Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.49 n.1 Pretoria 2019
http://dx.doi.org/10.4314/sajas.v49i1.5
ARTICLES
Evaluating horn traits of economic importance in sable antelope (Hippotragus niger niger)
G.C. JoslingI, #; A.A. LeporiI; F.W.C. NeserI; P.C. LuboutII; J.B. van WykI
IDepartment of Animal, Wildlife and Grassland Sciences, University of the Free State, P.O. Box 339, Bloemfontein, 9300, South Africa
IIWildlife Stud Services, Postnet Suite 489, Private Bag X025, Pretoria, South Africa
ABSTRACT
Much of the economic value of wildlife can be attributed to horn size, which is an important trait for trophy hunters. The main objective of the study was to estimate genetic parameters for the economically important horn traits of sable antelope that are currently being measured in the South African industry. To date, no quantitative genetic analysis has been done for any traits in sable antelope. The total number of records included in the evaluation were n = 1713 for horn length (SHL), n = 1503 for circumference (SHC), n = 1486 for tip to tip (SHTT), n = 1505 for tip length (SHT), and n = 1447 for rings (SHR). Males and females were considered separately in six-month age clusters. A Markov chain Monte Carlo (MCMC) multi-trait analysis was used to estimate (co)variance parameters for the horn traits. The results indicate a sex effect for all the traits and suggest that it is not economically viable to measure horn length of either sex after 54 months old. The horns of females are on average 40% shorter compared with bulls at maturity. Continuous horn growth throughout the lifetime of sable is suggested by the formation of ring posts, but is often masked by horn attrition and inadequate measuring techniques. An inbreeding coefficient of 0.0043 suggests adequate genetic diversity in the studied population. Heritability estimates of horn traits varied from 0.085 to 0.52, while genetic correlations ranged from 0.1 to 0.6 with the highest correlation being found between horn length and tip to tip. Further studies are recommended to confirm these results.
Keywords: Game, genetic evaluation, heritability
Introduction
Hippotragus niger niger is one of four recognized subspecies of sable antelope that are found in Africa (Matthee & Robinson, 1999). Historically they were distributed naturally over the northern savanna bushveld of South Africa (Furstenburg, 2008). Between 1930 and 1960 in the Letaba/Gravelotte area of Limpopo, the number of sable antelope decreased from an estimated 20 000 to 1 500 (Kriek Wildlife Group, 2017). According to Parrini et al. (2016), the development of livestock farms, deterioration of habitat and uncontrolled hunting were the main reasons for this dramatic decrease in animal numbers. Sable are generally sensitive to interspecific grazing competition from livestock and high-density wildlife species such as impala and wildebeest, while climate change (e.g. drought) and habitat mismanagement (bush encroachment, burning practices) are also responsible for the continuous loss of suitable habitat (Bothma et al., 2010; Parrini et al., 2016). Driver et al. (2012) reported that the intact natural habitat of sable antelope is highly threatened in South Africa, with only 10% currently being well protected. In the 1970s major relocation projects were carried out by the South African National Parks Board and Department of Nature Conservation to prevent extinction of the species (Oberem & Oberem, 2016). However, only in 1996 were sable declared lower risk/conservation dependent. They were moved to least concern on the International Union for Conservation (IUCN) red list in 2008 (Parrini et al., 2016).
After the implementation of the Game Theft Act No. 105 of 1991 which allowed private ownership of wildlife in South Africa (Simpson, 2012), sable were imported from Malawi, Zimbabwe and Zambia to rebuild the South African population (Kriek Wildlife Group, 2017), which marked the beginning of the sable ranching era in the country. Being a scarce species and one of the most visually appealing African antelopes, sable are currently popular high-value animals on game ranches because of their considerable contribution to the multibillion rand breeding and hunting industries (Cloete et al., 2015). Consequently, much of their economic worth is attributed to horn size. Factors that affect the horns are therefore of particular importance to wildlife managers. As a result, data recording of horn growth, pedigree and reproduction information has become an integral part of sable management.
Male and female sable both have black horns that curve backwards and are ringed on the upper surface. The cows' horns are more slender and significantly shorter than those of the bulls (Estes et al., 1999). The horns of live and trophy animals are classified and measured according to the general horn measuring methods of the Rowland Ward (RW) and Safari Club International (SCI) trophy hunting systems (Van Rooyen et al., 2016), including horn lengths, circumferences and tip-to-tip measurements. Alternative measurements such as horn tip length and ring counts are also taken to evaluate the horn performance of live animals.
Molecular genetic studies have been conducted on sable antelope to determine mitochondrial and nuclear DNA population structures (Matthee & Robinson, 1999; Jansen van Vuuren et al., 2010), while DNA markers have been developed for parentage verification (Miller et al., 2016). However, to date no information is available on quantitative genetic analysis for sable horn traits. According to the knowledge of the authors, the current study is the first of its kind and aims to i) provide descriptive statistics, and ii) estimate genetic parameters based on genomic verified pedigrees for horn traits of economic importance of sable antelope in South Africa.
Materials and Methods
On-farm horn performances were recorded by wildlife managers, and genomic verified pedigrees using microsatellite markers were extracted from the complete dataset (1537 animals from 76 herds across South Africa from January 2000 to May 2016) that was provided by Wildlife Stud Services. Guidelines regarding animal care and welfare were followed as stipulated and approved by the Animal Ethics Committee of the University of the Free State (Experiment No. UFS-AED2018/0054). Horn traits were measured according to the RW, SCI and other systems. Figure 1 illustrates the measurements of the economically important horn traits that were considered in the present study. The SCI horn traits included left (SHLL) and right horn length (SHLR), and left (SHCL) and right (SHCR) horn circumference. The tip-to-tip measurement of the RW system (SHTT) and other horn parameters such as tip length (SHTL, SHTR), and ring counts (SHRL, SHRR) of both horns were also considered.
Horn length is measured from the base along the front curve to the horn tip (A-B) without pressing the measuring tape down into the grooves (Figure 1). The circumference measurement is taken at the base of the horn as close to the head as possible (F). The tip-to-tip length of the horn (C-B) is determined as the straight line from the tip of the one horn to the tip of the other, while the tip length is measured from the first ring of the apex along the front curve to the horn tip (Van Rooyen et al., 2016). Ring count measurement is taken as the number of horizontal rings along the horn.
The original dataset was filtered using R statistical computing software (R Core Team, 2017), including animals within three standard deviations from the mean, and eliminating animals without date of birth. Animals in a contemporary group (as the interaction between herd and birth year) with fewer than five individuals were excluded. After correlations were established between left and right horn measurements using R statistical computing software (R Core Team, 2017), average measurements were calculated and used for further evaluation of the descriptive statistics for the traits, while bilateral traits were considered individually to establish genetic correlations. The total numbers of records included in the evaluation for each horn trait were n = 1713 for average length (SHL), n = 1503 for average circumference (SHC), n = 1486 for tip to tip (SHTT), n = 1505 for average tip length (SHT), and n = 1447 for average number of rings (SHR). For the descriptive statistics, males and females were considered individually and six-month age clusters were assigned.
All the independent variables were evaluated using a type III (ANOVA). A multi-trait repeated measurement animal model, using Markov chain Monte Carlo techniques (MCMC) was fitted. The R software package MCMCglmm (Hadfield, 2010) was utilised to estimate genetic parameters for and among these horn traits. Relative uninformative prior distributions for direct, permanent and residual variances were specified, resembling an inverse gamma distribution with a variance at the limit of 1 and a degree of belief parameter of 0.002, which is frequently used for variance components (Hadfield, 2017). The MCMC analysis was set up with a total of 200 000 iterations. Twenty thousand iterations were dropped at the beginning of the burning process, while one in five iterations was stored in memory. Convergence was assessed using the Cramer-von-Mises statistic test.
Expanded from Mrode (2005), in matrix notation the multi-trait animal model can be represented as:

Where: y is a vector that represents the observed phenotypic records of the i trait (1 to n)
Xiis an incidence matrix of fixed effects associated with trait i
biis a vector of the regression coefficients for the fixed effects of each trait i
Ziis an incidence matrix relating traits i records to animals in the pedigree
aiis the vector of additive random animal effects for trait i
Si is an incidence matrix of the permanent environmental effects
peiis the vector of permanent environmental effects for trait i
eiis a vector of trait's i random residual values (not considered by the fixed and other random effects)
The random effects were evaluated according to the assumption that they were normally distributed and independent.

Where: G, Q, and R are (co)variance matrices of genetic, permanent environmental and residual effects that are represented as:
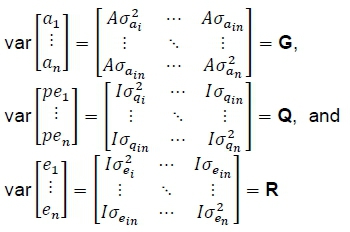
Where: I is an identity matrix accounting for the total number of evaluated animals, A is the relationship matrix defined by the pedigree,
the diagonal elements σαi.2, σqi.2and σei2are the genetic, permanent environmental and residual effects variances for traits i to n σαiη, σqinand σeiηare their corresponding co-variances. The definitive animal models for each trait were:
Where: yijklmoprefers to the ijklmopth observation of an animal in the ijklmopth subclass (e.g. herd-year)
μ corresponds to the population mean of the evaluated trait
sexidenominates the ith effect of sex
agejidentifies the jth effect of the age in months of every measurement
cskrepresents the kth calving season of the measured anima,
osi is the lth observation or measurement season
aorepresents the additive genetic component associated with the performance record,
pepequals the random permanent environmental effect
eijklmopcomprises the residual effect of the model, including environmental and non-additive genetic effect.
Based on Falconer & Mackay (1996), heritability estimates (h2) were calculated as the average of each individual posterior distribution heritability using the following equation:
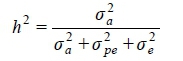
Where:σ2arepresenting the additive genetic variance
σ2pethe permanent environmental variance
σ2e the residual variance.
As shown by Wilson et al. (2010), the posterior genetic correlations rxywere obtained as the average of each posterior distribution correlation described as:
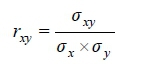
Where: σχy is the posterior additive genetic covariance between traits x and y
σχwith σyare the posterior genetic standard deviations of traits x and y, respectively.
Inbreeding coefficients were estimated according to the equations presented by Wright (1922).
Results and Discussion
To the knowledge of the authors, quantitative genetic evaluations for horn traits of sable antelope have not been published before. The type III (ANOVA) results showed that sex effect had a significant (P<0.05) influence on all the traits. A more detailed analysis of these sex differences is presented in Figures 2 and 3, which highlight the differences in age (clustered in 6-month periods starting from seven months old). Horn length growth in males and females stabilizes by 49 to 54 months old (Figure 2A). Horn length, however, differs between sexes, with male horns being on average 13 inches longer than female horns over the study period. In the active growing stage (0-54 months), males gain on average 0.81 inches per month, while females achieve approximately 60% of that (0.51 inches). Maximum growth occurs between 7 and 36 months and 7 and 30 months old for males and females, respectively. Horn tip length demonstrates large variation around the mean within age clusters with a downward trend in horn tip length over time.
The primary control of horn growth resides within the skin, pushing the older layers ahead towards the apex. Thus horns can theoretically continue to grow throughout the lifetime of an individual (Hall, 2015). However, in the present study a plateau state was reached (Figure 2A). Therefore it may be assumed that the growth in length ceases or that the rate of attrition equals and even exceeds the growth rate (Simpson, 1971). This statement might apply in particular to aggressive animals (correlated with high social hierarchy and age), as they tend to wear off their horns or even break them as a result of fighting or dominant behaviour (Thompson, 1993; Pelletier et al., 2003). The large variation in horn tip lengths (Figure 2B) can partly be explained by various rates of horn attrition from the tip, while discrepancies in defining the first horn ring of the measuring technique contributes to this variation. In addition, care must be taken to prevent accidental selection against unknown correlated traits when selecting exclusively for horn size. Instead, a holistic selection approach is recommended that combines horn growth with multiple traits such as fertility and body conformation (Hazel & Lush, 1942; Lush, 1943), while considering the social structure of the herd.
In males, the role of testosterone in growth, development and maintenance of horns has long been acknowledged (Davis et al., 2011). The results of the present study (Figure 2A) indicate that the end of the maximum horn growth period coincides with the onset of puberty at around 36 months for males and 24 months for females (Furstenburg, 2008). Interestingly, in his study of Spanish ibex and European mouflon, Toledano-Díaz et al. (2007) concluded that horn growth could be interrupted and episodic, which is attributed not only to testosterone but also to complex interactions between various hormones and other signalling factors., Figure 2A shows that horn length ontogeny changes throughout the lifetime of sables, as indicated by Davis et al. (2011) for bovidae animals.
Contrary to what evolutionary studies suggest (Erickson, 1996; Hall, 2015), the findings of the present study did not exhibit inferior horn development for animals bred in captivity compared with their wild relatives (Figures 2 and 3). This is supported by a population average (42.43") above the RW trophy standard for bulls between 48 and 54 months in the present study, comparable with the results reported by Crosmary et al. (2013) on trophy-hunted sable in the Matetsi Safari area of Zimbabwe. The assumption of inferior performance of captive bred herds is based on the possible effects of inbreeding in small populations (Scribner et al., 1989; Fitzsimmons et al., 1995), but the evaluation of these herds showed a low average level of inbreeding (0.0043).
Horn circumference reaches a developmental plateau at the same age cluster (31-36 months) for both sexes (Figure 3). Similar to the horn length traits, males showed a greater development at the circumference, nearly 2.7 inches on average more than females over the study period. It is also apparent that these traits are less variable within age group than length traits.
Simpson (1971) observed that post-mature horn growth in sable occurs characteristically at the base of the horn, is thicker in diameter, and lacks the distinct ridges (Figure 4) of sub-mature growth. Environmental effects like abrasion at the anterior side of the horn can mask this type of growth in some cases and might also explain why there is no increase in horn circumference after 36 months old in the present study (Figure 4). As discussed, the 36-month-old stage involves a range of physiological changes. For example, the bulls reaching puberty and females start to calve down, which contributes to the environmental effects that might disguise growth in horn circumference during this period. The possibility that horn growth can occur in terms of horn density (structural and weight changes) without affecting the horn dimensions should also be investigated.
Within age clusters it is evident that the tip-to-tip measurement (Figure 5) presents a large variation for both males and females, with males again displaying higher trait values on average.
Distinctive horn shapes may arise by modulating zones of keratin production at the base of the horn (Davis et al. 2011). The sable breeding and hunting industry prefers a symmetrical horn shape with average to above average tip to tip to horns with narrow tips and those that are asymmetrical.
Horn rings follow a similar development pattern to horn length, but reach a plateau state at a later age (61-66 months old) (Figure 6). Little variation between sexes is observed for horn ring counts from 7 to 42 months old, while relatively large variation is seen around the means within the age clusters. An increase in the number of horn rings is seen even after the growth in horn length seemed to have ceased. In terms of the downward trend in horn tip length with age, it has been suggested that horn attrition accounts for this phenomenon and horn growth is a continuous process throughout the lifetime of the animal (Simpson, 1971; Hall, 2015). The ring count seems to reach a plateau state, but measuring techniques do not consider horn ring abrasion or sub-mature growth, often referred to as 'posts' (Figure 4). In addition, the data of the present study indicate that horn ring count is not an accurate estimation of age for sable antelope because of the variation in means in age classes for both sexes.
Based on the posterior standard deviations values (SD) (Table 1), most variance components (except horn length and tip to tip) were shown to be accurate for horn traits. In the present study heritability estimates for horn length and circumference were found to be very low (below 0.1), but the heritability of the horn length was not estimated accurately according to the SD. On the other hand, the tip-to-tip measurement showed a heritability estimate that was considered medium to high (0.31), while tip length was highly heritable (±0.5).
Considering the low heritability estimates of horn circumference (Table 1) in this population, performance is probably affected by the environment largely. Environmental factors such as seasonal and annual cycles, disease, physiological status and stressors such as social hierarchy have been reported to influence horn growth (Frandson et al., 2003). Based on the phenotypic variation of the horn tip-to-tip (Figure 5) and the moderate heritability estimate, this suggests that genetic improvement can be made. However, the high SD values associated with the tip-to-tip trait (Table 1) warrant further investigation. For this study, the small sample sizes and the lack of a standard protocol for horn recording suggest that more data and additional studies are required to confirm these results.
Positive genetic correlations were estimated between horn length and other horn traits (Table 2). According to these values, circumference and tip length genes are weakly associated with the one controlling length expression. Rings were moderately correlated with length, while a moderate to high relationship was found between length and tip to tip.
The monetary value of live and trophy-hunted sable is determined largely by horn length (Du Toit et al., 2010; Van Rooyen et al., 2016). Naturally, it is worth exploring whether the final horn length performance can be predicted at an early age from other traits by considering the strength of the relationships between traits. Tip length and ring count was moderately correlated with horn length, while circumference was weakly correlated and tip to tip was moderately to highly correlated with horn length. None of the traits revealed a genetic (Table 2) or phenotypic correlation (data not shown) above 0.7 with horn length, indicating that a number of exceptions are likely to occur (Bourdon, 2000). Also, when considering the low heritability estimate of horn length it is evident that the expression of the trait is not strongly influenced by additive genetics, but is more closely associated with the environment.
Lastly, an inbreeding coefficient of 0.0043 suggests that this population retained adequate genetic diversity and management practices did not affect the genetic integrity or performance negatively.
Conclusions and Recommendations
The study indicated that horn traits of sable antelope are influenced by sex. Bulls have on average 40% longer horns than cows at maturity. Although the horns of sable grow continuously throughout their lifetime, this is masked by horn attrition. Therefore, it is not economically viable to measure horn length after 54 months old in either sex.
Heritability estimates for sable horn traits vary from low to high. However, the small sample sizes and protocol recording issues of some traits suggest that more data and further studies are required to confirm the results of the present study. Finally, proper management practices demonstrate that captivity does not affect the performance or genetic integrity of sable negatively.
Acknowledgements
The authors would like to acknowledge Wildlife Stud Services for providing the dataset on horn traits of sable antelope for evaluation. Dreyer Van Zyl Game is acknowledged for photographic illustrations of post-mature horn growth.
Authors' Contributions
AAL designed the experiment and carried out the analysis. GCJ and AAL structured the scientific content and drafted the manuscript. FWCN, PCL and JBvW assisted with the statistical analysis, while all authors provided editorial suggestions and approved the final manuscript.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
References
Bothma, J.duP., Van Rooyen, N. & Du Toit, J.G., 2010. Antelope and other smaller herbivores. In: J.duP. Bothma & J.G. du Toit (eds). Game Ranch Management. Fifth edition. Van Schaik, Pretoria. pp. 210-245. [ Links ]
Bothma, J.duP., Van Rooyen, N. & Du Toit, J.G., 2016. Antelope and other smaller herbivores. In: J.duP. Bothma & J.G. du Toit (eds). Game Ranch Management. Sixth edition. Van Schaik, Pretoria. pp. 210-245. [ Links ]
Bourdon, R.M., 2000. Understanding Animal Breeding, 2nd edition. Prentice-Hall, New Jersey. pp. 144-148. [ Links ]
Cloete, P.C., Van der Merwe, P. & Saayman, M., 2015. Game Ranch Profitability in South Africa: 2nd edition. ABSA, Cape Town. pp. 63-79. [ Links ]
Crosmary, W-G., Loveridge, A.J., Ndaimani, H., Lebel, S., Booth, V., Côté, S.D. & Fritz, H., 2013. Trophy hunting in Africa: Long-term trends in antelope horn size. Anim. Conserv. 16, 648-660. [ Links ]
Davis, E.B., Brakora, K.A. & Lee, A.H., 2011. Evolution of ruminant headgear: A review. Proc. R. Soc. B. 278, 2857-2865. [ Links ]
Driver, A., Sink, K.J., Nel, J.L., Holness, S., Van Niekerk, L., Daniels, F., Jonas, Z., Majiedt, P.A., Harris, L. & Maze, K., 2012. National Biodiversity Assessment 2011: An assessment of South Africa's biodiversity and ecosystems. Synthesis Report. South African National Biodiversity Institute and Department of Environmental Affairs, Pretoria. [ Links ]
Du Toit, J.G., Van Rooyen, J. & Bothma, J.duP., 2010. Trophy Hunting. In J.duP. Bothma & J.G. du Toit (eds). Game Ranch Management. Fifth edition. Van Schaik, Pretoria. pp. 643-650. [ Links ]
Erickson, G.M., 1996. Toothlessness in American alligators, Alligator mississippiensis. Copeia. 1996, 739-743. [ Links ]
Estes, R.D., Otte, D. & Fuller, K.S., 1999. The safari companion: A guide to watching African mammals including hoofed mammals, carnivores, and primates. Chelsea Green, USA. pp. 98-101. [ Links ]
Falconer, D.S. & Mackay, T.F.C., 1996. Introduction to Quantitative Genetics. Fourth edition. Longman, Harlow, Essex, UK. 464 pp. [ Links ]
Fitzsimmons, N.N., Buskirk, S.W. & Smith, M.H., 1995. Population history, genetic variability, and horn growth in Bighorn sheep. Conserv. Biol. 9 (2), 314-323. [ Links ]
Frandson, R., Wilke, W. & Fails, A., 2003. The integument. In: Anatomy and Physiology of Farm Animals. Sixth edition. Blackwell, USA. pp. 201-213. [ Links ]
Furstenburg, D., 2008. Naturally occurring common species: An overview. In: Wildlife Ranching in South Africa. Department of Agriculture, Private Bag X250, Pretoria, 0001 South Africa. pp. 34-60. [ Links ]
Hadfield, J.D., 2010. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R Package. J. Stat. Softw. 33, 1-22. [ Links ]
Hadfield, J.D., 2017. MCMCglmm course notes. CRAN - Package MCMCglmm. Available from: https://cran.r-project.org/web/packages/MCMCglmm/index.html. [ Links ]
Hall, B.K., 2015. Horns and Ossicones. In: Bones and Cartilage. Second edition. Academic Press, San Diego. pp. 113-122. [ Links ]
Hazel, L.N. & Lush, J.L., 1942. The efficiency of three methods of selection. J. Hered. 33, 393-399. [ Links ]
Jansen van Vuuren, B., Robinson, T.J., VazPinto, P., Estes, R. & Matthee, C.A., 2010. Western Zambian sable: Are they a geographic extension of the giant sable antelope? S. Afr. J. Wild. Res. 40, 35-42. [ Links ]
Kriek Wildlife Group, 2017. Game breeders journal. Unpublished. http://www.kriekwildlife.com/KWG%20Journal%20JAN%.pdf. Accessed 31 January 2018. [ Links ]
Lush, J.L., 1943. The genetic basis of variation. In: Animal Breeding Plans. The Iowa State College Press, USA. pp. 71-85. [ Links ]
Matthee, C.A. & Robinson, T.J., 1999. Mitochondrial DNA population structure of roan and sable antelope: Implications for the translocation and conservation of the species. Mol. Ecol. 8, 227-238. [ Links ]
Miller, S.M., Clarke, A.B., Bloomer, P., Guthrie, A.J. & Harper, C.K., 2016. Evaluation of microsatellites for common ungulates in the South African wildlife industry. Conserv. Genet. Resour. 8, 329-341. [ Links ]
Mrode, R., 2005. Linear models for the prediction of animal breeding values. Second edition. CABI, Oxfordshire, UK. 344 pp. [ Links ]
Oberem, P. & Oberem, P., 2016. The New Game Rancher. First edition. Briza, Pretoria. pp. 165-172. [ Links ]
Parrini, F., Koen, J., Dalton, D. & Eksteen, J., 2016. A conservation assessment of Hippotragus niger niger. In: M.F. Child, L. Roxburgh, E. Do Linh San, D. Raimondo & H.T. Davies-Mostert (eds). The red list of mammals of South Africa, Swaziland and Lesotho. South African National Biodiversity Institute and Endangered Wildlife Trust, South Africa. [ Links ]
Pelletier, F., Bauman, J. & Festa-Bianchet, M., 2003. Fecal testosterone in bighorn sheep (Ovis canadensis): Behavioural and endocrine correlates. Can. J. Zool. 81, 1678-1684. [ Links ]
R Core Team, 2017. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. Available from: https://www.R-project.org/. [ Links ]
Scribner, K.T., Smith, M.H. & Johns, P.E., 1989. Environmental and genetic components of antler growth in white-tailed deer. J. Mamm. 70, 284-291. [ Links ]
Simpson, C.D., 1971. Horn growth as a potential age criterion in some southern African antelopes. S. Afr. J. Wild. Res. 1, 20-25. [ Links ]
Simpson, I.F., 2012. The development of organised game ranching in South Africa. In: Mpunzi, dawn of a new era: A historical overview of the development of the game ranching industry in KwaZulu-Natal and Southern Africa in the 20th century. Ian Fleming Simpson, Cape Town. pp. 83-90. [ Links ]
Thompson, K.V., 1993. Aggressive behavior and dominance hierarchies in female sable antelope, Hippotragus niger: Implications for captive management. Zoo Biol. 12, 189-202. [ Links ]
Toledano-Díaz, A., Santiago-Moreno, J., Gómez-Brunet, A., Pulido-Pastor, A. & López-Sebastián, A., 2007. Horn growth related to testosterone secretion in two wild Mediterranean ruminant species: The Spanish ibex (Capra pyrenaica hispanica) and European mouflon (Ovis orientalis musimon). Anim. Reprod. Sci. 102, 300-307. [ Links ]
Van Rooyen, N., Van Rooyen, J. & Van Rooyen, N., 2016. Handling and measuring trophies. In: J.duP. Bothma & J.G. du Toit (eds). Game Ranch Management, Sixth edition. Van Schaik, Pretoria. pp. 719-753. [ Links ]
Wilson, A.J., Réale, D., Clements, M.N., Morrissey, M.M., Postma, E., Walling, C.A., Kruuk, L.E.B. & Nussey, D.H., 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13-26. [ Links ]
Wright, S., 1922. Coefficients of inbreeding and relationship. Am. Nat. 56, 330-338. [ Links ]
Received 10 May 2018
Accepted 28 August
First published online 2 March 2019
# Corresponding author: buitendachgc@ufs.ac.za














