Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.42 n.4 Pretoria Jan. 2012
Variety effect on composition, kinetics of fermentation and in vitro digestibility of oat (Avena sativa L.) straw and its neutral detergent fibre
F. KafilzadehI, #; N. HeidaryI; S. BahraminejadII
IDepartment of Animal Science, Faculty of Agriculture, Razi University, Kermanshah, Iran
IIDepartment of Agronomy and Plant Breeding, Faculty of Agriculture, Razi University, Kermanshah, Iran
ABSTRACT
Yield, chemical composition, in vitro digestibility and kinetics of fermentation of straw from 18 varieties of oats (Avena sativa L.) were studied. All the straw varieties were grown in three replicates under the same agronomic conditions. Significance differences were observed in the yield of straw (4.4 to 7.5 ton dry matter (DM)/ha) from different varieties. The proportion of seed/straw from these varieties varied from 0.28 to 1.02. Crude protein (CP), neutral detergent fibre (NDF), acid detergent fibre (ADF) and acid detergent lignin (ADL) content varied from 24.2 to 48.1, 626 to 708, 437 to 533 and 52.0 to 92.4 g/kg DM, respectively in the straws. In vitro organic matter digestibility (IVOMD) differed among varieties and varied from 400 to 539 g/kg DM. The mean value of digestible organic matter yield (DOM) was 2.34 ton/ha. A significant difference was observed in the potential gas production (A) and lag time (L) among varieties. The fractional rate of gas production (c, /h) ranged from 0.030 to 0.034. The results emphasized that in any evaluation of oat varieties, kinetics of digestion or fermentation should be taken into consideration as well as yield and digestibility.
Keywords: Oats, straw yield, composition, digestibility, gas production kinetics
Introduction
Cereals are cultivated to obtain grain for human consumption or for animal feed. Straw as the residue of cereals after harvesting can represent a substantial amount of biomass. Despite its abundance, straw has generally been overlooked as animal feed, in many cases owing to insufficient knowledge of its potential feeding value. Under severe shortage of hay, straw can become valuable low-cost forage that can be used effectively, especially in extensive ruminant production systems based on low inputs (López et al., 2005). It has been estimated (Kossila, 1984) that the amount of straw produced for each unit of grain is 0.6 for wheat, 0.72 for barley, 0.78 for oats and 1.2 for rye. Oats (Avena sativa L.) ranks around sixth in the world cereal production, producing in excess of 23 million tons annually worldwide (FAO, 2011) leaving about 20 million tons of straw. To provide balanced diets that include straw, it is important to know the nutritive value of this roughage and its variability, as straw sources vary in their nutrient content and digestibility. Crude protein (CP) content of cereal straw varies from 24 up to 54 g/kg dry matter (DM) (Theander & Aman, 1984). Capper (1988) and Capper et al. (1988) reported that in vitro DM digestibility for wheat, barley and oats straw were 360, 400, 450 g/kg, respectively. Mulholland et al. (1994) showed DM intake for oats, wheat and barley straw with sheep to be 660, 450 and 320 g/day, which indicates a large variability in the nutritive value of cereal crop residues. Cuddeford (1995) suggested that oat straw has higher digestible organic matter (OM) and metabolizable energy (ME) contents than other cereals in terms of available energy. He also suggested that oat straw is the most palatable and nutritious, followed by barley straw, wheat straw and rye straw. Varietal differences have also been reported in the nutritive value of the residues from wheat (White et al., 1981; Kernan et al., 1984; Tolera et al., 2008), barley (Bediye et al., 1998) and corn (Harika & Sharma, 1994; Tolera et al., 1999).
The objective of this study was to evaluate the nutritive value of straw from 18 oat varieties in terms of composition, digestibility and kinetics of fermentation for better understanding of varietal differences for ruminant nutritionists and crop breeders.
Material and Methods
Straw from 18 varieties (Table 1) of oats provided by the South Australian Research and Development Institute (SARDI) was used in the study. They were grown under similar agronomic condition in three replicates in a randomized complete block design at the Research Farm of the School of Agriculture, Razi University, Kermanshah, Iran. The blocks were designed based on the non-uniformity of the field (in vertical to non-uniformity slope). Each experimental unit consisted of five rows by 2 m. Straw was obtained after harvesting the oat grain. The plants were cut by hand at about 10 cm above soil surface. The weight of grain and straw of each plot was determined. A subsample of approximately 450 g was randomly taken from the harvested portion of each plot and dried at 60 °C for further analysis and at 100 °C for 24 h to determine DM yield per hectare.
The straw samples were ground with a laboratory mill to pass a 1-mm screen. Standard methods as described in AOAC (1990) were used to determine DM, ash and CP levels. The neutral detergent fibre (NDF), acid detergent fibre (ADF) and acid detergent lignin (ADL) levels were determined according to Van Soest et al. (1991).
In vitro digestibility was determined as described by Tilley & Terry (1963). Rumen liquid was collected from three rumen cannulated sheep (receiving a mixture of lucerne hay and straw) before the morning feeding. The rumen liquid from the sheep was mixed on a volume basis, and filtered through four layers of cheesecloth.
The incubation inoculum was prepared by diluting the rumen liquid with a buffer solution (Tilley & Terry, 1963) in a 1 : 4 (vol/vol) ratio. Mixed inoculum was stirred in a water bath at 39 °C with purging CO2 until used (10 to 15 min later). About 250 mg (1 mm ground) of each sample was placed into 50-mL sterile tubes, and 20 mL of the incubation inoculum was added. The tube was stoppered with a Bunsen valve and incubated for 48 h at 39 °C. The tubes were gently swirled by hand every 8 h. Each sample was incubated in three replicates. At the end of the 48 h of incubation, the tube contents were acidified using 6 M HCl to reach a final pH of 1.3 to 1.5. After a few seconds, when the foam subsided, pepsin (EC 3.4.23.1) powder was added to a final concentration of 0.2% (wt/vol). The tubes were re-incubated for an additional 48 hours. The tubes were then centrifuged at 2500 rpm for 15 min, and the supernatant was discarded. The tubes containing the pellets were dried in a forced air oven at 60 °C for 48 h to determine the residual DM weights. In vitro DM and OM digestibility were calculated respectively as the DM and OM that disappeared from the initial weight inserted into the tube.
The method used for gas production measurements was as described by Theodorou et al. (1994). All samples were ground to pass a 1-mm screen. About 125 mg of each sample were weighed into tubes kept at approximately 39 °C and flushed with CO2 before use. Each sample was incubated in three replicates. Fifteen mL of buffered rumen fluid (20% rumen fluid + 80% buffer solution) were prepared (as described in in vitro digestibility section) and were anaerobically dispensed in each tube at 39 °C. All the tubes were flushed with CO2, crimped with rubber stoppers and aluminium seals, placed in an incubator at 39 °C, and shaken at regular intervals. The pressure of gas produced in each tube was recorded using a pressure transducer (Testo 512 digital manometer) at 2, 4, 6, 8, 12, 18, 24, 48, 72, 96 and 120 h after the start of incubation. To estimate the kinetics of gas production, data on cumulative gas volume produced, were fitted using the generalized Mitscherlich model proposed by France et al. (1993):
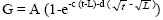
where G (mL) denotes cumulative gas production at time t, A (mL) is asymptotic gas production, c (/h) and d (/h) are rate constants and L (h) is lag time. The half-life (t1/2, h) of the fermentable fraction of each substrate was calculated as the time taken for gas accumulation to reach 50% of its asymptotic value. All gas volumes were adjusted to a common sample weight of 200 mg DM (Lopez et al, 2007). The volume of gas produced (GP) (mL/200 mg) after 24 h incubation was used with CP content to estimate metabolizable energy (ME) concentration (MJ/kg DM), based on the following equation reported by Menke & Steingass (1988) for roughage feeds:
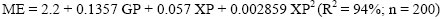
where:
ME = metabolizable energy (MJ/kg DM); GP = gas production after 24 h (mL/200 mg DM); XP = crude protein (%).
A shorter gas production test was done to determine in vitro true DM degradability (IVTDMD) and NDF degradability (NDFD). Rumen digesta collection, sample and buffer preparations, and incubation techniques were as described above. Incubation was stopped at 30 h after recording the gas volume and the entire residue in the incubation tubes was drained into 600 mL spotless beakers and refluxed with neutral detergent solution to determine IVTDMD and NDFD. This determination is the Goering & Van Soest (1970) modification of Tilley & Terry (1963), as described by Blummel & Becker (1997). The partitioning factor (PF) was calculated as the ratio of mg substrate truly degraded/mL gas produced by it, according to Blummel et al. (1997).
Data of gas production parameters and in vitro dry matter digestibility (IVDMD) were analysed using multi-observational data analysis with three replicates and three samples each. Since there were no significant differences among samples, the mean of three samples of each replicate was analysed, based on RCBD with three replicates such as the other agronomical traits. Analysis of variance was carried out using SAS (2000) and significant differences between treatments were identified using Duncan multiple-range test (Duncan, 1955).
Results and Discussion
Yield of straw, grain, grain/straw ratio and total biomass from the 18 varieties of oats are shown in Table 2. Yield of grain from these varieties varied from 2.1 to 5.8 ton DM/ha. Tamm (2003) reported that yield of grain from different varieties of oats ranged from 3.3 to 5.8 ton DM/ ha. There was a difference (P <0.01) in the yield of straw from different varieties. Maximum straw yield was obtained from V8 and minimum yield was recorded for V12. A positive correlation was observed between grain yield and total biomass produced (r = 0.83, P <0.01). The grain/straw ratio varied from 0.28 to 1.02, indicating a wide variation in the proportion of straw to grain produced.
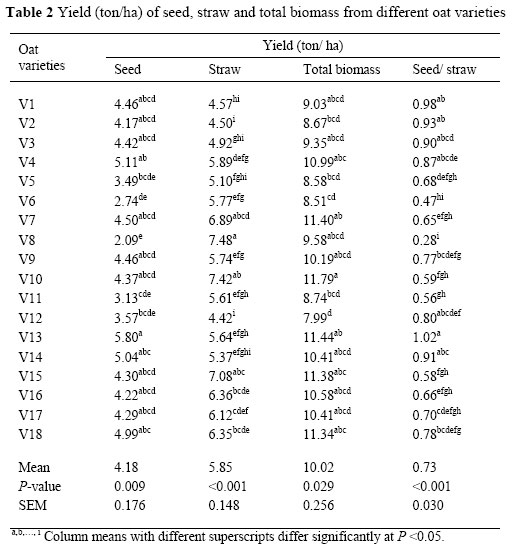
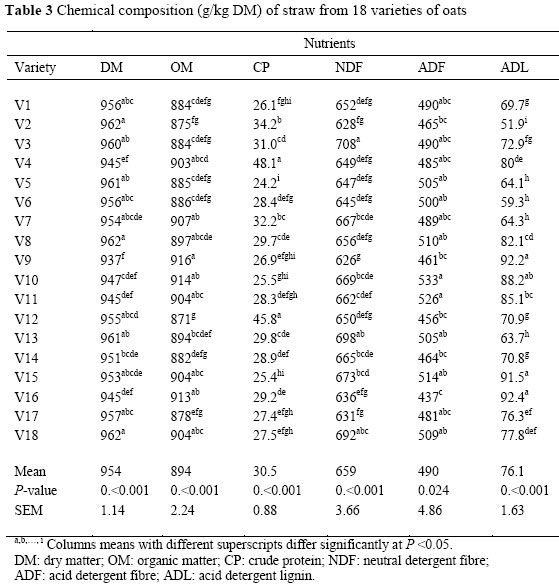
The chemical composition of different varieties of oats is presented in Table 2. There were significant (P <0.01) differences between varieties in terms of CP, NDF, ADF and ADL levels. Crude protein level (ranging from 24 to 48.1 g/kg DM) of the oat varieties used in the present experiment were similar to those reported by Pearson et al. (2001), Lopez et al. (2005) and Anderson & Hoffman (2006).
The mean NDF, ADF and ADL levels in the oat straw were 659, 490 and 76.1 g/kg DM, respectively. The greatest difference between the highest and the lowest value in cell wall fractions was observed in lignin levels (92.4 vs. 51.9 g/kg DM), followed by hemicellulose (217.9 vs. 135.3 g/kg DM) and cellulose (445.2 vs. 334.6 g/kg DM). The NDF levels of the oat varieties in the present study were lower than the values reported by Pearson et al. (2001) and Lopez et al. (2005), but the obtained ADF and ADL levels were consistent with their values.
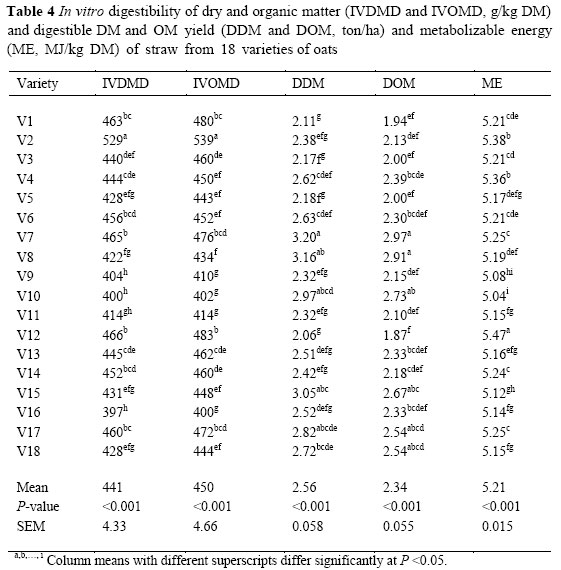
The result of in vitro digestibility (Table 3) showed that there were differences (P <0.01) between DM and OM digestibility of straws from the different varieties. The IVOMD ranged from 400 to 539 g/kg DM. The in vitro DM digestibility of oat straws (from 397 to 529 g/kg DM) was similar to the values reported by Lopez et al. (2005) and higher than the values reported by Brown & Almodares (1976) and Jung et al. (1992). Differences in the digestibility of straws from different varieties may be due, not only to the chemical composition (Dias-da-silva & Guedes, 1990) but also to stem, leaf and seed ratios (Bhargava et al, 1988). Crude protein was positively correlated to IVOMD (r = 0.33, P <0.05). There were significant negative correlations between IVOMD and cell wall fractions, particularly ADL (r = -0.77, P <0.001). Variety 16 with the highest ADL had the lowest IVOMD, while V2 with the lowest ADL had the highest IVOMD. It is accepted that forage degradation in the rumen is affected mainly by the cell wall content and its lignification, as lignin is an indigestible fraction and acts as a barrier, limiting the access of microbial enzymes to the structural polysaccharides of the cell wall. Ammar (2002) reported that NDF, ADF and ADL levels were negatively correlated with in vitro digestibility. The mean value of digestible OM yield (DOM) was 2.3 ton/ha. Varieties 7 and 8 had the highest yield of DOM (2.9 ton/ha) while V12 produced the lowest DOM yield (1.9 ton/ha). Other factors known to affect the composition and digestibility of straw are variety and cultivar (Mould et al., 2001; Kafilzadeh & Maleki, 2011), environmental and seasonal effects (Mathison et al, 1999) and proportion of morphological fractions of the straw (Agbagla et al, 2001).
The ME (MJ/kg DM) levels of straw from different varieties were calculated from the amount of gas produced at 24 h incubation with the supplementary analysis of CP. There were significant (P <0.01) differences among ME of straw from these varieties. Metabolizable energy was negatively correlated with ADL concentrations (r = -0.51, P <0.01) and positively correlated with the CP level (r = 0.79, P <0.01).
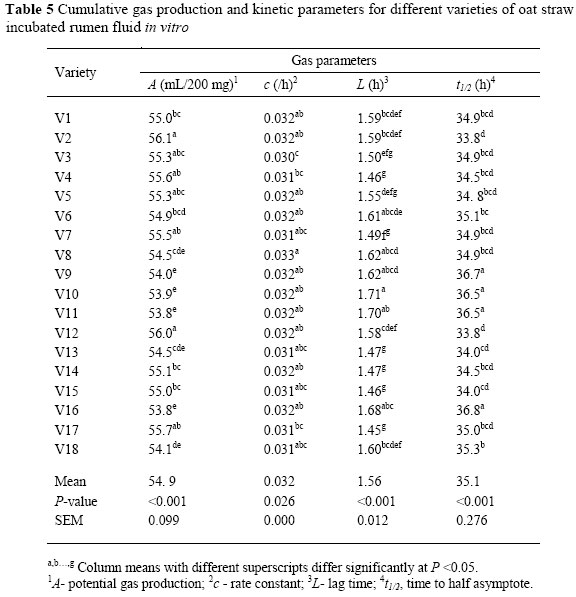
Gas production kinetic parameters of straw from oat varieties are presented in Table 5. There were differences (P <0.01) among varieties in asymptotic gas production (A) and lag time (L). Potential gas production (A) ranged from 53.8 to 56.0 mL/200 mg. The constant rate (c) was also different (P <0.05) among varieties. The differences in gas production characteristics may partly be due to differences in CP, NDF and ADF concentrations. Nsahlai et al. (1994) and Larbi et al. (1998) reported that there was a positive correlation between CP and the rate of gas production, and negative correlations between NDF and ADF with the rate and extent of gas production. Asymptotic gas production (4) was lowest in V16 and highest in V2. These findings were in line with the in vitro digestibility results in which V16 and V2 showed the lowest and the highest IVOMD, respectively (r = 0.53, P <0.01). The rate at which gas was produced (c) was not altered much in different varieties. Therefore, it appeared that the rate of gas production was not responsible for the differences in the total gas production. Varieties with higher gas production had a shorter half time (t1/2). The negative correlation found between asymptotic gas production (4) with either NDF and ADF (r = -0.47, r = -0.50, P <0.01) is consistent with the results of Haddi et al. (2003). The negative effect of cell wall content on gas production could be the result of a reduction of microbial activity through increasing the adverse environmental conditions as incubation time progresses. The values of potential gas production and fractional rate of gas production (c) of experimental varieties of oats were consistent with those (56.6 mL/200 mg DM and 0.031/h, respectively) reported by Lopez et al. (2005).
Digestibility of NDF is an important component of forage quality. Increased NDF digestibility (NDFD) may result in reduced physical fill in the rumen over time, and allows greater voluntary feed intake (Dado & Allen, 1995). Oba & Allen (1999) reported that one unit increase in forage NDFD in vitro or in situ was associated with a 0.17 kg increase in dry matter intake (DMI). In vitro true DM degradability (IVTDMD) and NDFD were determined after terminating the incubation at 30 h and were calculated from the truly undegraded substrate. The IVTDMD varied from 44.9% to 57.2%, while NDFD varied from 17.3% to 31.9%. The partitioning factor (PF), an index of the substrate dependent variation, is the ratio of substrate - degraded to gas volume produced by it at 30 h incubation. This ratio is reported to be valuable in improving the accuracy of voluntary DMI prediction of temperate, tropical crop residues and Mediterranean hays (Blummel et al, 1997), and forages with high PF had high DMI (Blummel et al., 2005). Blummel et al. (1997) noted that in forage fermentation, PF values between 2.75 and 4.41 mg/mL do correspond to YATP's from 10 to 32 mg, and a YATP of 32 mg is considered maximum microbial efficiency. Calculated PF from varieties in the present experiment ranged from 2.77 to 3.23. Blummel et al. (2005) reported 37.6% and 2.90 mg/mL for IVTDMD and PF (24 h) in oat straw, respectively, and forages with high PF had high DMI.
Conclusion
Considering the importance of straw in diets for ruminants in the world, it is suggested that selection of new oat varieties should take into consideration the nutritive value of the straw as well as the quantities of seed and straw produced. The study showed the presence of considerable varietal differences in nutritive value of straw from 18 varieties of oats. Cell wall content, digestibility and potential gas production of fermentation of straws were affected by variety. There were significant negative correlations between IVOMD, gas production and cell wall fractions. These variations indicate that in any evaluation of oat varieties, not only yield, but digestible OM yield of straw and partitioning factor as index of intake, as well - as the digestibility of straw, should be taken into consideration, particularly in areas where straw from these grasses is considered an important feedstuff for ruminants.
References
Agbagla-Dohnani, A., Noziere, P., Clement, G. & Doreau, M., 2001. In sacco degradability, chemical and morphological composition of 15 varieties of European rice straw. Anim. Feed Sci. Technol. 94, 15-27. [ Links ]
Ammar, H., 2002. Composition química, digestibilidad y cinética de fermentation ruminal in vitro de arbustos. PhD tesi, Universidad de León, Spain. [ Links ]
Anderson, T.P. & Hoffman, P., 2006. Nutrient composition of straw used in dairy cattle diets. University of Wisconsin Extension Focus on Forage. Vol 8. No. 1. [ Links ]
AOAC, 1990. Official Methods of Analysis. 15th Association of Official Analytical Chemists, Arlington, USA. [ Links ]
Bediye, S., Sileshi, Z., Assefa, G. & Gebre, H., 1998. Effect of cultivar and fertilizer application on yield and quality of barley straw. Proceedings of 6th conference of Ethiopian Society of Animal Production, 1415 May 1998. ESAP, Addis Abada, Ethiopia. pp. 145-152. [ Links ]
Bhargava, P.K., ∅rskov, E.R. & Walli, T.K., 1988. Rumen degradation of straw. Selection and degradation of morphological component of barley straw by sheep. Anim. Prod. 47, 105-110. [ Links ]
Blummel, M. & Becker, K., 1997. The degradability characteristics of fifty-four roughages and roughage neutral-detergent fibers as described by in vitro gas production and their relationship to voluntary feed intake. Br. J. Nutr. 77, 757-768. [ Links ]
Blummel, M., Makkar, H.P.S. & Becker, K., 1997. In vitro gas production: a technique revisited. J. Anim. Physiol. Anim. Nutr. 77, 24-34. [ Links ]
Blummel, M., Cone, J.W., Van Gelder, A.H., Nshalai, I., Umunna, N.N., Makkar, H.P.S. & Becker, K., 2005. Prediction of forage intake using in vitro gas production methods: Comparison of multiphase fermentation kinetics measured in an automated gas test, and combined gas volume and substrate degradability measurements in a manual syringe system. Anim. Feed Sci. Technol. 123-124, 517-526. [ Links ]
Brown, A.R. & Almodares, A., 1976. Quantity and quality of triticale forage compared to other small grains. Agron. J. 68, 264-266. [ Links ]
Capper, B.S., 1988. Genetic variation in the feeding value of cereal straw. Anim. Feed Sci. Technol. 21, 127-140. [ Links ]
Capper, B.S., Thomson, E.F. & Herbert, F., 1988. Genetic variation in the feeding value of barley and wheat straw. In: Plant breeding and the nutritive value of crop residues. Eds Reed J.D., Capper B.S. & Neate, J.H., Addis Ababa, International livestock for Africa. pp. 177-193. [ Links ]
Cuddeford, D., 1995. Oats for animal feed. In: The Oat Crop. Ed. Welch, R.W., Chapman and Hall, UK. pp. 321-368. [ Links ]
Dado, R.G. & Allen, M.S., 1995. Intake limitation, feeding behavior, and rumen function of cows challenged with rumen fill from dietary fiber or inert bulk. J. Dairy. Sci. 78, 118-113. [ Links ]
Dias-da-silva, A.A. & Guedes, C.V.M., 1990. Variability in the nutritive value of cultivars of wheat, rye and triticle and response to area treatment. Anim. Feed Sci. Technol. 28, 79-89. [ Links ]
Duncan, D.B., 1955. Multiple range and multiple F - tests. Biometrics 11, 1-42. [ Links ]
FAO, 2011. http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor [ Links ]
France, J., Dijkstra, J., Dhanoa, M.S., Theodorou, M.K., Lister, S.J., Davies, D.R. & Isac, D.A., 1993. A model to interpret gas accumulation profiles associated with in vitro degradation of ruminant feeds. J. Theor. Biol. 163, 99-111. [ Links ]
Goering, H.K. & Van Soest, P.J., 1970. Forage Fiber Analysis. In: Agricultural Handbook No. 379. Agricultural Research Service, US Department of Agriculture, Washington, D.C., USA. [ Links ]
Haddi, M.L., Filacorda, S., Meniai, K., Rollin, F.P. & Susmel, P., 2003. In vitro fermentation kinetics of some halophyte shrubs sampled at three stages of maturity. Anim. Feed Sci. Technol. 104, 215-225. [ Links ]
Harika, A.S. & Sharma, D.D., 1994. Quality and yield differences in maize stover due to varieties and stage of harvesting. In: Variation in the Quantity and Quality of Fibrous Crop Residues. Eds Joshi, A.L., Doyle, P.T. & Oosting, S.J., Proceedings of the National Seminar held at the BAIF Development Research Foundation, 8-9 February 1994, Pune (Maharashtra, India). pp. 20-28. [ Links ]
Jung, H.G., Valdez, F.R., Abad, A.R., Blanchette, R.A. & Hatfield, R.D., 1992. Effect of white rot basidiomycetes on chemical composition and in vitro digestibility of oat straw and alfalfa stems. J. Anim. Sci. 70, 1928-1935. [ Links ]
Kafilzadeh, F. & Maleki, E., 2011. Chemical composition, in vitro digestibility and gas production of straws from different varieties and accessions of chickpea. J. Anim. Physiol. Anim. Nutr. DOI: 10.1111/j.1439-0396.2011.01131.x [ Links ]
Kernan, J.A., Coxworth, E.C., Crowle, W.L. & Spurr, D.T., 1984. The nutritional value of crop residue components from several wheat cultivars grown at different fertilizer levels. Anim. Feed Sci. Technol. 11, 301-311. [ Links ]
Kossila, V.L., 1984. Location and potential feed use. In: Straw and Other Fibrous By-products as Feeds. Eds Sundstol l, F. & Owen, E., Elsevier, Amsterdam. pp. 4-24. [ Links ]
Larbi, A., Smith, J.W., Kurdi, I.O., Adekunle, I.O., Raji, A.M. & Ladipo, D.O., 1998. Chemical composition, rumen degradation, and gas production characteristics of some multipurpose fodder trees and shrubs during wet and dry seasons in the humid tropics. Anim. Feed Sci. Technol. 72, 81-96. [ Links ]
López, S., Davies, D.R., Giráldez, F.J., Dhanoa , M.S., Dijkstra, J. & France, J., 2005. Assessment of nutritive value of cereal and legume straws based on chemical composition and in vitro digestibility. Anim. Feed Sci. Technol. 85, 1550-1557. [ Links ]
López, S., Dhanoa, M.S., Dijkstra, J., Bannink, A., Kebrea, E. & France, J., 2007. Some methodological and analytical considerations regarding application of the gas production technique. Anim. Feed Sci. Technol. 135, 139-156. [ Links ]
Mathison, G.W., Soofi-Siawash, R., Okine, E.K., Helm, J. & Juskiw, P., 1999. Factors influencing composition and ruminal degradability of barley straw. Can. J. Anim. Sci. 79, 343-351. [ Links ]
Menke, K.H. & Steingass, H., 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28, 7-55. [ Links ]
Mould, F.L., Hervás, G., Owen, E., Wheeler, T.R., Smith, N.O. & Summerfield, R.J., 2001. The effect of cultivar on the rate and extent of combining pea straw degradability examined in vitro using the Reading pressure technique. Grass. Forage Sci. 56, 374-382. [ Links ]
Mulholland, J.G., Coombe, J.B. & Mcmanus, W.R., 1994. Intake and live weight response of sheep fed three ground and pelleted cereal straw. Aust. Exp. Agric. Anim. Husb. 14 (69), 449-453. [ Links ]
Nsahlai, I.V., Siaw, D.E.K.A. & Osuji, P.O., 1994. The relationship between gas production and chemical composition of 23 browses of the genus Sesbania. J. Sci. Food Agric. 65, 13. [ Links ]
Oba, M. & Allen, M.S., 1999. Evaluation of the importance of NDF digestibility: effects on dry matter intake and milk yield of dairy cows. J. Dairy. Sci. 82, 589-596. [ Links ]
Pearson, R.A., Archibald, R.F. & Muirhead, R.H., 2001. The effect of forage quality and level of feeding on digestibility and gastrointestinal transit time of oat straw and alfalfa given to ponies and donkeys. Br. J. Nutr. 85, 599-606. [ Links ]
SAS, 2002. Statistical Analyses Systems Users Guide. SAS Institute Inc., Cary, N.C., USA. [ Links ]
Tamm, I., 2003. Genetic and environmental variation of grain yield of oat varieties. Agron. Res. 1, 93-97. [ Links ]
Theander, O. & Aman, P., 1984. Anatomical and chemical characteristics. In: Straw and other Fibrous Byproducts as Feed. Eds Sundstol, F. & Owen, E., Elsevier, Amsterdam. pp. 45-78. [ Links ]
Theodorou, M.K., Williams, B.A., Dhanoa, M.S., McAllan, A.B. & France, J., 1994. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 48, 185-197. [ Links ]
Tilley, J.M.A. & Terry, R.A., 1963. A two-stage technique for the in vitro digestion of forage crops. J. Br. Grassl. 18, 104-111. [ Links ]
Tolera, A. & Sundstól, F., 1999: Morphological fractions of maize stover harvested at different stages of grain maturity and nutritive value of different fractions of the stover. Anim. Feed Sci. Technol. 81, 1-16. [ Links ]
Tolera, A., Tsegaye, B. & Berg, T., 2008. Effects of variety, cropping year, location and fertilizer application on nutritive value of durum wheat straw. J. Anim. Physiol. Anim. Nutr. 92, 121-130. [ Links ]
Van Soest, P.J., Robertson, J.B. & Lewis, B.A., 1991. Methods of dietary fiber, neutral detergent fiber and non-starch polysaccharide in relation to animal nutrition. J. Dairy Sci. 74, 3583-3597. [ Links ]
White, L.M., Hartman, G.P. & Bergman, J.W., 1981. In vitro digestibility, crude protein, and phosphorus content of straw of winter wheat, spring wheat, barley, and oat cultivars in eastern Montana. Agron. J. 73, 117-121. [ Links ]
Copyright resides with the authors in terms of the Creative Commons Attribution 2.5 South African Licence. See: http://creativecommons.org/licenses/by/2.5/za
Condition of use: The user may copy, distribute, transmit and adapt the work, but must recognise the authors and the South African Journal of Animal Science.
# Corresponding author: kafilzadeh@razi.ac.ir














