Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.49 n.2 Pretoria 2019
http://dx.doi.org/10.4314/sajas.v49i2.18
ARTICLES
Carcass and meat quality attributes of Malawi Zebu steers fed Vachellia polyacantha leaves or Adansonia digitata seed as alternative protein sources to Glycine max
G. ChingalaI, II; E. RaffrenatoI; K. DzamaI; L.C. HoffmanI, III; C. MapiyeI, #
IDepartment of Animal Sciences, Stellenbosch University, Private Bag X1, Matieland 7602, South Africa
IILilongwe University of Agriculture and Natural Resources, P.O. Box 219, Lilongwe, Malawi
IIICentre for Nutrition and Food Sciences, Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Coopers Plains, QLD 4108, Australia
ABSTRACT
To enhance smallholder beef farmers' capacity to cope with animal feed shortages, especially dietary protein induced by climate change, it is important to evaluate the feeding value of low-cost protein sources naturally available in the environment. The aim of the study was to evaluate carcass and meat quality of Malawi Zebu steers fed diets containing Adansonia digitata (baobab) seed meal or Vachellia polyacantha (white thorn tree) leaf meal as alternative protein sources to Glycine max (soybean) under feedlot conditions. Thirty Malawi Zebu steers (181 ± 21.4 kg body weight; 29 months old) were individually fed forage-based diets made up of rangeland hay and maize bran, with either baobab seed meal, Vachellia leaf meal or soybean meal as a protein source for 120 days. At the end of the feeding trial, animals were slaughtered and the longissimus thoracis et lumborum muscle sampled for meat quality analyses. Steers fed soybean and baobab diets had higher subcutaneous fat thickness, carcass weights than those fed the Vachellia diet. Meat from steers fed the Vachellia and baobab diets had higher ultimate pH and water-holding capacity, and lower drip and cooking losses than meat from steers fed the soybean diet. Feeding baobab and Vachellia diets produced beef with lower lightness (L*) values than the soybean diet, characteristic of dark, firm and dry (DFD) beef. Steers fed the baobab diet had the highest gross profits followed by those fed the Vachellia and soybean diets, respectively. Overall, feeding the baobab and Vachellia diets improved gross profit but produced DFD beef compared to the soybean diet.
Keywords: beef cattle, baobab seed, soybean meal, smallholder production
Introduction
Smallholder beef farming dominated by the small-framed, indigenous Malawi Zebu cattle breed provides over 90% of the beef consumed in Malawi (Chingala et al., 2017a). Compared to Bos taurus (British and European) cattle breeds, Bos indicus (Zebu) cattle breeds are tolerant to heat, disease and parasites and valorise fibrous diets better and have low water requirements, which are key adaptation traits in the face of climate change in tropical areas (Strydom, 2008). Despite the Malawi Zebu possessing desirable adaptation traits, smallholder beef production is low and market options are limited in Malawi (Dzanja et al., 2013). Alternative strategies already exist in Malawi (Nkhonjera et al., 1988) and other parts of the world (Abate et al., 1992; Kaliba et al., 1997; Stür et al., 2013) to increase the competitiveness of smallholder cattle producers in both informal and formal markets. In Vietnam, for example, smallholder farmers intensified livestock production by adopting a stall-fed system as an alternative to the traditional grazing system, which enabled them to be more competitive in the formal livestock markets (Stür et al., 2013).
Malawi, like many other countries in the tropics, is experiencing decreases in precipitation and increases in ambient temperatures (IPCC, 2007). These climatic changes lead to reduction in crop yields including grain legumes and oilseeds (Conway et al., 2015), which in turn limit their availability for use as protein supplements for smallholder beef production. Use of abundantly available natural protein sources including indigenous browse legume leaf meals and tree-borne oilseeds such as Vachellia tree species and Adansonia digitata (baobab), respectively (Chíngala et al., 2018) could help mitigate severe shortages of protein supplements for smallholder beef cattle production. Vachellia leaf meal and baobab seeds have crude protein levels of 15% to 20% and 30% to 35%, respectively, which is comparable to the conventional grain legume and oil-seed crops (Assogbadjo et al., 2012; Mapiye et al., 2009; 2010). Therefore, use of these tree-borne protein supplements could improve beef production and quality and consequently, contribute to food, nutrition, income and social security of resource-poor households in dry environments (Mlambo & Mapiye, 2015).
Currently, the use of tree-borne, protein-rich feed resources is restricted by the presence of plant secondary compounds, particularly polyphenols, which compromise feed intake and nutrient digestibility when fed at high concentrations (>60 g/kg DM; Makkar, 2003). Nonetheless, feeding ruminants with leaf and seed meals containing moderate levels of polyphenolic compounds have been shown to improve growth attributes (Chingala et al., 2018), meat fatty acid composition (Mapiye et al., 2011) and shelf-life stability (Mlambo & Mapiye, 2015; Jerónimo et al., 2016). While some reports on effects of supplementing forage-based diets with Vachellia trees leaf-meals on carcass (Mapiye et al., 2009) and beef quality (Mapiye et al., 2010) are available, the effects of baobab seed on beef quality have not been investigated. Therefore, the objective of the current study was to evaluate the effects of feeding Malawi Zebu steers under feedlot conditions with diets containing baobab seed meal or V. polyacantha leaf meal as alternative protein sources to soybean meal on carcass and meat quality attributes.
Materials and Methods
The study was carried out at the Beef Unit of the Department of Animal Science, Lilongwe University of Agriculture and Natural Resources (LUANAR) in Malawi (14° 11.013* S 33 ° 47.95' E). The area experiences subtropical climate with three seasons; cool dry (May-August), hot dry (September-November) and hot wet seasons (December-April). The University Beef Unit receives 800 - 1000 mm of precipitation and has average daily minimum and maximum temperatures of 18 °C and 27 °C, respectively. The present study was performed between July and November 2016 with the approval of the Research Ethics Committee on Animal Care and Use of Stellenbosch University, Stellenbosch, South Africa (Protocol Number: SU-ACUD14-00075).
Thirty Malawi Zebu (Bos indicus) steers (weighing 181 ± 21.4 kg; 29 months old) were individually housed in covered pens, measuring 2 χ 4 m. Prior to the experiment, the animals were tagged, dewormed and dipped in acaricides. The steers were randomly assigned to three diets (10 steers/ diet) containing rangeland hay and maize bran with either V. polyacantha leaf meal (Vachellia diet), baobab seed meal (baobab diet) or soybean meal (soybean diet, the control) as a protein source (Table 1). The rangeland hay mainly comprised of Cynodon nlemfuensis, Chloris gayana and Brachiaria decumbens harvested from the same area at booting stage in the hot wet season (February 2016). Maize bran, a by-product of shelling, was purchased from local maize grain millers. The leaf meal of V. polyacantha, formerly Acacia polyacantha (white thorn tree), was harvested at the leaf maturity stage around the Beef Unit at LUANAR. A batch of baobab seeds, waste products of baobab beverage-processing, were purchased from a local baobab beverage manufacturing company in Lilongwe, Malawi. Isonitrogenous diets were fed to steers as total mixed rations for 120 days ad libitum. Samples of feed were collected weekly for determination of chemical composition of the diets.
For all the diets, dry matter (method 934.01), ash (method 942.05) and crude fat by acid hydrolysis (method 954.02) were analysed following the AOAC International (2006) procedures. Crude protein was analysed using the Dumas Method with a macro-nitrogen analyser (LECO® FP528, LECO Corporation, Miami, USA). Starch was measured using a commercial assay (Total starch Megazyme kit KTSTA, Megazyme International Ireland Ltd., Wicklow, Ireland) according to Hall (2009). Neutral detergent fibre (NDF) and lignin were determined according to Mertens et al., (2002) and Raffrenato & Van Amburgh (2011), respectively. The in vitro digestibility of NDF (ivNDFd) (30-h incubation) were according to Goering & Van Soest (1970). The total phenolic compounds and total tannins were analysed according to Makkar (2000) while condensed tannins were quantified according to Porter et al. (1985).
Feed offered and refused were measured daily to determine average daily DM intake (DMI). Live weights were measured every three weeks using a digital scale (Sasco Africa, South Africa). Average daily gain (ADG) for individual steers was calculated as coefficient of a linear regression of body weight against time of the data points. Feed conversion ratio (FCR) was estimated as ratio of DMI to ADG. Growth performance and carcass attributes of Malawi Zebu steers fed diets containing different protein sources are shown in Table 2. The findings of growth performance were reported by Chíngala et al. (2018) and will not be discussed further.
On day 120 of the feeding experiment, animals were weighed and transported to Kanengo Abattoir, Lilongwe, Malawi where they were deprived of feed except water for approximately 16 hours. The steers were then slaughtered according to the commercial abattoir practices, and no electrical stimulation was applied post-mortem. The carcasses were dressed, inspected and split into left and right halves. Approximately 45 min post-mortem pH and temperature of the carcasses were recorded on the loin of the right half of the carcasses using a Crison pH meter (Lasec Pty Ltd, Cape Town, South Africa). In addition, fat thickness was measured 5 cm laterally from the mid-line cut between the 10th and 11th ribs. The carcasses were weighed and chilled for 24 h at 4 °C. The pH was recorded again at 24 h post-mortem at the same anatomical position as for the 45-min post-mortem pH. The cold carcass weight was estimated by subtracting 2% from the warm carcass weight following the practice of the abattoir. Dressing percentage was calculated by dividing the warm carcass weight by the final live body weight and express the quotient as a percentage. The carcasses were graded using the Malawian Grading System by a qualified government meat grader (Malawi Meat and Products Act of 1985). Steaks from longissimus thoracis et lumborum (LTL) muscle between the 9th and 11th ribs were excised from left side of the carcasses. The steaks were sliced for a measurement of colour, drip loss, water-holding capacity, cooking loss, Warner-Blatzler shear force (WBSF) and proximate composition.
Colour measurements were performed directly on the meat surface after blooming for 30 min. Lightness (L*), redness (a*) and yellowness (b*) parameters were recorded using the CIELAB colour meter: Colour Guide 45°/0° Colorimeter (BYK-Gardner GmbH, Gerestried, Germany) with a 11-mm diameter aperture, using an illuminant/observer of D65/ 10° observer settings. The measurements were taken three times at different locations per sample. The hue angle (H°) and chroma (C*) values were calculated as:

The drip loss samples were weighed in duplicate and suspended in an inflated plastic bag. Drip loss was estimated as a proportion of weight loss to initial weight after meat samples were stored for 24 h at 4°C (Honikel, 1998).
Water holding capacity (WHC) was determined using the filter pressing method of Grau & Hamm (1953). Beef samples, weighing 0.5 g each were finely chopped and pressed onto filter paper between two Perspex plates (pressure: 588N). The photographs of the pressed samples on the filter paper were taken from the same camera settings. The ImageJ software (National Institute of Mental Health, Bethesda, Maryland, USA) was used to estimate the area of purge (outer circle) and meat (inner circle) on photographs. The water holding capacity was calculated as the difference between the outer and inner circle area divided by the outer circle area and expressed as a percentage. The analyses was performed in duplicate.
The cooking loss was determined by boiling LTL steaks (in duplicate) in plastic bags at 80 °C in a water-bath for an hour to reach an internal temperature of 75 °C monitored using a thermocouple attached to a handheld digital temperature monitor (Hanna Instruments Bellville, South Africa; Honikel, 1998). The bags were cooled to ± 4 °C and LTL slices were removed and blotted dry with paper towels. The cooking loss was estimated as the difference between initial and final weights. The cooked samples were cooled at 4 °C for 24 h before the determination of WBSF. A minimum of six cores from each meat sample were removed in the direction of the muscle fibres using a sharp, stainless steel 1.27 cm diameter borer. Core shear force was determined using a V-shaped, 1 mm thick Warner Bratzler cutting blade. The blade moved at a cross speed of 3.33 mm/s and was connected to an electric scale to compute the maximum weight (force) in kg per 1.27cm Φ diameter required to shear the samples. The mean of the six readings was calculated in kg/1.27cm Φ diameter.
The LTL steaks for proximate analyses were trimmed of all subcutaneous fat before being homogenized using a knife mill (Knifetec™ 1095, Höganäs., Sweden), vacuum-packed and stored at -20 °C for chemical analyses. Moisture (Method 934.01) and ash (Method 942.05) were determined according to the (AOAC, 2006). Total fat was extracted using 2:1 chloroform/methanol (v/v) solvent and determined according to Lee et al. (1996). Protein content was determined using DUMAS method (LECO® FP528, LECO Corporation, Miami, USA). Samples were analysed in duplicate.
A gross profit analysis was conducted to determine the financial viability of incorporating Vachellia and baobab seed in beef cattle finisher diets. Gross profit was calculated as the difference between total revenue and total variable costs. In the present study, total variable costs were exclusively based on the major contributory factors to expenses, which were cost of labour and total feed consumed per steer. All the other expenses were similar across the different diets, therefore, excluded from the gross profit analyses. Total revenue for each treatment was determined as the income received from the selling of each carcass. All data were analysed in SAS 9.4 (SAS Institute, Cary, NC, USA). Proc univariate statement was used to test for data normality. All data were normally distributed and had equal variance among treatments. The fixed effect of diet on DMI, ADG, carcass and meat quality attributes was analysed using Proc GLM with initial weight as a covariate. The means were separated using the PDIFF option with the Tukey-Cramer Adjustment. Significance was declared at <0.05. The following statistical model was used:

where: 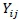 = dependent response;
= dependent response;
μ= overall mean;
βι= fixed effect of the ith diet (i = baobab, Vachellia, soybean diets) and;
= random error
Results
Briefly, the diets had a comparable (P >0.05) CP content but starch and fat contents were slightly lower (P <0.05) in the Vachellia diet than in the other two diets (Table 1). The Vachellia diet had the highest NDF, ADL and phenolic compound levels followed by the baobab and soybean diets, respectively (P <0.05; Table 1). The in vitro NDF digestibility values of the baobab and soybean diets were higher P <0.05) than that of the Vachellia diet.
Diet influenced (P <0.05) DMI, ADG and slaughter weight (Table 2). Steers fed the soybean and baobab diets had higher (P <0.05) DMI, ADG and slaughter weight than those fed the Vachellia diet. Diet had an effect (P <0.05) on subcutaneous fat thickness, warm and cold carcass weights with steers fed soybean and baobab diets having thicker (P <0.05) subcutaneous fat and heavier (P <0.05) carcasses than the steers fed the Vachellia diet (Table 2). Diet did not affect (P = 0.858) dressing percentage (Table 2). All carcasses were graded as Standard (Table 2), a grade given to carcasses from steers, cows and bulls of any age, moderately fleshed, and covered with fat.
Physico-chemical meat quality characteristics of Malawi Zebu steers fed the three different sources of protein are presented in Table 2. The ultimate pH (pHu) and WHC was influenced (P <0.05) by the diet with steers fed Vachellia and baobab diets having higher (P <0.05) values than steers fed the soybean diet. For colour values, only lightness (L*) was influenced (P = 0.027) by diet. Baobab- and Vachellia-fed steers had lower (P <0.05) L* values than soybean-fed steers. Furthermore, diet affected (P <0.05) drip and cooking losses. Steers fed Vachellia and baobab diets had lower (P <0.05) drip and cooking losses than soybean-fed steers. The WBSF values were not influenced (P = 0.648) by diet. Of the proximate parameters, only moisture and intramuscular fat contents were influenced (P <0.05) by diet. The meat moisture content was greater (P <0.05) in beef of steers fed baobab and Vachellia diets than in those fed the soybean diet. Baobab diet produced beef with higher (P <0.05) intramuscular fat than the Vachellia and soybean diets.
The results of the gross profit analysis of Malawi zebu steers fed diets of different protein sources are presented in Table 3. The Vachellia diet had the least total variable costs while the baobab diet had moderate costs and the soybean diet had the highest costs (P < 0.05). Steers fed the baobab and soybean diets had high total revenue compared to the Vachellia diet. The baobab-fed steers had the highest gross profit followed by Vachellia- and Soybean-fed steers, respectively.
Discussion
The lower subcutaneous fat and carcass weights reported for steers fed the Vachellia diet could be because of their low DMI, energy, nutrient digestibility and ADG. This was a result of the low starch, high lignin and tannin contents of the former diet as reported by Chingala et al. (2018). Starch provides readily fermented carbohydrates to rumen microbes to digest fibrous carbohydrates (Allen, 2000). Lignin acts as physical barrier against the action of ruminal microbial enzymes on hemicellulose and cellulose (Moore & Jung, 2001). Tannins normally affect animal performance by reducing feed palatability because of their astringent taste, inhibiting microbial growth and activity by forming a stable tannin-nutrient complex that is not readily digestible in the rumen (Makkar, 2003).
The Standard Grade which was awarded to all the steers despite them having less than four permanent incisors was solely influenced by the lower carcass weights of less than 146 kg. Malawi Zebu is a slow-growing breed which hardly qualifies for top grade at a young age under the Malawian Grading System (Chingala et al., 2017b). Grading the animals based solely on the carcass weight may disadvantage the farmers who may strive to finish the slow-growing Malawi Zebu under feedlot conditions, especially using forage-based diets.
The higher pHu and low L* values in beef from animals that consumed the Vachellia diet may be because of low muscle glycogen levels in the animals at slaughter originating from the low DMI, energy and nutrient digestibility of the diet chiefly due to high lignin and tannin content in the diet as explained previously. Low muscle glycogen in animals at slaughter may lead to a reduced rate of glycolysis, thus a slower build-up of lactic acid, and a slower rate of pH decline post-slaughter (Zarasvand et al., 2012). The high pHu minimizes meat pigment losses and denaturation, thereby increasing light absorbance, which gives the meat darker colour (Matarneh et al., 2017). Similarly, lambs fed tannin-rich diets had a high pHu due to low dietary energy supply because of the interference of tannins with ruminal fermentation in the lambs (Priolo et al., 2000). Also, Mtynek & Gulinski (2007) reported similar results and ascribed them to variation in muscle fibre type composition in response to growth rates. Slower animal growth rates result in higher contents of type I muscle fibres and lower contents of type II muscle fibres (Mtynek & Gulinski, 2007). Type I muscle fibres have high oxidative capacity, and are lower in glycogen content and glycolytic enzyme activity (Lefaucheur, 2010). In such muscles, lactic acid production is reduced, muscle acidification slows down and darker colour is produced (Mtynek & Gulinski, 2007).
The higher WHC, lower drip and cooking losses of the meat from steers fed the Vachellia and baobab diets compared to those fed the soybean diet could be attributed to the high muscle pHu and high dietary tannin concentration recorded for the former diet. Meat pHu is positively related to WHC and inversely related to drip and cooking loss (Warris, 2010). The rate of proteolysis of muscle proteins increases as the pH declines leading to a reduction in the ability of proteins to bind water (Warris, 2010). Tannins also have the ability to prevent the loss of membrane integrity and protein cross-links by inhibiting/or reducing the rate of oxidation in red meat (Estévez, 2011; Gómez-Cortés et al., 2018) thereby maintaining the integrity of myofibrillar proteins to bind to water molecules. The high water-retention and low cooking losses are a characteristic of DFD meat (Warriss, 2010). Although no difference was found between diets, the WBSF values for all diets were below the 4.4 kg threshold used for denoting superior tenderness of beef (Howard et al., 2013). The tender beef in the current trial was probably due to the higher meat pHu as it is known that high meat pHu increases meat tenderness (Yu & Lee, 1986) and/or the steers' young age at slaughter, which was less than three years old.
The highest gross profit reported for baobab-fed steers were a result of both higher total revenue and low input costs of the diets. Although, steers on the Vachellia diet had low production costs compared to the baobab-fed steers, the slow live weight gain of the animals on the diet resulted in lower carcass weights, thereby reducing the total revenue. The lowest gross profit for the soybean-fed steers was due to the high total input costs, particularly of the soybean itself, despite the group achieving high final weights. Use of baobab seed and Vachellia as protein supplements for steers are economically viable. It is, however, difficult to make recommendations based on current results because this study used a small sample size, and the diets that were not balanced for energy. Iso-energetic diets can be made by adding starch to the Vachelia diet that may subsequently increase metabolisable energy and glycogen reserves in animals consuming the diet and ultimately improve meat pHu and the associated meat quality attributes (Warriss, 2010). The other limitation of the study was the use of V. polyacantha, which had high tannin content. In that regard, further research to determine the optimal inclusion levels of Vachellia leaf meal to minimise DFD beef could be important. It may also be important to identify cost-efficient methods of reducing tannins in V. polyacantha before feeding or co-feeding it with ingredients that are low in tannins.
Conclusion
Based on the present results, steers fed the baobab diet had comparable carcass weights to those fed the soybean diet. Inclusion of baobab seed meal and Vachellia leaf meal in cattle finishing diets produced DFD beef compared to the soybean diet. However, feeding steers' diets containing baobab seed meal and Vachellia leaf meal improved gross profits. Further studies should investigate optimal inclusion levels of baobab seed and Vachellia leaf meal to minimise DFD beef.
Acknowledgements
The authors acknowledge the financial support of The Royal Kingdom of Norway through The Royal Norwegian Embassy in Malawi and Norwegian University of Life Sciences. CM and LCH acknowledges funding from the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (UID number: 84633).
Authors' Contributions
GC, ER, KD, LCH and CM conceived the study. GC drafted the manuscript with inputs and editorial assistance of C.M. ER, KD and LCH critically reviewed the manuscript.
Conflict of Interest Declaration
The authors have declared that no competing interests exist.
References
Abate, A, Dzowela, B.H. & Kategile, J.A., 1992. Intensive animal feeding practices for optimum feed utilization. In: J.A. Kategile & S. Mubi (eds). Future of Livestock Industries in East and Southern Africa. International Livestock Centre for Africa. pp. 9-19. Nairobi, Kenya. [ Links ]
Allen, M.S., 2000. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J Dairy Sci. 83, 1598-1624. [ Links ]
Assogbadjo, A.E., Chadare, F.J., Kaka'í, R.G., Fandohan, B. & Baidu-Forson, J.J., 2012. Variation in biochemical composition of baobab (Adansonia digitata) pulp, leaves and seeds in relation to soil types and tree provenances. Agric. Ecosyst. Environ. 157, 94-99. [ Links ]
Chingala, G., Mapiye, C., Raffrenato, E., Hoffman, L.C. & Dzama, K., 2017a. Determinants of smallholder farmers' perceptions of impacts of climate change on beef production and quality in Malawi. Clim. Change 142, 129-141. [ Links ]
Chingala, G., Raffrenato, E., Dzama, K., Hoffman, L.C. & Mapiye, C., 2017b. Towards a regional beef carcass classification system for Southern Africa. S. Afr. J. Anim. Sci. 47, 408-423. [ Links ]
Chingala, G., Raffrenato, E., Dzama, K., Hoffman, L.C. & Mapiye, C., 2018. Intake, digestibility, rumen protein synthesis, and growth performance of Malawi Zebu steers fed diets containing rangeland-based protein sources. Trop. Anim. Health Prod. 51, 199-204. [ Links ]
Conway, D., Van Garderen, E.A., Deryng, D., Dorling, S., Krueger, T. & Dalin, C., 2015. Climate and southern Africa's water-energy-food nexus. Nat. Clim. Change 5, 837-846. [ Links ]
Dzanja, J., Kapondamgaga P. & Tchale, H., 2013. Value chain analysis of beef in central and southern Malawi (case studies of Lilongwe and Chikhwawa Districts). Int. J. Bus. Soc. Sci. 4, 92-102. [ Links ]
Estévez, M., 2011. Protein carbonyls in meat systems: A review. Meat Sci. 89, 259-279. [ Links ]
Goering, H.K. & Van Soest, P.J., 1970. Forage fiber analyses (apparatus, reagents, procedures, and some applications). USDA Agriculture Handbook No. 379. [ Links ]
Gómez-Cortés, P., Guerra-Rivas, C., Gallardo, B., Lavín, P., Mantecón, A.R., de la Fuente, M.A. & Manso, T., 2018. Grape pomace in ewes diet: Effects on meat quality and the fatty acid profile of their suckling lambs. Food Res. Int. 113, 36-42. [ Links ]
Grau R. & Hamm R. 1953. Eine einfache Methode zur Bestimmung der Wasserbindung im Muskel. Naturwissenschaften 40, 29-30. [ Links ]
Hall, M.B., 2009. Determination of starch, including maltooligosaccharides, in animal feeds: Comparison of methods and a method recommended for AOAC collaborative study. J. AOAC Int. 92, 42-49. [ Links ]
Honikel, K.O., 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49, 447-557. [ Links ]
Howard, S.T., Woerner, D.R., Scanga, J.A., Van Overbeke, D.L., Mafi, G.G., Igo, J.L., Salman, M.D., Tatum, J.D. & Belk, K.E., 2013. North American Beef Tenderness Survey 2011-2012: Benchmarking tenderness and sample shipping procedures. J. Anim. Sci. 91, 5981-5988. [ Links ]
IPCC, 2007. Climate change 2007: Impacts, adaptation and vulnerability: Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel, Genebra, Suíça. Doi:10.1256/004316502320517344 [ Links ]
Kaliba, A.R.M., Featherstone, A.M .& Norman, D.W., 1997. A stall-feeding management for improved cattle in semiarid central Tanzania: Factors influencing adoption. Agric. Econ. 17,133-146. [ Links ]
Jerónimo, E., Pinheiro, C., Lamy, E., Teresa Dentinho, M., Sales-Baptista, E., Lopes, O. & Capela Silva, F., 2016. Tannins in ruminant nutrition: Impact on animal performance and quality of edible products. In: C.A. Combs (ed). Tannins: Biochemistry, Food Sources and Nutritional Properties. pp. 121-168. Nova Science Publishers Inc., NY, USA [ Links ]
Lee, C.M., Trevino, B. & Chaiyawat, M., 1996. A simple and rapid solvent extraction method for determining total lipids in fish tissue. J. AOAC Int. 79, 487-492. [ Links ]
Lefaucheur, L., 2010. A second look into fibre typing - Relation to meat quality. Meat Sci. 84, 257-570. [ Links ]
Makkar, H., 2000. Quantification of tannins in tree foliage: A laboratory manual for the FAO/IAEA co-ordinated research project on use of nuclear and related techniques to develop simple tannin assays for predicting and improving the safety and efficiency of feeding ruminants on the tanniferous tree foliage. Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture. FAO, Rome, Italy. [ Links ]
Makkar, H.P.S., 2003. Effects and fate of tannins. in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 49, 241-256. [ Links ]
Mapiye, C., Chimonyo, M. Dzama, K., Hugo, A., Strydom, P.E. & Muchenje, V., 2011. Fatty acid composition of beef from Nguni steers supplemented with Acacia karroo leaf-meal. J. Food Comp. Analy. 24, 523-528. [ Links ]
Mapiye, C., Chimonyo, M., Dzama, K., Muchenje, V. & Strydom, P.E., 2010. Meat quality of Nguni steers supplemented with Acacia karroo leaf-meal. Meat Sci. 84, 621-627. [ Links ]
Mapiye, C., Chimonyo, M., Dzama, K., Strydom, P.E., Muchenje, V. & Marufu, M.C., 2009. Nutritional status, growth performance and carcass characteristics of Nguni steers supplemented with Acacia karroo leaf-meal. Livest. Sci. 126, 206-214. [ Links ]
Matarneh, S.K., England, E.M., Scheffler, T.L. & Gerrard, D.E., 2017. The conversion of muscle to meat. In: Lawrie's Meat Science. Edited by F. Toldra. 8th edition. Elsievier. Duxford, UK. [ Links ]
Mertens, D.R., Allen, M., Carmany, J., Clegg, J., Davidowicz, A. & Wolf, M., 2002. Gravimetric determination of amylase- treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 85, 1217-1240. [ Links ]
Mlambo, V. & Mapiye, C., 2015. Towards household food and nutrition security in semi-arid areas: What role for condensed tannin-rich ruminant feedstuffs? Food Res. Int. 76, 953-961. [ Links ]
Mtynek, K. & Gulinski, P., 2007. The effect of growth rate and age at slaughter on dressing percentage and colour, pH48 and microstructure of longissimus dorsi muscle in Black-and-White (BW) bulls vs commercial crossbreds of BW with beef breeds. Anim. Sci. Pap. Reports 5 (2), 65-71. [ Links ]
Moore, K.J. & Jung, H.G., 2001. Lignin and fibre digestion. J. Range Manage. 54, 420-430. [ Links ]
Nkhonjera, L., Agymang, K. & Butterworth, M.H., 1988. Performance of cattle stall-fed for beef in Malawi. Trop. Anim. Health Prod. 20, 155-160. [ Links ]
Porter, L.J., Hrstich, L.N. & Chan, B.G., 1985. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochem. 25, 223-230. [ Links ]
Priolo, A., Waghorn, G.C., Lanza, M., Biondi, L. & Pennisi, P., 2000. Polyethylene glycol as a means for reducing the impact of condensed tannins in carob pulp: Effects on lamb growth performance and meat quality. J. Anim. Sci. 78, 810-816. [ Links ]
Raffrenato, E. & Van Amburgh, M.E., 2011. Technical note: Improved methodology for analyses of acid detergent fiber and acid detergent lignin. J. Dairy Sci. 94, 3613-3617. [ Links ]
Strydom, P.E., 2008. Do indigenous Southern African cattle breeds have the right genetics for commercial production of quality meat? Meat Sci. 80, 86-93. [ Links ]
Stür, W., Khanh, T.T. & Duncan, A., 2013. Transformation of smallholder beef cattle production in Vietnam. Int. J. Agric. Sustain. 11, 363-381. [ Links ]
Warriss, P.D., 2000. Meat Science: An Introductory Text. CABI, UK. [ Links ]
Yu, L.P. & Lee, Y.B., 1986. Effects of postmortem pH and temperature on bovine muscle structure and meat tenderness. J. Fd Sc. 51, 774-780. [ Links ]
Zarasvand, S.A., Kadivar, M., Aminlari, M. & Shekarforoush, S.S., 2012. A comparative study of physico-chemical and functional properties, and ultrastructure of ostrich meat and beef during aging. CyTA - J. Fd, 10, 201-209. [ Links ]
Received 2 November 2018
Accepted 16 March 2019
First published online 9 May 2019
# Corresponding author email: cmapiye@sun.ac.za














