Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.49 no.2 Pretoria 2019
http://dx.doi.org/10.4314/sajas.v49i2.4
ARTICLES
Prebiotic effects of oligosaccharides extracted from palm kernel expeller on different levels of Salmonella typhimurium infection in chicks
S. RezaeiI, II; W.L. ChenI; S.C.L. CandyrineI, III; R.Q. FooI; M.F. JahromiI, IV; A.S. FarjamI; I. ZulkifliI; J.B. LiangI, #
IInstitute of Tropical Agriculture and Food Security, University of Putra Malaysia, Serdang, 43400 Selangor, Malaysia
IICentre of Excellence for Emerging and Re-emerging Infectious Diseases in Animals, Faculty of Veterinary Science, Chulalongkorn University, Bangkok 10330, Thailand
IIIFaculty of Sustainable Agriculture, University Malaysia Sabah, 90509, Sandakan, Sabah
IVAgriculture Biotechnology Research Institute of Iran (ABRII), East and North-East Branch, P.O. Box 91735/844, Mashhad, Iran
ABSTRACT
Prebiotic effects of oligosaccharides extract from palm kernel expeller (OligoPKE) on Salmonella typhimurium were investigated in chicks in two experiments. Results of the first experiment showed that OligoPKE reduced the colonization of Salmonella typhimurium in the cecum and increased the immunoglobulin A (IgA) production in the blood and jejunum. The second experiment further investigated the prebiotic efficacy of dietary supplementation of OligoPKE on three levels of Salmonella typhimurium infection (1.0 x 104 Colony-forming unit (CFU)/mL; 1.0 x 106 CFU/mL and 1.0 x 108 CFU/mL) in chicks. OligoPKE reduced the colonization of Salmonella in the medium and high levels of infection. IgA level in serum and jejunum content increased significantly in all the three levels of infection when chicks received OligoPKE in their diet. Interleukin 8, and 10; interferon-α; and tumour necrosis factor genes were up-regulated in the jejunum of the infected chicks, and OligoPKE down-regulated these gene expressions. Results of the current study indicated that OligoPKE, an oligosaccharides extract from palm kernel expeller, is capable of reducing colonization of Salmonella typhimurium in young chicks, and boosted their immunity.
Keywords: chicken, immunoglobulin, immune gene, microbial population
Introduction
Salmonellosis can be caused by Salmonella contamination in poultry products at any stage of the production line, such as processing, distribution, retail marketing, handling and preparation (El-Aziz, 2013). In the Philippines, nearly 95% of human cases of salmonellosis are caused by contaminated poultry products (Sheffield et al., 2014). Annually, in the United States, 600 deaths and 1.4 million illnesses are reported because of Salmonella infection (Wang et al., 2015). One of the common non-typhoidal Salmonella strains that can cause salmonellosis is Salmonella typhimurium. Salmonella typhimurium is a pathogenic gramnegative bacterium that is found predominately in the gastro-intestinal tract. This strain of Salmonella has a wide range of animal reservoirs, high ability to spread, can survive in different environments, and is known for its rapid resistance to multiple drugs and antibiotics (Aktas et al., 2007). The use of prebiotics is one of the alternative approaches to prevent development of antibiotic resistance resulted from S. typhimurium infection in livestock farms.
Several oligosaccharides, such as fructo-oligosaccharides (FOS) and mannan-oligosaccharides (MOS), inhibit the adherence of pathogens to epithelial cells and are classified as prebiotic sources (Gourbeyre et al., 2011; Ibuki et al., 2011; Samal & Behura, 2015). We recently reported that OligoPKE which contains mainly MOS exhibits prebiotic properties in experimental mice and chickens (Jahromi et al., 2015). In chickens, the highest mortality from S. typhimurium infection occurs in chicks less than two weeks old, when their immune system is not yet fully developed (Dunkley et al., 2009), Therefore, in this study, two experiments were designed to examine the prebiotic effects of OligoPKE on S. typhimurium at different infection rates on the immune systems of broiler chicks at neonatal age.
Materials and Methods
Oligosaccharides were extracted from raw palm kernel expeller (PKE) using water (without enzyme) as solvent, following the procedure of Rezaei et al. (2015). Briefly, 1 L distilled water was added to 200 g ground PKE in a one-litre Scott bottle, shaken for 1 hour at room temperature, and later autoclaved. The insoluble materials were removed by centrifugation at 3000 g χ 5 min. The supernatant was filtered (Whatman filter paper no. 1) and the excess water was evaporated with a rotary evaporator (Hei-Vap Plug and Play Value 1, Heidolph Instruments, Swabach, Germany). Fatty acids were removed from the extraction with chloroform (two solvents/one extraction, shaken for 10 min, repeated two times). The excess solvent was evaporated using a rotary evaporator. Proteins were also removed from the extraction with acetonitrile (two solvents/one extraction, shaken for 10 min, repeated two times), and the excess solvent was again evaporated with a rotary evaporator. The extract was freeze-dried (Freezone 6 Plus, Labconco, USA). The solid extract (OligoPKE) was stored in capped containers at -80 °C. This process was repeated until sufficient OligoPKE was obtained for the study.
This study was conducted as two experiments at Biosafety Laboratory level III (BSL III) of Veterinary Research Institute (VRI), Ipoh, Malaysia. The Institutional Animal Care and Use Committee (IACUC) of VRI approved the experimental protocol (AEC VRI 05/2014).
The first experiment was designed to examine the general effects of OligoPKE on the immune systems of chickens infected with a fixed level of S. typhimurium. Rezaei et al. (2015) demonstrated that 1% OligoPKE is the most appropriate amount as a supplement in chicken diet. Consequently, 1% OligoPKE was used in this experiment. Fifty-six one-day-old male broiler chicks, which were provided by VRI, were randomly allocated to four treatment groups with seven replicates per treatment (two birds per replicate) in a completely randomized design. From day 1, two treatments were fed a corn-soy-based starter diet (22% crude protein (CP) and 3000 kcal metabolizable energy (ME)/kg) and the other two treatments received the same diet supplemented with 1% OligoPKE. Clean drinking water was freely available to the birds. On day 3, chicks in one group of each treatment were administered orally with 1 mL distilled water and the other groups received 1 mL S. typhimurium (1.0 x 109 CFU/mL) (Table 1). This strain of Salmonella (previously isolated from infected chickens) was provided by the Microbiology Laboratory of VRI.
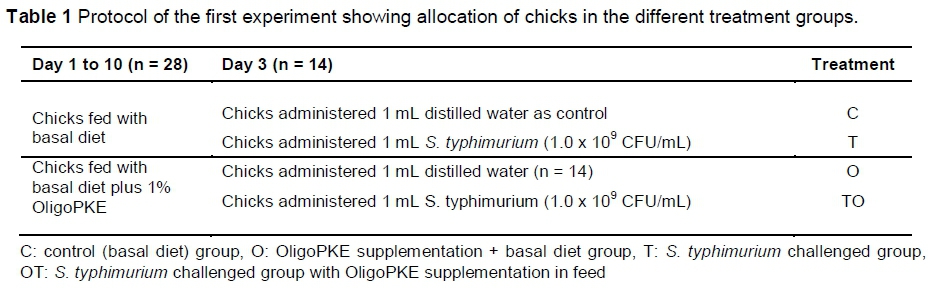
The second experiment was designed to further investigate the effects of OligoPKE in chicks infected with three levels of S. typhimurium infection. A total of 112 one-day-old male broiler chickens provided by the VRI were randomly allocated to eight treatment groups with seven replicates per treatment (two chicks per replicate) in a CRD experiment. From day 1, the chicks in four treatment groups were fed a corn-soy-based starter diet (22% CP and 3000 kcal ME/kg) and birds in the remaining four groups received the same diet supplemented with 1% OligoPKE. Clean drinking water was freely available to the birds. On day 3, the first group of chicks in each dietary group were administered orally with 1 mL distilled water and the remaining three groups with 1 mL S. typhimurium at 1.0 x 108 CFU/mL (high infection level), 1.0 x 106 CFU/mL (medium infection level) and 1.0 x 104 CFU/mL (low infection level) (Table 2).
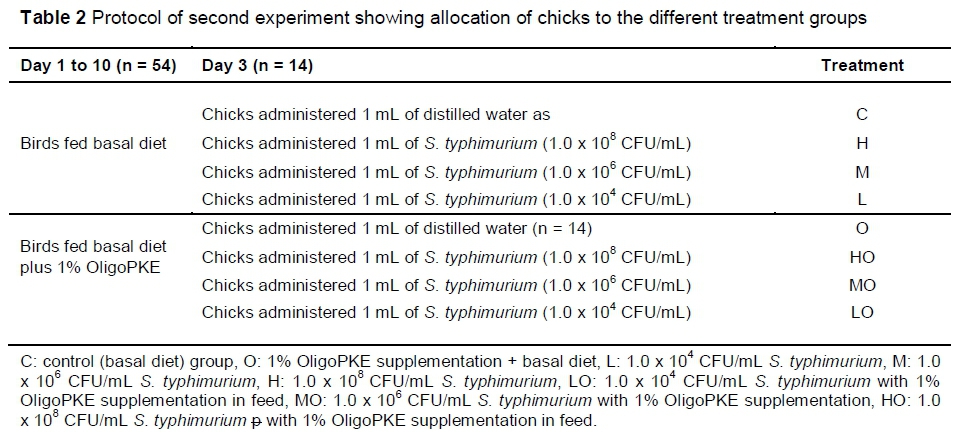
On day 10 of each experiment, all chicks were decapitated. The caecal contents of each bird were collected in 2 mL centrifuge tubes, and stored at -20 °C, pending bacterial quantification. For jejunal samples, the jejunum contents were also collected in 2 mL centrifuge tubes and stored at -20 °C, pending IgA assay and bacterial quantification. The jejunum tissue was rinsed with distilled water and collected in 2 mL centrifuge tubes containing RNAse-free solution, then stored at -80 °C for gene expression.
Concurrently, fresh blood samples were collected from all chicks in 2 mL centrifuge tubes containing EDTA buffer, centrifuged at 3000 g for 5 min (refrigerated centrifuge, Hitachi CT15RE, Hitachi, Japan) to collect serum. Then, serum samples were stored in 2 mL centrifuge tubes, and kept at -20 °C for immunoglobulin analysis.
Bacterial quantification was conducted according to the method described by Rezaei et al. (2015). In brief, DNA was isolated from the caecum contents by QIAamp® DNA Stool Mini kit, (QIAGEN, Germany). PCR products were purified using the MEGA-quick-spin TM (Intron Biotechnology, South Korea). The purity and concentration of DNA in each sample were measured using a Nanodrop ND-1000 spectrophotometer, Thermo Scientific, USA, and the number of copies of a template DNA per mL of elution buffer was calculated. Standard curves were constructed using serial dilution of PCR products from pure cultures of each bacterial group.
The populations of S. typhimurium, Lactobacillus, Bifidobacterium and Enterobacter were estimated by Quantitative Real-Time Polymerase Chain Reaction (q-PCR). The primers are shown in Table 3.
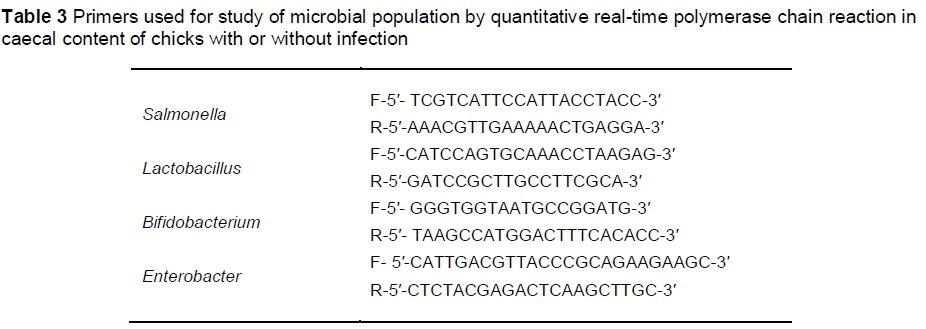
Real-time PCR was performed with the BioRad CFX96 Touch (BioRad, USA) using optical-grade plates. The PCR reaction was performed on a total volume of 25 μL using the iQTM SYBR Green supermix (BioRad, USA). Each reaction included 12.5 μL SYBR Green supermix, 1 μL of each primer, 1 μL of DNA samples and 9.5 μL H2O. The reaction conditions for amplification of DNA were 94 °C for 5 min, 40 cycles of 94 °C for 20 seconds, 58 °C for 30 seconds, and 72 °C for 20 seconds. To confirm the specificity of amplification, melting curve analysis was carried out after the last cycle of each amplification.
Fresh blood samples were collected individually in 2 mL tubes containing EDTA buffer. Samples were centrifuged at 3000 g for 5 min, and the serum was collected and stored at -20 °C for immunoglobulin assay. Jejunum contents were collected and stored in 2 mL tubes for IgA assay. IgA (chicken) ELISA kit (Abnova, version 05, Taiwan) was used to measure the IgA in the serum and jejunum tissue following the manufacturer's protocol with 5000-fold dilution.
After the jejunum samples had been removed and rinsed, they were transferred immediately to RNAlater (QIAGEN, Germany) and stored at -80 °C. Total RNA was extracted from the samples using the RNeasy Plus Midi Kit (QIAGEN, Inc., Valencia, CA), according to the manufacturer's instructions. Total RNA was quantified at 260/280 nm using a NanoDropND-1000 spectrophotometer and stored at -80 °C. The quality and integrity of the extracted RNA was checked through agarose gel (1%) electrophoresis. The amount of 100 ng total RNA was reverse-transcribed using a cDNA synthesis kit, according to the manufacturer's instructions (Maxime RT-PCR PreMix Kit, iNtRON Biotechnology Co, Ltd, Korea). The cDNA that was obtained was subjected to quantitative real-time PCR using the Maxima SYBR Green qPCR master mix (Thermo Scientific, USA) with a BioRad CFX96 real-time cycler (BioRad Laboratories, Inc, Hercules, USA). Each PCR reaction was performed on a total volume of 25 μL, consisting of 12.5 μL SYBR Green master mix, 1 μL of each primer (Table 4), 1 μL of each cDNA sample, and 9.5 μL nuclease-free water. The PCR process was performed under these conditions: initial denaturation at 95 °C for 2 min, 40 cycles of denaturation at 95 °C for 20 seconds, annealing for 30 seconds and extension at 72 °C for 20 seconds. To confirm the specificity of amplification, melting curve analysis was carried out after the last cycle of each amplifications as described for microbial quantification. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as reference gene. The cycle numbers at which amplified DNA samples exceeded a computergenerated fluorescence threshold level were normalized and compared with determined relative gene expression. Higher cycle number values indicated lower initial concentrations of cDNA, and thus lower levels of mRNA expression. Each sample was run in duplicate, and averaged duplicates were used to assign cycle threshold (CT) values. The AACT method was used to determine relative gene expression (Livak & Schmittgen, 2001). The ACT is the difference between the CT value of the target gene and the CT value of the reference gene (GAPDH). The AACT is the ACT of OligoPKE-treated samples minus the ACT of the untreated control. Data are presented as fold change expression in the target gene of a treatment sample compared with the normal sample.
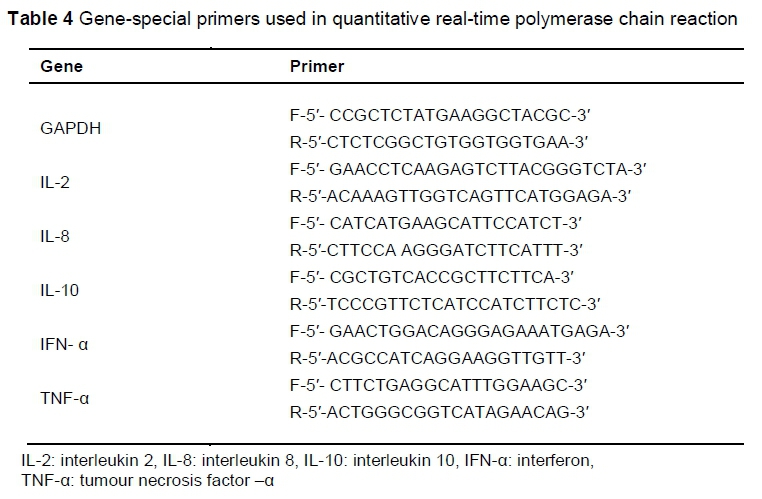
Data analyses were performed by comparing the birds that were fed OligoPKE with the control for each parameter, using a completely randomized design with equal group variance with SAS Statistical Software (2008). The significance level was set at P <0.05.
Results
Results of the first experiment showed no significant changes in beneficial bacteria (Bifidobacterium and Lactobacillus) and in pathogenic Enterobacter. OligoPKE supplementation reduced the Salmonella population in the unchallenged chicks as compared with the control group. A drastic increase in the Salmonella population in the groups with infection, compared with the control, while OligoPKE supplementation reduced the population of this pathogen (Table 5).
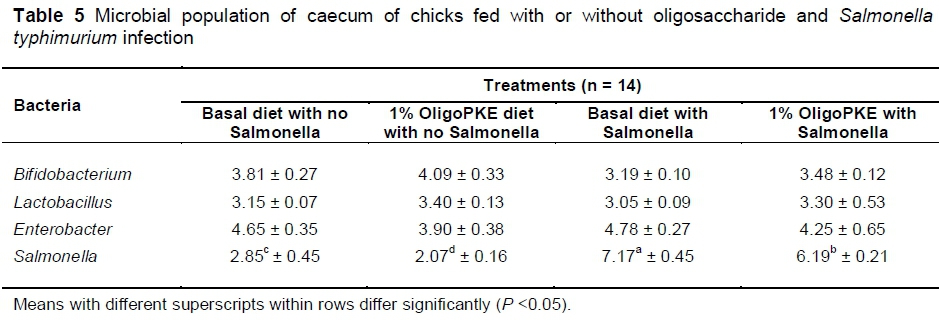
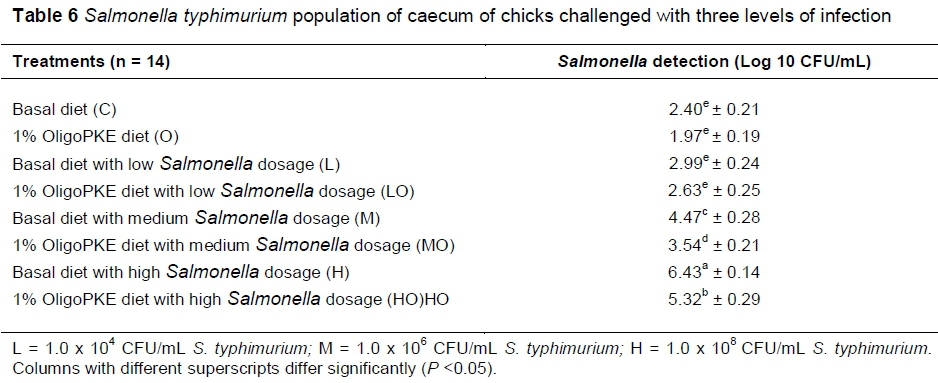
Results of the second experiment showed that colonization of S. typhimurium in the low level infection (1.0 x 104 CFU/mL) did not differ with the control. However, Salmonella colonization in the medium (1.0 x 106 CFU/mL) and high (1.0 x 108 CFU/mL) levels of infection manifested themselves differently. OligoPKE supplementation did not significantly altered Salmonella colonization in the low infection group, however, OligoPKE supplementation significantly reduced Salmonella colonization in the high and medium infection groups (Table 6).
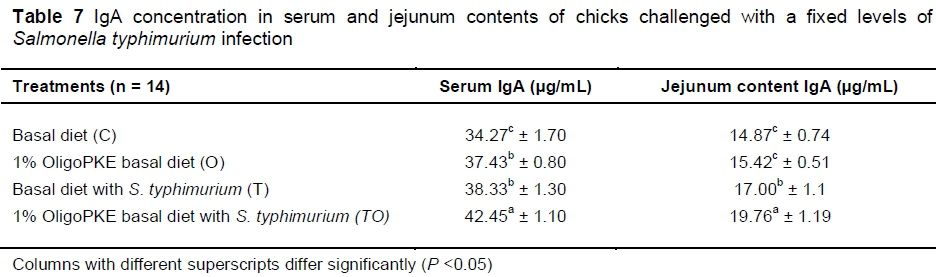
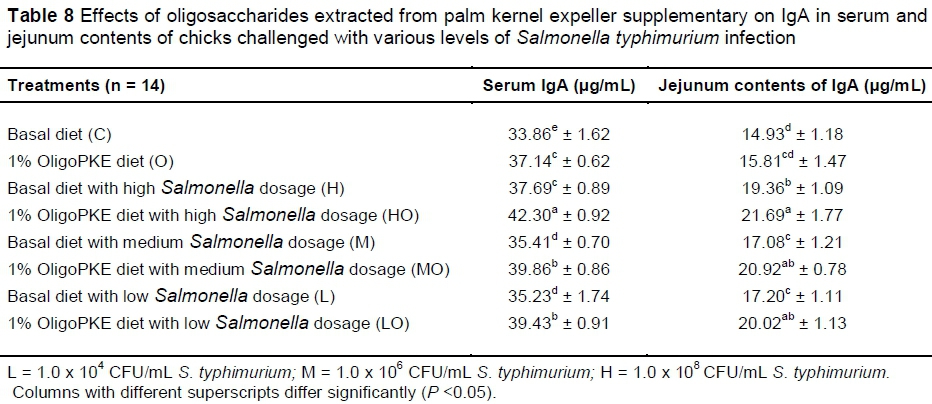
Supplementation of OligoPKE increased the IgA level in blood serum significantly (Table 7). A comparison between the IgA level of groups with OligoPKE supplementation and infected groups without supplementation indicated that OligoPKE increased the serum IgA of chickens without infection to the same level as chicks with infection. This result confirms that OligoPKE in feed increased the animals' immunity to unpredicted environmental infections. The increase in IgA secretion was significantly higher when infected chickens received OligoPKE in feed (Table 7). These groups were more able to confront the pathogen. The results also indicated that in normal conditions without infection, IgA secretion in the jejunum contents remained steady, whether chickens received OligoPKE or not. As expected, this secretion increased with S. typhimurium infection and OligoPKE increased the release of IgA in the jejunum significantly (Table 8).
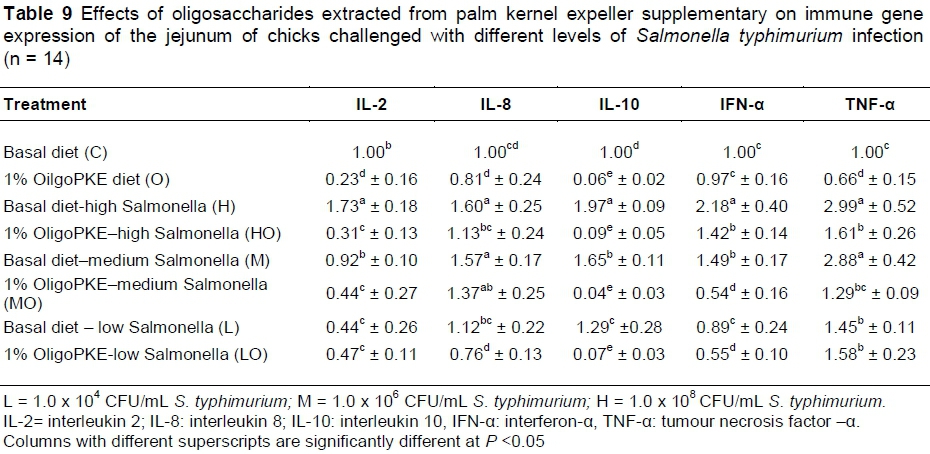
OligoPKE supplementation down-regulated the production of interleukin 2 (IL-2) compared with the control. IL-2 manifested significantly in the jejunum when chicks were challenged with high level of S. typhimurium (1.0 x 108 CFU/mL), however, OligoPKE supplementation maintained the IL-2 level in the jejunum. The moderate and low infections of S. typhimurium did not significant altered the expression of IL-2 in the jejunum (Table 9). Chicks infected with S. typhimurium showed up-regulation in IL-8 production and OligoPKE supplementation reduced IL-8 expression, but the reduction remained higher than the normal range (Table 9). Interleukin 10 (IL-10) expression was increased according to infection level, and OligoPKE supplementation in all cases reduced this expression to almost zero. Interferon-α (IFN-α) remained at the same level in the low infection, control and OligoPKE supplementation groups (Table 9). When chickens were challenged with low and medium levels of S. typhimurium and received OligoPKE in their feed, their IFN-α expression was even lower than the control group. Our results indicated that IFN-α expression increased dramatically in the high and medium infections groups without supplementation, but OligoPKE was able to reduce this expression, with the medium infection group showed IFN-α expression that was even lower than the control (Table 9). Tumour necrosis factor-α (TNF-α) expression was up-regulated when chicks were challenged with the pathogen, regardless of the level of infection. This up-regulation was similar in the high and medium infection groups and again OligoPKE was able to reduce this up-regulation.
Discussion
Several mechanisms have been proposed how prebiotics could act to suppress the colonization of Salmonella and other pathogens. Prebiotics, such as OligoPKE, may mimic host receptors and therefore reduce pathogen attachment to the intestinal cell surface (Badia et al., 2013) and thus suppress their growth and multiplication. FOS and MOS, due to their β-linkages, are able to bypass hydrolysis in the upper gastrointestinal tract to reach the caecum, where most of the oligosaccharides are fermented and produce short chain fatty acids (SCFAs) (Donalson et al., 2008) and these SCFAs exhibit bactericidal properties, which can negatively affect species belonging to several genera, including Salmonella (Gibson & Wang, 1994). Fernandez et al. (2002) reported that increasing beneficial bacteria such as Bifidobacterium and Lactobacillus with supplementation of MOS and palm kernel meal (the same product as PKE but with a different oil extraction method) could create unfavourable conditions for pathogens and reduce them in the caecum of chickens. In addition, the authors noted reduced susceptibility to colonization of Salmonella in young chickens.
The reduction of Salmonella colonization in normal conditions without infection, can be caused by increased production of lactic acid from the increased population of lactobacillus, because a low pH and acidic environment is not suitable for pathogens, and could lead to their reduction in the intestine and caecum (Allen et al., 1997). Rezaei et al. (2015) and Chen et al. (2014) reported that oligosaccharides from PKE increased the population of beneficial Bifidobacterium (P <0.05) and decreased (P <0.05) pathogenic Enterobacter in caecal samples in chickens and rats, respectively. The results of the present study (Table 5) showed that OligoPKE supplementation did not significantly alter (P >0.05) the population of beneficial bacteria (Lactobacillus, Bifidobacterium) or pathogenic Enterobacter (Table 5). However, the inconsistency between the present study and those reported earlier is not surprising because in this study chicks were challenged with Salmonella, while the animals used by Rezaei et al. (2015) and Chen et al. (2014) were unchallenged with pathogens. In normal (unchallenged) conditions, probiotics would compete with enteric pathogens for substrates and attachment sites, and in addition, produce compounds that would inhibit the growth and activity of pathogens (Patterson & Burkholder, 2003). On the other hand, when birds were infected experimentally with pathogens, the microflora in the gut would be altered to favour the pathogens, resulting in a lower proportion of beneficial bacteria in the gut. However, the results of this study demonstrated that although it was not able to increase the population of beneficial bacteria, OligoPKE was capable of offsetting the sudden invasion of Salmonella and maintaining the population to avoid a net decrease of the two beneficial bacteria in the cecum.
Approximately 25% of the intestinal mucosa is made up of lymphoid tissue, which produces IgA antibodies in the intestine (Schley & Field, 2002). The main function of IgA is to prevent the attachment of pathogens to the intestinal wall (Schley & Field, 2002). The results of this experiment agree with those of Muir et al. (1998), who reported an increase in IgA production in the intestines of chickens challenged with S. typhimurium. The authors attributed the increased levels of IgA to the intestine being the first line of self-defence of the gastrointestinal tract. Lymphoid tissues act as a mucosal immune system, and thus are normally located in areas with high risk of contact with external pathogens, such as respiratory, urinary and reproductive tracts, as well as the gut in the form of gut-associated lymphoid tissues, which protect the intestine (Schley & Field, 2002). The results of this study showed that OligoPKE increased IgA production in the jejunum particularly that of the Salmonella challenged chicks. This could help the inhibition of the attachment and penetration of Salmonella in the intestine, increase the production of mucus, and prevent inflammation, which could cause epithelial tissue damage (Kim et al., 2011).
OligoPKE acts as a prebiotic that could enhance the immune system in chickens as is evident from the significant increase in their plasma IgA concentration (Rezaei et al., 2015). A similar response in plasma IgA was reported in chickens challenged with S. enteritidis (Seo et al., 2003). The authors noted that levels of IgA in the chickens challenged with S. enteritidis increased progressively to day 17 before declining on day 24 post challenge. Generally, the results of this study concurred with these two studies. That is, chickens challenged with S. typhimurium had higher plasma IgA than the control birds. In addition, the IgA level was higher (P <0.05) in chicks that received OligoPKE, suggesting its positive effect in modulating the immune response in chicks challenged with S. typhimurium.
Interleukins have a variety of actions, depending on the type of interleukin and their target cells. IL-2 is produced by T helper 1 cells (Th1 cell), which are responsible for the activation of T cells and B cells. IL-8 is produced by macrophages, lymphocytes and epithelial cells, B cells, and Th1 cells. Lastly, IL-10 is produced by monocytes, T cells, B cells and macrophages (Khadka, 2014). Interferon-α (IFN-α) is released by infected cells, causing nearby cells to extend their anti-viral defences. On the other hand, it activates natural killer cells (NK) and macrophages and in general increases the cell defence mechanism. Tumour necrosis factor is responsible for the immune response and activation of several immune cells, such as lymphocytes and NK cells, in the event of inflammation (Shokryazdan et al., 2017).
Research has shown that consumption of oligosaccharides (with prebiotic characteristics) improves immune gene expression. These effects occur as down-regulating consequences on the expression of several immune genes. Lecerf et al. (2012) reported that supplementation with inulin and Xylo-Oligosaccharides for four weeks in healthy volunteers reduced the expression of IL-8 and TNF-α. Vulevic et al. (2008) found that consumption of the galactans (5.5 g/d) for 10 weeks reduced the production of pro-inflammatory cytokine TNF-α. In another study on human beings (Dehghan et al., 2014), inulin was compared with maltodextrin and caused a significant decrease in the level of TNF-α. In another study, Faghfoori et al. (2011) showed that levels of TNF-α and IL-8 decreased in the group that received germinated barley foodstuff (a prebiotic consisting of insoluble glutamine-rich protein and dietary fibre), compared with the control group. In a study on mice, animals that received prebiotics showed lower cytokine (TNF-α, INF, IL-10) expression (Cani et al., 2009). According to the results of this study, targeted immune genes up-regulated the level of infections. As predicted, the highest changes in immune gene expression were observed at 1.0 x 108 CFU/mL of Salmonella infection, which were higher than all other treatments. Meanwhile, OligoPKE supplementation in all the treatments showed that the immune system did not produce the immune gene as much as chicks with the same level of infection without supplementation. This result indicates that OligoPKE supplementation is able to increase body immunity against S. typhimurium infection.
Conclusion
The results of this study showed OligoPKE tended to increase beneficial Bifidobacterium and Lactobacillus populations in both the control and Salmonella infected groups but the increase was not significant (P >0.05), however, OligoPKE supplementation reduced (P <0.05) the population of Salmonella in the caeca of the experimental chicks. The immunoglobulin study showed that OligoPKE increased serum and jejunum IgA in chicks infected with S. typhimurium. This study demonstrated for the first time that OligoPKE, an oligosaccharide extract from palm kernel expeller could enhance the immune responses in chicks infected with Salmonella.
Acknowledgments
This study was supported by LRGS Fasa 1/2012 (University of Putra Malaysia) provided by the Ministry of Education, Malaysia.
Conflict of Interest Declaration
The authors declared no conflict of interest.
References
Aktas, Z., Day, M., Kayacan, C.B., Diren, S. & Threlfall, E.J., 2007. Molecular characterization of Salmonella typhimurium and Salmonella enteritidis by plasmid analysis and pulsed-field gel electrophoresis. Int. J. Antimicrobial Agents 30, 541-545. [ Links ]
Allen, V.M., Fernandez, F. & Hinton, M.H., 1997. Evaluation of the influence of supplementing the diet with mannose or palm kernel meal on salmonella colonisation in poultry. Br. Poult. Sci. 38, 485-488. [ Links ]
Badia, R., Lizardo, R., Martínez, P. & Brufau, J., 2013. Oligosaccharide structure determines prebiotic role of β-galactomannan against Salmonella enterica ser. typhimurium in vitro. Gut Microbes 4 (1), 72-75. [ Links ]
Cani, P.D., Possemiers, S., Van, D.W.T., Guiot, Y., Everard, A., Rottier, O. & Muccioli, G.G., 2009. Changes in gut microbiota control inflammation in obese mice through a mechanism involving Glp-2-driven improvement of gut permeability. Gut 58 (8), 1091-1103. [ Links ]
Chen, W.L., Liang, J.B., Jahromi, M.F., Abdullah, N., Ho, Y.W. & Tufarelli, V., 2014. Enzyme treatment enhances release of prebiotic oligosaccharides from palm kernel expeller. BioResources 10, 196-209. [ Links ]
Dehghan, P., Gargari, B.P. & Jafar-Abadi, M.A., 2014. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized controlled clinical trial. Nutrition 30, 418-423. [ Links ]
Donalson, L.M., McReynolds, J.L., Kim, W.K., Chalova, V.I., Woodward, C.L., Kubena, L.F. & Ricke, S.C., 2008. The influence of a fructooligosaccharide prebiotic combined with alfalfa molt diets on the gastrointestinal tract fermentation, Salmonella enteritidis infection, and intestinal shedding in laying hens. Poult. Sci. 87, 1253-1262. [ Links ]
Dunkley, K.D., Callaway, T.R., Chalova, V.I., McReynolds, J.L., Hume, M.E., Dunkley, C.S. & Ricke, S.C., 2009. Foodborne salmonella ecology in the avian gastrointestinal tract. Anaerobe 15 (1), 26-35. [ Links ]
El-Aziz, D.M.A., 2013. Detection of Salmonella typhimurium in retail chicken meat and chicken giblets. Asian Pacific J. Trop. Biomed. 3 (9), 678-681. [ Links ]
Faghfoori, Z., Navai, L., Shakerhosseini, R., Somi, M.H., Nikniaz, Z. & Norouzi, M.F., 2011. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-α, interleukin-6 and-8 in patients with ulcerative colitis. Ann. Clin. Biochem. 48, 233-237. [ Links ]
Fernandez, F., Hinton, M. & Gils, B.V., 2002. Dietary mannan-oligosaccharides and their effect on chicken caecal microflora in relation to Salmonella enteritidis colonization. Avian Path. 31, 49-58. [ Links ]
Gibson, G.R. & Wang, X., 1994. Regulatory effects of Bifidobacteria on the growth of other colonic bacteria. J. Appl. Microbiol. 77, 412-420. [ Links ]
Gourbeyre, P., Denery, S. & Bodinier, M., 2011. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J. Leukocyte Biol. 89, 685-695. [ Links ]
Ibuki, M., Kovacs-Nolan, J., Fukui, K., Kanatani, H. & Mine, Y., 2011. β 1-4 mannobiose enhances salmonella-killing activity and activates innate immune responses in chicken macrophages. Vet. Immun. Immunopath. 139, 289-295. [ Links ]
Jahromi, M.F., Liang, J.B., Abdullah, N., Goh, Y.M., Ebrahimi, R. & Shokryazdan, P., 2015. Extraction and characterization of oligosaccharides from palm kernel cake as prebiotic. BioResources 11, 674-695. [ Links ]
Khadka, A., 2014. Interleukins in therapeutics. PharmaTutor 2 (4), 67-72. [ Links ]
Kim, G.B., Seo, Y.M., Kim, C.H. & Paik, I.K., 2011. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 90, 75-82. [ Links ]
Lecerf, J.M., Dépeint, F., Clerc, E., Dugenet, Y., Niamba, C.N., Rhazi, L. & Jacobs, H., 2012. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 108, 1847-1858. [ Links ]
Livak, K.J. & Schmittgen, T.D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2- AACT method. Methods 25, 402-408. [ Links ]
Muir, W.I., Bryden, W.L. & Husband, A.J., 1998. Evaluation of the efficacy of intraperitoneal immunization in reducing Salmonella typhimurium infection in chickens. Poult. Sci. 77, 1874-1883. [ Links ]
Patterson, J.A. & Burkholder, K.M., 2003. Application of prebiotics and probiotics in poultry production. Poult. Sci. 82, 627-631. [ Links ]
Rezaei, S., Jahromi, M.F., Liang, J.B., Zulkifli, I., Farjam, A.S., Laudadio, V. & Tufarelli, V., 2015. Effect of oligosaccharides extract from palm kernel expeller on growth performance, gut microbiota and immune response in broiler chickens. Poult. Sci. 94, 2414-2420. [ Links ]
Samal, L. & Behura, N.C., 2015. Prebiotics: an emerging nutritional approach for improving gut health of livestock and poultry. Asian J. Anim. Vet. 10, 724-739. [ Links ]
Schley, P.D. & Field, C.J., 2002. The immune-enhancing effects of dietary fibers and prebiotics. Br. J. Nutr. 87, S221-S230. [ Links ]
Seo, K.H., Holt, P.S., Vaughn, L.E., Gast, R.K. & Stone, H.D., 2003. Detection of Salmonella enteritidis-specific immunoglobulin A antibodies in crop samples from chickens infected with Salmonella enteritidis. Poult. Sci. 82, 67-70. [ Links ]
Sheffield, C.L., Crippen, T.L., Beier, R.C. & Byrd, J.A., 2014. Salmonella typhimurium in chicken manure reduced or eliminated by addition of LT1000. J. Appl. Poult. Res. 23, 116-120. [ Links ]
Shokryazdan, P., Jahromi, M.F., Navidshad, B. & Liang, J.B., 2017. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 206, 1-9. [ Links ]
Vulevic. J., Drakoularakou, A., Yaqoob, P., Tzortzis, G. & Gibson, G.R., 2008. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 88, 1438-1446. [ Links ]
Wang, Y., Yang, B., Cui, Y., Alali, W.Q., Xia, X., Xi, M. & Meng, J., 2015. Subtyping of Salmonella isolates on retail raw chicken in china by pulsed-field gel electrophoresis and plasmid analysis. Food Control 47, 420-426. [ Links ]
Received 4 June 2018
Accepted 10 January 2018
First published online 3 April 2019
# Corresponding author: jbliang@upm.edu.my














