009).
In terms of genotype, a higher egg weight and egg production percentage were observed in RB and BR crosses than in RR and BB crosses, which could be attributed to heterosis. In crossbreeding, favourable alleles from RNN and BNN masked the less favourable alleles. Saadey et al. (2008) also reported that crossbreeding is useful to obtain offspring that combine the characteristics of their parental ancestries and ultimately produce an animal with hybrid vigour. Ahmad et al. (2019) reported higher egg weights from RNN (53.16 g) and BNN (53.13 g) compared to purebred Naked Neck (46.68 g), and likewise that egg production percentage was higher for BNN (60.71) and RNN (60.21) compared to Naked Neck (54.13) chickens. In the present study, egg weight and production percentage were further improved in the F2 generation. Egg weights in RB (52.84 g) and BR (53.04 g) were higher than for RR (50.18 g) and BB (49.69 g). Furthermore, the egg production percentage of RB (61.99) and BR (62.04) was also higher than RR (57.73) and BB (58.25). Another possible reason for the improvement of egg production and egg weight in crossbred chickens is breed complementarity. For example, Razuki et al. (2011) explained the improvement in egg production and egg weight after crossbreeding between White Leghorn, Iraq Brown, and New Hampshire as the result of breed complementarity.
In this study, overall egg quality was better for birds housed in EC. This might be attributed to an increase in nutrients for egg formation and growth. A reduction in energy expenditure for locomotor activities in this environment might result from reduced stress as a result of enhanced expression of natural behaviours. Another possible reason for the higher egg weight in EC is the greater bodyweights of chickens in this system, because egg weight and bodyweight are positively correlated (Nigussie et al., 2011). Higher egg surface area, egg volume, and Haugh unit score in EC might be due to a proportional correlation of egg components with egg weight. Differences in egg quality characteristics could be a manifestation of genetic and environmental discrepancies (Falconer & Mackay, 1996). The egg quality might be influenced by the genotype and breeder age (Monira et al., 2003), production system and environment (Travel et al., 2010).
In the initial stage of the production cycle at 26 weeks old, the present study revealed higher egg weight and egg volume in RB and BR than in BB and RR. The surface area was also higher in BR than in other breed crosses. When egg quality was evaluated at the end of experiment at 46 weeks old, RB and BR had higher egg weight, egg surface area and egg volume than BB and RR. The Haugh unit score was higher in RB than in other crosses. Higher egg weight in RB and BR crosses could be because of higher bodyweights in these crosses, as it is well known that heavier breeds lay larger eggs (Du Plessis & Erasmus, 1972; Nigussie et al., 2011). Khawaja et al. (2016) found higher egg weight in crosses between White Leghorn and Fayoumi than in crosses of Fayoumi with Rhode Island Red and attributed the increase in egg weight to its positive correlation with bodyweight.
In the present study, higher surface area and egg volume in RB and BR could be attributed to the shapes of their eggs with the long axis being slightly longer than the short one. Variations in internal and external egg quality traits could be attributed to the variations in genetic makeup of the birds. Monira et al. (2003) explained that variations in egg quality traits were mainly the result of differences on genotype and age. Variations in egg volume and egg surface area in the eggs of broiler breeder strains and Aseel varieties were explained by Rayan et al. (2010) and Rehman et al. (2017), respectively. Differences in egg surface area were observed to vary among strains (Anderson et al., 2004) and breeds (Islam et al., 2010. Dunga (2013) observed different Haugh unit scores in Aseel and Naked Neck chickens. On the other hand, nonsignificant differences were reported in Haugh unit score among breed types (Rajkumar et al., 2009). Rayan et al. (2010) and Van Den Brand et al. (2004) found differences in the egg shape index of commercial layers and indigenous chickens, whereas Rehman et al. (2017) reported differences in the egg shape index of Aseel varieties. In the present study, non-significant differences were observed in shell thickness and egg yolk index among crosses. Similarly, non-significant differences in shell thickness of breed types were observed in numerous studies (Hocking et al., 2003; Dukic-Stojcic et al., 2009; Rehman et al., 2017). However, significant variations in the yolk index among frizzle chickens and Naked Neck were also reported (Dunga, 2013). A lower yolk index in eggs of Naked Neck chicken than normal feathered chicken was reported by Rajkumar et al. (2009).
Cost effectiveness of a hatchery enterprise is determined mainly by the fertility and hatchability of the flock (Peters et al., 2008b). Environmental temperature and mating combination are important factors in determining the hatchability of eggs (Mo et al., 2007). In the present study, significant differences were observed in hatching traits of breed crosses reared in three production systems. When the production systems were compared, fertility and hatchability percentages were higher in AV than those of CC and EC systems. On the other hand, infertility and early embryonic mortality were same in the CC and EC systems and low in AV. However, late embryonic mortality was similar in all production systems. The most appropriate reasons behind the higher fertility and hatchability percentage in AV are more space per bird and lower stocking density which enhanced the mating activity of the chickens. Ahmad et al.'s (2019) findings support the present outcomes, in which the highest fertility and hatchability percentages were obtained in chickens reared in a free-range system followed by the chickens reared in semi-intensive and intensive systems. However, better semen quality resulted in an ultimately better fertility percentage in Botswana chicken breed crosses when reared under an intensive system (Mothibedi et al., 2016).
Among the breed crosses in this study, fertility and hatchability were highest in the RB chickens. The variations in hatching traits among the breed crosses might be attributed to the use of crossbreeding. Ahmad et al. (2019) reported a higher fertility in RNN (87.4%) and BNN (86.7%) crossbreds than purebred Naked Neck (81.7%). Furthermore, hatchability was higher in RNN (71.6%), followed by BNN (69.2%) and purebred Naked Neck (64.1%). In the present trial, fertility and hatchability percentages were improved after reciprocal crossing of RNN and BNN (RB and BR) rather than inter mating (BB and RR). The fertility percentage was highest in RB (88.9%), followed by BR (86.8%), RR (85.9%), and BB (83.7%). Similarly, the hatchability percentage was highest in RB (74.4%), followed by BR (73.0%), RR (71.3%) and BB (68.57%). Peters et al. (2008a) explained the variations in fertility and hatchability percentages among pure and crossbreds of indigenous Nigerian chickens as being because of the gene segregation effect. In another study, Peters et al. (2008b) found comparable genetic variations between the semen quantity and quality traits of local and exotic cocks. However non-significant breed differences in hatchability of eggs have been reported (Islam et al., 2002). Peters et al. (2004) reported the highest fertility and hatchability percentage in frizzle-feathered followed by formal feathered and Naked Neck chickens. Similarly, the higher fertility percentage (90.5%) in frizzle-feathered chickens compared with normal feathered chickens (84.4%) revealed the variation in hatching traits among breed crosses (Adeleke et al., 2012). Merat (1986) reported higher (up to 10%) embryonic mortality in homozygous Naked Neck chicken than heterozygous partial feathered chickens. Reduction in embryonic survival up to 6.1% in Naked Neck chickens has been reported compared with normal-feathered chicken (Peters et al., 2008a) which might have happened during last incubation stages and caused a higher dead-in-shell percentage (21.2%) than dead germ percentage in pure Naked Neck (Singh et al., 2001). The effects of crossbreeding in INRA44 female duck also revealed a higher fertility percentage (85.5%) in purebred than in crossbreds (66.4%) (Brun & Larzul, 2003). Sellier et al. (2005) attributed this variation in response to the differences in fecundity according to the genetic type.
Conclusions
Among alternative production systems, hens housed in EC had higher production performance and egg quality than hens housed in AV or CC. However, AV may improve fertility and hatchability. In terms of breed crosses, RB and BR had increased performance relative to BB and RR.
Acknowledgements
The authors gratefully acknowledge Dr. Sohail Ahmad (lecturer) and the Administration at Indigenous Chicken Genetic Resource Centre, University of Veterinary and Animal Sciences, Ravi Campus, Pattoki, Pakistan. for their help during the biological trial and manuscript write up.
Author's Contributions
MU conducted this study as part of his Ph.D. research under the supervision of AM, JH and AJ. AM and AJ helped in reviewing the manuscript. JH helped in statistical analysis and formatting of manuscript.
Conflict of Interest Declaration
The authors declare no potential conflicts of interest.
References
Adebambo, A.O., Ikeobi, C.O.N., Ozoje, M.O., Oduguwa, O.O. & Adebambo, O.A., 2011. Combining abilities of growth traits among pure and crossbred meat type chickens. Arch. Zootech. 60, 953-963. [ Links ]
Adeleke, M.A., Peters, S.O., Ozoje, M.O., Ikeobi, C.O.N., Bamgbose, A.M. & Adebambo, O.A., 2012. Effect of crossbreeding on fertility, hatchability and embryonic mortality of Nigerian local chickens. Trop. Anim. Health Prod. 44, 505-510. [ Links ]
Ahammed, A., Chae, B.J., Lohakare, J., Keohavong, B., Lee, M.H., Lee, S.J., Kim, D.M., Lee, J.Y. & Ohh, S.J., 2014. Comparison of aviary, barn and conventional cage raising of chickens on laying performance and egg quality. Asian-Aust. J. Anim. Sci. 27, 1196-1203. [ Links ]
Ahmad, S., Mahmud, A., Hussain, J. & Javed, K., 2019. Productive performance, egg characteristics and hatching traits of three chicken breed crosses under free range, semi intensive and intensive housing systems. Braz. J. Poult. Sci. 21, 1-10. [ Links ]
Anderson, K.E., Tharrington, J.B., Curtis, P.A. & Jones, F.T. 2004. Shell characteristics of eggs from historic strains of single comb white Leghorn chickens and the relationship of egg shape to shell strength. Int. J. Poult. Sci. 3, 1719. [ Links ]
Anonymous, 2019. Economic survey of Pakistan, 2018-19. Agriculture, Chapter 2. III. Livestock and Poultry. b. Poultry. p. 28-29. [ Links ]
Appleby, M.C., 1998. The Edinburgh modified cage: Effects of group size and space allowance on brown laying hens. J. Appl. Poult. Res. 7, 152-161. [ Links ]
Brun, J.M. & Larzul, C., 2003. Inheritance of reproductive traits of female common ducks (Anas platyrhynchos) in pure-breeding and in inter-generic crossbreeding with Muscovy ducks (Cairina moschata). Brit. Poult. Sci. 44, 1-6. [ Links ]
Du Plessis, P.H.S. & Erasmus, J., 1972. The relationship between egg productions, egg weight and bodyweight in laying hens. World's Poult. Sci. J. 28, 73-78. [ Links ]
Dukic-Stojcic, M., Peric, L., Bjedov, S. & Milosevic, N., 2009. The quality of table eggs produced in different housing systems. Biotech. Anim. Husb. 25, 1103-1108. [ Links ]
Dunga, G.T., 2013. The effect of the Naked Neck (Na) and frizzling genes on the fertility, hatchability, egg quality and pterylosis of locally developed commercial layer parent lines. PhD thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. [ Links ]
Englmaierova, M., Tumova, E., Charvatova, V. & Skrivan, M., 2014. Effects of laying hens housing system on laying performance, egg quality characteristics, and egg microbial contamination. Czech J. Anim. Sci. 59, 345-352. [ Links ]
Falconer, D.S. & Mackay, T.F.C., 1996. Introduction to quantitative genetics. 4th edition. Benjamin Cummings, London, UK. ISBN-13: 9780582243026. p. 464. [ Links ]
Garces, A., Casey, N.H. & Horst, P., 2001. Productive performance of naked neck, frizzle and dwarf laying hens under various natural climates and two nutritional treatments. S. Afr. J. Anim. Sci. 31, 174-180. [ Links ]
Gikunju, M.M., Kabuage, L.W., Wachira, A.M., Oliech, G.W. & Gicheha, M.G., 2018. Evaluation of pure breeds, crossbreeds and indigenous chicken egg quality traits in Kenya. Livestock Res. Rur. Dev. 30, 170. [ Links ]
Grobbelaar, J.A.N., Sutherland, B. & Molalakgotla, N.M., 2010. Egg production potentials of certain indigenous chicken breeds from South Africa. Anim. Gen. Res. 46, 25-32. [ Links ]
Hidalgo, A., Rossi, M., Clerici, F. & Ratti, S., 2008. A market study on the quality characteristics of eggs from different housing systems. Food Chem. 106, 1301-1308. [ Links ]
Hocking, P., Bain, M., Channing, C., Fleming, R. & Wilson, S., 2003. Genetic variation for egg production, egg quality and bone strength in selected and traditional breeds of laying fowl. Brit. Poult. Sci. 44, 365-373. [ Links ]
Islam, M.S., Howlider, M.A.R., Kabir, F. & Alam, J., 2002. Comparative assessment of fertility and hatchability of barred Plymouth Rock, White Leghorn, Rhode Island Red and White Rock hen. Int. J. Poult. Sci. 1, 85-90. [ Links ]
Islam, S.M. & Dutta, R.K., 2010. Egg quality traits of indigenous, exotic and cross bred chickens (Gallus domesticus L.) in Rajshahi, Bangladesh. J. Life Earth Sci. 5, 63-67. [ Links ]
Jones, D.R., Cox, N.A., Guard, J., Fedorka-Cray, P.J., Buhr, R.J., Gast, R.K., Abdo, Z., Rigsby, L. L., Plumblee, J. R., Karcher, D. M., Robison, C. I., Blatchford, R. A. & Makagon, M.M., 2014. Microbiological impact of three commercial laying hen housing systems. Poult. Sci. 94, 544-551. [ Links ]
Khawaja, T., Khan, S.H, Mukhtar, N., Parveen, A. & Fareed, G., 2016. Production performance, egg quality and biochemical parameters of three way crossbred chickens with reciprocal F1 crossbred chickens in sub-tropical environment locomotor activity. Ital. J. Anim. Sci. 12, 127-132. [ Links ]
Lay, D.C., Fulton, R.M., Hester, P.Y., Karcher, D.M., Kjaer, J.B., Mench, J.A., Mullens, B.A., Newberry, R.C., Nicol, C.J., O'Sullivan, N.P. & Porter, R.E., 2011. Hen welfare in different housing systems. Poult. Sci. 90, 278-294. [ Links ]
Leeson, S. & Summers, J.D., 2005. Commercial poultry nutrition. 3rd edition Nottingham University Press, Nottingham. Pp. 297-305. [ Links ]
Leyendecker, M., Hamann, H., Hartung, J., Kamphues, J., Ring, C., Glunder, G., Ahlers, C., Sander, I., Neuman, U. & Distl, O., 2001. Analysis of genotype environment interactions between layer lines and housing systems for performance traits, egg quality and bone breaking strength: 1st communication: Performance traits. Zuchtungskunde. 73, 290-307. [ Links ]
Mallia, J.G., 1999. Observation on family poultry units in parts of Central America and sustainable development opportunities. Livestock Res. Rur. Dev. 11, 3. http://www.fao.org/ag/AGA/AGAP/frg/FEEDback/lrrd/lrrd11/3/mal113.htm [ Links ]
Mench, J.A. & Blatchford, R.A., 2014. Determination of space use by laying hens using kinematic analysis. Poult. Sci. 93, 794-798. [ Links ]
Mench, J.A., Sumner, D.A. & Rosen-Molina, J.T., 2011. Sustainability of egg production in the United States-the policy and market context. Poult. Sci. 90, 229-240. [ Links ]
Menge, E.O., Kosgey, I.S., Kahi & A.K., 2005. Bio-economic model to support breeding of indigenous chicken in different production systems. Int. J. Poult. Sci. 4, 1-13. [ Links ]
Merat, P., 1986. Potential usefulness of the Na (naked neck) gene in poultry production, World's Poult. Sci. J. 42, 124 - 142. [ Links ]
Mo, D., Li, K., Qiangba, Y., Tang, Z., Zhu, M., Xu, R., Fan, B. & Liu, B., 2007. Effect of mating combination and environmental factor on hatchability of chicken eggs in Tibet. Front. Agric. China. 1, 214. [ Links ]
Monira, K.N., Salahuddin, M. & Miah, G., 2003. Effect of breed and holding period on egg quality characteristics of chicken. Int. J. Poult. Sci. 2, 261-263. [ Links ]
Mothibedi, K., Nsoso, S.J., Waugh, E.E. & Kgwatalala, P.M., 2016. Semen characteristics of purebred Naked Neck Tswana and Black Australorp * Naked Neck Tswana crossbred chickens under an intensive management system in Botswana. Amer. J. Res. Comm. 4, 38-47. [ Links ]
Neijat, M., House, J.D., Guenter, W. & Kebreab, E., 2011. Production performance and nitrogen flow of Shaver White layers housed in enriched or conventional cage systems. Poult. Sci. 90, 543-554. [ Links ]
Nigussie, D., Waaij, E.H.V. & Johan, A.M.V.A., 2011. Genetic and phenotypic parameter estimates for bodyweights and egg production in Horro chicken of Ethiopia. Trop. Anim. Health Prod. 43, 21-28. [ Links ]
Nwachukwu, E.N., Ibe, S.N. & Ejekwu, K., 2006. Short term egg production and egg quality characteristics of main and reciprocal crossbred normal local, naked-neck and frizzle chicken X exotic broiler breeder stock in a humid tropical environment. J. Anim. Vet. Adv. 5, 547-551. [ Links ]
Onbasilar, E.E., Unal, N., Erdem, E., Kocakaya, A. & Yaranoglu, B., 2015. Production performance, use of nest box, and external appearance of two strains of laying hens kept in conventional and enriched cages. Poult. Sci. 94, 559-564. [ Links ]
Peters, S.O., Omidiji, E.A., Ikeobi, C.O.N., Ozoje, M.O. & Adebambo, O.A., 2004. Effects of Naked neck and Frizzled genes on egg traits, fertility and hatchability in local chicken. In: J.O. Ogunji, I.I. Osakwe, V.U. Ewa, S.O. Alaku, M.O. Otuma & B.O. Nweze, (eds), Proceedings of the 9th annual conference of Animal Science Association of Nigeria, Abakaliki. p. 262-264. [ Links ]
Peters, S.O., Ilori, B.M., Ozoje, M.O., Ikeobi, C.O.N. & Adebambo, O.A., 2008a. Gene segregation effects on fertility and hatchability of pure and crossbred chicken breed crosses in the humid tropics. Int. J. Poult. Sci. 7, 954-958. [ Links ]
Peters, S.O., Shoyebo, O.D., Ilori, B.M., Ozoje, M.O., Ikeobi, C.O.N. & Adebambo, O.A., 2008b. Semen quality traits of seven strains of chickens raised in the humid tropics. Int. J. Poult. Sci. 7, 949-953. [ Links ]
Preisinger, R., 2005. Development, state and perspectives of poultry production. Zuchtungskunde 77, 502-507. [ Links ]
Rajkumar, U., Sharma, R.P., Rajaravindra, K.S., Niranjan, M., Reddy, B.L.N., Bhattacharya, T.K. & Chatterjee, R.N., 2009. Effect of genotype and age on egg quality traits in Naked Neck chicken under tropical climate from India. Int. J. Poult. Sci. 8, 1151-1155. [ Links ]
Rayan, G.N., Galal, A., Fathi, M.M. & El-Attar, A.H., 2010. Impact of layer breeder flock age and strain on mechanical and ultrastructural properties of eggshell in chicken. Int. J. Poult. Sci. 9, 139-147. [ Links ]
Razuki, W.M., Mukhlis, S.A., Jasim, F.H. & Hamad, R.F., 2011. Productive performance of four commercial broiler breed crosses reared under high ambient temperatures. Int. J. Poult, Sci. 10, 87-92. [ Links ]
Rehman, M.S., Mahmud, A., Mehmood, S., Pasha, T.N., Javed, K., Hussain, J. & Khan, M.T., 2016. Production performance of Aseel chicken under free range, semi intensive and confinement rearing systems. J. Anim. Plant Sci. 26, 1589-1596. [ Links ]
Rehman, M.S., Mahmud, A., Mehmood, S., Pasha, T.N., Hussain, J. & Khan, M.T., 2017. Comparative evaluation of egg morphometry and quality in Aseel hens under different rearing systems. J. Appl. Poult. Res. 26, 401 -409. [ Links ]
Rodenburg, T.B., Tuyttens, F.A.M., De Reu, K., Herman, L., Zoons, J. & Sonck, B., 2008. Welfare assessment of laying hens in furnished cages and non-cage systems: assimilating expert opinion. Anim. Welfare 17, 355-361. [ Links ]
Saadey, S.M., Galal, A., Zaky, H.I. & Zein El-Dein, A., 2008. Diallel crossing analysis for bodyweights and egg production traits of two native Egyptian and two exotic chicken breeds. Int. J. Poult. Sci. 7, 64-71. [ Links ]
Sellier, N., Brun, J.M., Richard, M.M., Batellier, F., Dupy, V. & Brillard, J.P., 2005. Comparison of fertility and embryo mortality following artificial insemination of common ducks females (Anas platyrhynchos) with semen from common or Muscovy (Cuirina moschata) drakes. Theriogen 64, 429-439. [ Links ]
Shafik, B.M.N., El-Bayomi, K.M., Sosa, G.A. & Osman, A.M.R., 2013. Effect of crossing Fayoumi and Rhode Island Red on growth performance, egg and reproductive traits under Egyptian conditions. Benha Vet. Med. J. 24, 11-18. [ Links ]
Singh, C.V., Kumah, D. & Singh, Y., 2001. Potential usefulness of the plumage reducing Naked neck (Na) gene in poultry production at normal and high ambient temperatures. World's Poult. Sci. J. 57, 139-156. [ Links ]
Singh, R., Cheng, K.M. & Silversides, F.G., 2009. Production performance and egg quality of four strains of laying hens kept in conventional cages and floor pens. Poult. Sci., 88, 256-264. [ Links ]
Sosnowka-Czajka, E., Herbut, E. & Skomorucha, I., 2010. Effect of different housing systems on productivity and welfare of laying hens. Ann. Anim. Sci. 10, 349-360. [ Links ]
Tactacan, G.B., Guenter, W., Lewis, N.J., Rodriguez-Lecompte, J.C. & House, J.D., 2009. Performance and welfare of laying hens in conventional and enriched cages. Poult. Sci. 88, 698-707. [ Links ]
Tauson, R., Wahlstrom, A. & Abrahamsson, P., 1999. Effect of two floor housing systems and cages on health, production, and fear response in layers. J. Appl. Poult. Res. 8, 152-159. [ Links ]
Travel, A., Nys, Y. & Lopes, E., 2010. Productivity and environmental factors affecting egg quality. INRA Productions Animales. 23, 155-166. https://www6.inra.fr [ Links ]
Tukey, J.W., 1953. The problem of multiple comparisons. In: The collected works of John W. Tukey VIII. Multiple comparisons. New York: Chapman and Hall. [ Links ]
Van Asselt, E.D., van Bussel, L.G.J., van Horne, P., van der Voet, H., van der Heijden, G.W.A.M. & van der Fels-Klerx, H.J., 2015. Assessing the sustainability of egg production systems in The Netherlands. Poult. Sci. 94, 1742-1750. [ Links ]
Van Den Brand, H., Parmentier, H.K. & Kemp, B., 2004. Effect of housing system (outdoor vs cages) and age of laying hens on egg characteristics. Brit. Poult. Sci. 45, 745-752. [ Links ]
Submitted 14 December 2019
Accepted 25 March 2020
Published 24 September 2020
# Corresponding author: musman@uvas.edu.pk














