Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Animal Science
versión On-line ISSN 2221-4062
versión impresa ISSN 0375-1589
S. Afr. j. anim. sci. vol.39 no.5 Pretoria ene. 2009
Effect of All-lac xcl 5x, Acid-pak 2x, Bio-mos® and Zinc Bacitracin on nutrient digestibility and gastrointestinal morphology of broiler chickens
A.M. Ngxumeshe#; R.M. Gous
Animal and Poultry Science, School of Agriculture and Agribusiness, University of KwaZulu-Nata, Scottsville 3209, South Africa
ABSTRACT
An experiment was conducted with Ross broiler chickens from day-old to 42 d of age. A prebiotic (Bio-Mos®), probiotic (All Lac XCL 5x), organic acid (Acid pak 2x), individually or in combination were used to supplement an antibiotic growth promoter (Zinc Bacitracin). The chickens were challenge with Clostridium perfringens (CP) at 21, 22 and 23 days of age to determine the efficacy of these additives for replacing antibiotics in hindering the effects of CP on the villus surface area. Feed additives in this experiment prevented the negative effects of CP as the treated birds did not have lesions on their villus surfaces.
Keywords: Clostridium perfringens, prebiotic, probiotic, organic acid, broiler chicken, villus surface area
Introduction
The chicken's gastro-intestinal tract is simply composed of stomach, small intestine, and large intestine. Digestion of feedstuffs must be completed before reaching the ileo-caecal junction and the nutrients must be absorbed across the villi to satisfy the nutritional needs of the animal. Any undigested feed residue passing into the colon will be used as a substrate by the microflora (Bedford, 2000; Hong, 2002). This will decrease the ratio of beneficial to pathogenic microflora, which in turn will result in problems such as necrotic enteritis (NE), caused by Clostridium perfringens (Wilson et al., 2005). Necrotic enteritis suppresses chick growth by interfering with the absorption process in the intestine and is responsible for high mortalities in the broiler industry. The removal of antibiotic growth promoters in the animal industry has aggravated the risk of this disease. Therefore, there is a clear need for safe alternatives to antibiotic growth promoters in the poultry industry. Owens et al. (2003) showed that when yeast extract, organic acids and enzyme were added in combination they behaved synergistically. The aim of this study was to evaluate the effect of All-Lac XCL 5x (a probiotic), Acid-pak 2x (an organic acid) and Bio-Mos® (prebiotic), either individually or in combination, and compare them with a commonly used antibiotic (Zinc Bacitracin), on controlling NE caused by C. perfringens on the broiler chicken villi.
Materials and Methods
A randomised block design was used. There were four blocks, which were chosen down the length of the house, to account for variation in temperature that prevails in a longitudinally ventilated house. The pens in the house measured 5 m2, yielding a stocking density of 10-birds/m2 at six weeks of age. Temperature was reduced stepwise from 31 ºC at day-old to 21 ºC at day 21, and maintained there for the rest of the experimental period. The humidity in the house was dependent on exchange of air provided by the tunnel ventilation system.
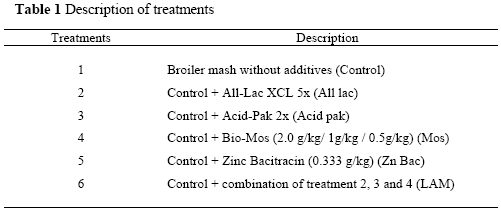
Two thousand eight hundred and eighty sexed day-old Ross chicks were used for this study. The chicks were randomly allocated to 48 pens in groups of 60 and assigned to one of the six diets; the control diet with no feed supplements and five other diets contained supplements (Table 1). Each treatment was replicated eight times.
Clostridium perfringens was cultured by Dr Roger Horner of Allerton Regional Laboratories, and used to challenge the birds at 21 d of age. The feed consumption of birds on the day prior to the first inoculation was measured (feeders in six pens were weighed) and 85% of that quantity of feed for each inoculation day was used. Inoculation was performed twice a day, three days in a row, via the feed (inoculation at 09:30 and at 14:00). Food was withdrawn from the feeders at least one hour before inoculation, so that all the chickens eat simultaneously. Half the calculated daily feed quantity was used for each inoculation.
The feed and broth were blended manually into plastic buckets. Blending was rapidly done for a maximum time of five minutes and the feed was delivered to the relevant pens quickly, to ensure that the bacterium was still active when consumed by the birds. The quantity of each feed was weighed out ahead of time, to minimize that blending time. In the evening the feeders were topped up with sufficient food to ensure an ad libitum supply during the night.
For each feed inoculation approximately 20 mL of a C. perfringens culture containing 1.1 x 10 to the 10 bacteria per mL (to allow 20 x 4 twice daily) was used. The 20 mL was diluted in an isotonic saline (0.9% Na) to bring it to 1200 mL and each of the pens was inoculated with 100 mL of that solution.
At the end of the experiment one chick was sacrificed and dissected to expose the visceral organs. A piece of the jejunum (part of the intestinal tract extending from the ligament of Treitz to Meikel's diverticulum) was removed for analysis. The tissue was cut into pieces of 3 cm and fixed in gluteraldehyde. The tissues were further cut into pieces of 3 mm and were transferred into cacodylate buffer twice for 30 minutes. Thereafter the tissues were fixed in 2% osmium for two hours and then rinsed twice with a cacodylate buffer for 30 minutes. The tissues were dehydrated successively with 30%, 50%, 70%, 80%, 90% and three times with 100% ethanol for 10 minutes each. The tissues undergone critical drying in a critical point dryer at 33 ºC and 70 atm for 1.5 h and thereafter mounted on slides and viewed on a microscope and digitised.
The data were analysed for significant differences using the analysis of variance of Minitab (Minitab, 2000). The differences between the mean values were determined by the use of least significant difference (P <0.05).
Results and Discussions
The surface area of the villi at 21 d and 42 d of age is presented in Figure 1. There were no lesions observed before the challenge by Clostridium perfringens (21 d-old, C1 and E1). After the challenge (42 d-old, C2 and E2) the control group (C2) showed lesions on the surface area, which were not observed in other treatments. At the younger age the villus is bent and thin but at the old age it is thicker and straight. The present study was designed to test whether the different feed additives replacing antibiotics can effectively hinder the negative effects of Clostridium pefringens on the GIT of the chickens. After the challenge by CP the chickens on unsupplemented diet have their villus damaged (C2). Therefore the feed additives (Bio-mos, All lac XCL, Acid Pak and Zinc Bacitracin) were effective in hindering the ulcerating effect of CP on the intestines of the chickens. These results are in agreement with findings by (Hofacre et al., 2003). The bending of the villus at a young age might be due to them not yet matured. The thinning of the villi surface is good for digestion and absorption purposes (NRC, 1984).
Conclusions
The results from this study demonstrated that antibiotics could be replaced by Bio-Mos®, Acid pak and All-Lac in preventing the ulcerating effects of Clostrium perfringens. Bio-Mos also resembled antibiotic treatment in thinning the villi so as to improve the digestive/absorptive site.
References
Bedford, M.R., 2000. Exogenous enzymes in monogastric nutrition-their current value and future benefits. Anim. Feed Sci. Technol. 86, 1-13. [ Links ]
Hofacre, C.L., Beamaize, T., Collett, S. & Mathis, G., 2003. Using competitive exclusion, mannan-oligosaccharide and other intestinal products to control necrotic enteritis. J. Appl. Poult. Res. 12, 60-64. [ Links ]
Hong, D., Burrows, H. & Adeola, O., 2002. Addition of enzyme to starter and grower feeds for ducks. Poult. Sci. 81, 1842-1849. [ Links ]
Minitab., 2002. Minitab release 13.1. Minitab Inc. State College, P.A., USA. [ Links ]
NRC, 1984. Nutrient Requirements of Poultry. 8th ed. National Academy Press, Washington D.C.USA. [ Links ]
Owens, B., Ellis, S.M. & Mcckracken, K.J., 2003. Effects of yeast extracts, with and without oxygenisedTM water, on the performance and gut histology of broiler chickens. Br. Poult. Sci. 44, S21-2. [ Links ]
Wilson, J., Tice, G., Brash, M.L. & St. Hilaire, S., 2005. Manifestations of Clostridium perfringens and related bacterial enteritides in broiler chickens. World's Poult. Sci. J., 61, 435-49. [ Links ]
# Corresponding author. Email: ngxumeshea@arc.agric.za














