Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.35 no.3 Pretoria 2005
Effect of two commercial preparations of condensed tannins on the survival of gastrointestinal nematodes of mice and goats in vitro
R.A. MaxI, II, #; D. WakelinI; J. CraigonI; A.A. KassukuII; A.E. KimamboII; L.A. MtengaII; P.J. ButteryI
IUniversity of Nottingham, School of Biosciences, Sutton Bonington Campus, Loughborough, Leicestershire, LE12 5RD, United Kingdom
IISokoine University of Agriculture, P.O. Box 3017, Morogoro, Tanzania
ABSTRACT
In vitro studies were carried out to investigate anthelmintic activities of two commercial tannin preparations, quebracho (QT) and wattle (WT), against a freshly isolated mouse nematode (Heligmosomoides polygyrus) and goat nematodes (Haemonchus contortus and Oesophagostomum columbianum). The worms were incubated at 38 - 39 °C in culture media containing varying concentrations of the tannins. Worm activity was monitored for 48 h and the time taken for individual worms to die was recorded. Survival of the nematodes was significantly reduced by the addition of the tannin preparations to the culture media. Addition of polyethylene glycol (PEG), which binds and inactivates tannins, to media containing tannins significantly increased the survival time of the worms, suggesting that the toxicity of the extracts was at least partially due to the tannins. Worms dying in cultures containing QT or WT had darkly stained guts, an indication that death was due to ingestion of the tannins in the media. These results support the hypothesis that tannins in commercial extracts could have direct toxic effects on parasitic nematodes.
Keywords: Tannins, survival assay, mouse and goat nematodes
Introduction
The ever-increasing problem of anthelmintic resistance (Prichard, 1994) and concern about chemical residues in the environment add impetus to the continuous search for helminth control strategies that are less reliant on chemotherapeutics (Gill & Le Jambre, 1996). Also, poor availability and unaffordable costs of synthetic anthelmintic drugs particularly to resource-poor farmers in the developing countries have exacerbated helminth control strategies based on chemical anthelmintics (Hammond et al., 1997). Gastrointestinal (GI) infections associated with parasitic nematodes are among the most important factors limiting production of small ruminants raised in the tropical and subtropical regions of the world (Waller, 1997). If effective, the use of naturally occurring substances/agents as alternative measures to control these parasites could prove to be less expensive and potentially sustainable. Anthelmintics of plant origin have long been known in human medicine but more work is needed to validate their use for veterinary purposes. Field studies by Niezen et al. (1993; 1998) demonstrated that forages rich in condensed tannins (CT) improved the general performance of sheep infested with worms by reducing their worm burden. It has been shown that the dietary inclusion of CT in the form of a quebracho extract could significantly reduce parasitic egg output and the worm burden of sheep infested with Trichostrongylus colubriformis (Athanasiadou et al., 2000; Butter et al., 2000) and Haemonchus contortus (Max et al., 2005). In several in vitro studies tannin extracts from different plant species have been shown to exhibit inhibiting effects on different stages of GI nematode development. Decreased larval motility (Paolini et al., 2004) and the death of nematodes due to direct toxic effects of CT (Molan et al., 2000b) have been reported. Despite the clear association between CT intake and improved animal performance during nematode infestation, the mode of action and efficacy of CT is still equivocal. Therefore, an in vitro study was conducted to test the activity against gastrointestinal nematodes of two readily available sources of CT, quebracho tannin and wattle tannin.
Materials and Methods
Two commercial tannin preparations, quebracho (QT) and wattle (WT) extracts were obtained as single batches from Hodgesons Chemicals Ltd., UK and Wattle Tannin Company Ltd., Tanzania, respectively. Quebracho tannin is a reddish brown powder extracted from the heartwood of the tropical tree species, Schinopsis balansae, and according to the supplier, one kg contains ca. 730 g CT, 190 g simple phenolics and 80 g water. Wattle extract resembles QT in colour and is extracted from the bark of a tropical tree species, Acacia mearnsii, and contains ca. 700 g CT/kg. Both these commercial preparations are used in the leather industry.
The intestinal nematode, Heligmosomoides polygyrus, of adult mice, and abomasal (H. contortus) and large intestinal (Oesophagostomum columbianum) nematodes from goats were used in the study. A population of H. polygyrus was established in a colony of mice kept at the University of Nottingham, UK. Each time a worm survival assay (Table 1) was to be performed, two or three mice were humanely killed and adult worms were extracted immediately from the small intestines. The worms were suspended in freshly prepared lukewarm Hanks' balanced salt solution (HBSS) and used within three hours in the survival assay. Adult H. contortus and O. columbianum were maintained as a mixed infestation in goats kept at the Sokoine University of Agriculture, Tanzania. A goat was slaughtered humanely to supply worms for each in vitro assay. At slaughter, the entire gastrointestinal tract was removed and ligatures were applied to isolate the abomasum and the large intestine. The content of each compartment was emptied into a separate container and washed gently with lukewarm water through a 0.2 mm aperture sieve to retain the worms while reducing the amount of debris. The worms were picked from the retained debris using a mounted needle, and placed in Petri dishes containing lukewarm phosphate buffered saline (PBS). The entire procedure was carried out within 30 - 45 minutes of slaughter to ensure that the worms were viable and active prior to the survival assay.

Culture media were prepared by dissolving one of the commercial tannin extracts (QT or WT) in phosphate buffered saline (PBS at ~40 °C). Polyethylene glycol (PEG: molecular weight 3000-4000, Fisher Scientific UK, Ltd) was included in some of the media for each tannin source to test the effect of the tannin in the presence or absence of PEG. PEG binds to and inactivates CT. Therefore differences between the toxicity of a tannin preparation without PEG and one with PEG would indicate that toxicity was responding to the media's CT content. Three sets of culture media were prepared. Set 1: Tannin alone at 0, 1, 2, 4, 8 and 12 g/100 mL, Set 2: PEG alone at 0, 1.4, 2.8 and 5.6 g/100 mL and Set 3: QT or WT with PEG at 0 tannin [0 PEG], 1 [1.4], 2[2.8] and 4[5.6] g/100 mL of final solution. The amount of PEG in the media was based on the suggestion that ca. two parts by weight of PEG are required to neutralise one part of CT (Barry & Forss 1983; Yu et al., 1995). The two extracts were assumed to contain ca. 700 g CT/kg. Addition of PEG to culture media containing CT resulted in cloudy solutions that made it difficult to see the worms under a microscope. Thus, the effect of PEG was not tested in media containing 8 or 12 g tannin/100 mL and all the remaining test media were centrifuged at 3000 x g for 12 min to obtain clear supernatants prior to being used in the survival assay.
Size and the presence of copulatory aids or eggs in utero were used to determine if the worms were mature and their sex. A known number of male and female worms (10 to 15 of each) was placed in 20 mL of freshly prepared culture medium in duplicate Petri dishes and incubated at 37-39 °C for ca. 48 h. The effect of a particular medium on motility and survival of the worms was monitored under a stereomicroscope.
Motility and death were assessed by gently prodding the worms using a pointed probe. Worms were considered biologically dead when there was little or no reaction to touch. The number of dead worms was recorded after 0.5, 1, 2, 4, 6, 8, 10 h and four-hourly thereafter. Dead worms were removed from the medium as soon as they were identified. In addition, 10 male and female worms each were incubated in media containing 0, 2 and 12 g QT/100 mL for 22 hours and immediately preserved in formal-saline (10% formaldehyde (v/v) in normal saline) for histological examination. The preserved worms were prepared for histological sectioning as described by (Wilson & Gamble, 2002) to determine if the worms had ingested the tannin-containing medium during the incubations.
The data were analysed using the Genstat, (release 6.1) statistical package (GenStat, 2002). The effect of tannin concentration on the survival time of the worms was analysed using survival analysis for censored data. A censoring approach was adopted because some of the worms were still alive after the incubation period. For each worm, one time, either the time of death or the last time it was seen alive, was recorded and each time given a censoring score of 0 if it was a time of death and 1 if the worm was still alive. A generalised linear model assuming Poisson errors and a logarithm link were fitted to the censor scores with log(time) as an offset and the treatment groups as explanatory variables (Crawley, 1993). The Weibull survival distribution:
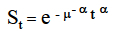
where St = survival proportion at time t
e = the base of natural logarithms (2. 718282)
|μ = the mean survival time and
α = a constant that defines the shape of the survival curve,
was used because it takes into consideration that a range of survivorship curve shapes exists in nature. The linear model was also used to predict estimated mean values of the response variable, survival time, with their standard errors (s.e.). The values and the s.e. were back-transformed to calculate combined parameter values and their 95% confidence limits.
Results
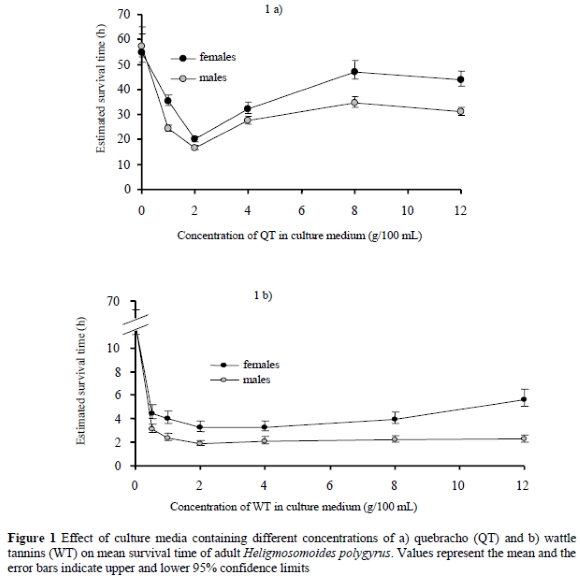
Estimated mean survival times of the adult nematode, H. polygyrus, in culture media containing varying concentrations of QT and WT are shown in Figures 1a & b, respectively. Worms incubated in the control medium (PBS) had estimated average survival times of 55 and 57 hours for the males and females, respectively. Addition of tannin to the culture media at all concentrations significantly (P < 0.001) reduced survival time compared to that of the controls. The average survival times of H. polygyrus in culture media containing 2 g QT/100 mL and 2 g WT/100 mL, were 18 and 3 h respectively, indicated that WT was approximately six times more potent than QT. An unexpected trend associating survival time and tannin concentration in the media was observed. Worm survival dropped, with increasing concentration of tannin, to the lowest value in cultures containing 2 g/100 mL and then started to increase (QT) or level off (WT) as the concentration increased above this value.
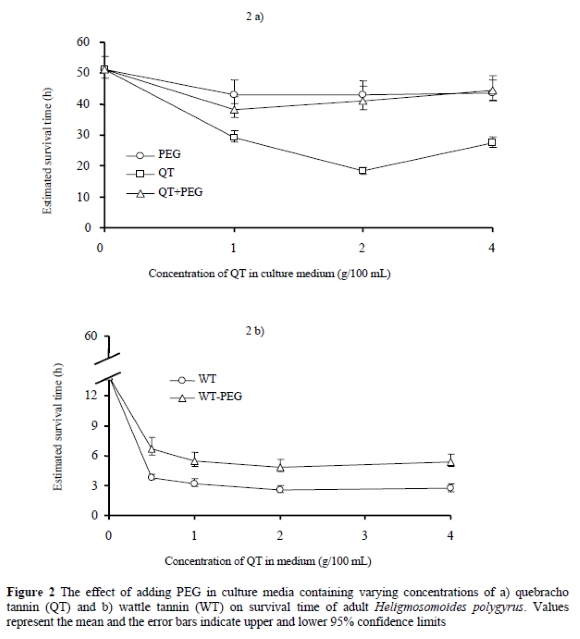
Addition of the condensed tannin deactivator, PEG, in the test media containing tannins at 1, 2 and 4 g/100 mL significantly (P < 0.05) prolonged the survival time of worms by averages of 65% and 83% for QT and WT, respectively (Figures 2a & b). There was no significant difference (P > 0.05) in survival times (which ranged from 42.9 - 43.7 h) among the three concentrations of PEG for media containing PEG alone without QT
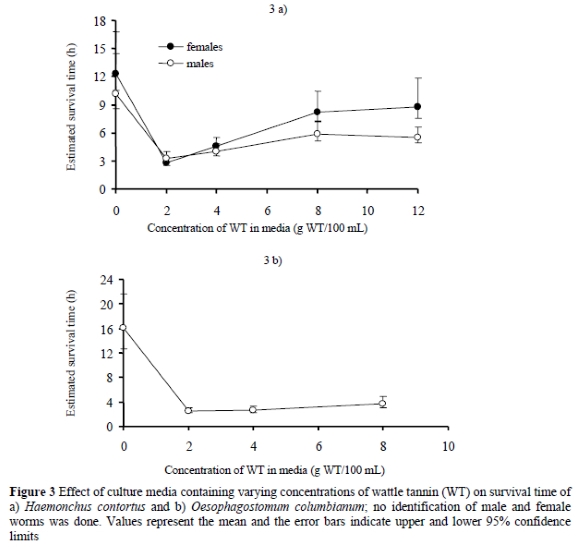
Dead worms from QT-containing media stained darker with haematoxylin-eosin than the worms incubated in the control media. Examination of worm sections showed that the gut contents of worms incubated in QT-containing media stained deep purple whereas the contents in the control worms stained pink. The effect of WT on the survival of the goat nematodes (H. contortus and O. columbianum) is shown in Figures 3a & b, respectively. The two species of nematodes, H. contortus and O. columbianum, were capable of surviving outside their host in a simple control culture medium for 11 and 16 h, respectively. Addition of WT in the media reduced (P < 0. 001) the survival of both worm species at all concentrations tested. Culture media containing 2 g WT/100 mL were the most toxic with an indication that toxicity decreased as the concentration of tannin increased. This effect of the higher concentrations of the WT was less than that seen with QT.
Apart from significant reductions in survival times, some features were common to all worms tested regardless of the type of tannin used. For instance survival of the worms was influenced by sex (survival time x sex: P < 0.05, Figures 1 & 2b). Females had longer survival times than their male counterparts. The behaviour of worms as they were introduced into tannin-containing media was similar in that motility increased sharply with worms uncoiling rapidly; an activity pattern not observed in the control media. Motility continued to diminish with time to a moribund stage and finally death.
Discussion
The in vitro studies demonstrated that the two sources of CT had varying degrees of anthelmintic activity against the tested nematodes, H. polygyrus, H. contortus and O. columbianum, as judged by reduction in worm survival times. The toxicity of WT against H. polygyrus was found to be about six times more potent than that of QT. In general, the present findings are consistent with those from several other studies investigating the effect of CT on parasitic nematodes (Molan et al., 2000a; Athanasiadou et al., 2001; Butter et al., 2001; Molan et al., 2003; Paolini et al., 2004). Culture media containing 2 g/100 mL of either QT or WT were found to be more effective in reducing worm survival than where higher or lower concentrations were tested. This resulted in positive correlations between worm survival time and tannin concentrations of 2 g/100 mL and above as follows: H. polygyrus in QT-containing media (R2 = 0.69, P = 0.17) and WT (R2 = 0.95, P = 0.03) as well as H. contortus in WT-containing media (r2 = 0.88, P = 0.06). This effect was more pronounced with QT than with WT.
The positive correlation between survival time and tannin concentration was an interesting and unexpected finding, which implied that after a maximum effect at 2 g/100 mL, survival increased with increasing CT levels in the culture medium. This was contrary to results from other studies (Molan et al., 2000a; Butter et al., 2001) in which CT reduced survival in a dose-dependent manner at all concentrations used. However, a tendency whereby toxicity decreases as concentration of the test drug increases has been observed with conventional anthelmintics in vitro and it is referred to as a 'recovery phenomenon'. Recovery from paralysis when adult N. brasiliensis or H. contortus larvae were incubated in increasing concentrations of levamisole has been reported (Coles et al., 1975; Coles et al., 1988). Also by using a larval development assay, Kotze et al. (1999) observed a significant recovery and continued development of various T. colubriformis and H. contortus larvae at high levamisole and pyrantel concentrations, resulting in a parabolic dose-response curve. Reasons for the recovery phenomenon are not clear but Coles et al. (1975) suggested receptor involvement leading to very rapid development of tolerance or immunity to the effects of a drug. An alternative explanation for the current observations could be that the tannins caused the worms to ingest less QT or WT at higher concentrations.
The dramatic increase in survival time of worms when PEG was added to the tannin-containing media indicated that CT in the extracts contributed to the observed toxicity. Similar results were reported by Molan et al. (2000b) who demonstrated that PEG was capable of eliminating up to 90% of the CT activity against migrating nematode larvae. Paoloni et al. (2004) also observed significant restorations in larval migration and motility of adult worms in tannin-containing extracts from hazel tree, oak and sainfoin when PEG was added. Differences in survival times between sexes were evident in the current experiments. We have no clear explanation for this observation, but the sheer difference in size between males and females suggests that the bigger female worms could have more energy reserves than males and are therefore better survivors. Worms incubated in media containing QT or WT were darkly stained compared to those from the control medium. Histological examination of these worms revealed that the colour was a result of staining in intestinal tissues rather than the cuticle. It is suggested that the worms died following ingestion of tannins present in the culture medium. The mechanisms involved in the toxicity of CT to nematodes have not been clearly established but a study investigating the effect of ellagitannin preparations on the free-living nematode, Caenorhabditis elegans, revealed some fatal disruption of internal organs including the gonads, uterine wall and the intestines (Mori et al., 2000). The affinity of CT for proteins has led to a suggestion that tannins could interfere with some enzymatic activities that are vital to the survival of the nematodes. Extracts from four Nigerian plants used as traditional medicines against GI nematodes found in animals and man were tested on two recombinant nematode glutathione S-transferases (GST) (Fakae et al., 2000). The results showed that all plants contained heat-stable inhibitory activities against the GSTs in vitro but the two most potent plant species contained significant amounts of CT.
This study demonstrated that QT and WT were toxic to mouse and goat nematodes in vitro. The histological findings also suggested that worms were affected after ingesting the media containing tannins; the latter could have disrupted vital metabolic processes or organs leading to death. Tanniniferous plants and commercial tannin preparations are readily available in the tropics. In vivo studies to investigate the potential of using tannins as a novel alternative in the control of nematode infections of sheep and goats in the tropics are underway.
Acknowledgements
The United Kingdom Department of International Development (DFID) funded this work for the benefit of developing countries. The views expressed are not necessarily those of DFID.
References
Athanasiadou, S., Kyriazakis, I., Jackson, F. & Coop, R.L., 2000. Effects of short-term exposure to condensed tannins on adult Trichostrongylus colubriformis. Vet. Rec. 146, 728-732. [ Links ]
Athanasiadou, S., Kyriazakis, I., Jackson, F. & Coop, R.L., 2001. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: in vitro and in vivo studies. Vet. Parasitol. 99, 205-219. [ Links ]
Barry, T.N. & Forss, D.A., 1983. The condensed tannin content of vegetative Lotus pedunculatus, its regulation by fertiliser application, and effect upon protein solubility. J. Sci. Food Agric. 34, 1047-1056. [ Links ]
Butter, N.L., Dawson J.M., Wakelin, D. & Buttery, P.J., 2000. Effect of dietary tannin and protein concentration on nematode infection (Trichostrongylus colubriformis) in lambs. J. Agric. Sci., Camb. 134, 89-99. [ Links ]
Butter, N.L., Dawson J.M., Wakelin, D. & Buttery, P.J., 2001. Effect of dietary condensed tannins on gastrointestinal nematodes. J. Agric. Sci., Camb. 137, 461-469. [ Links ]
Coles, G.C., East, J.M. & Jenkins, S.N., 1975. The mechanism of action of the anthelmintic levamisole. Gen. Pharmac. 6, 309-313. [ Links ]
Coles, G.C., Tritschler II, J.P., Giordano, D.J., Laste, N.L. & Schmidt, A.L., 1988. Larval development test for the detection of anthelmintic resistant nematodes. Res. Vet. Sci. 45, 50-53. [ Links ]
Crawley, M.J., 1993. Survival analysis. In: GLIM for Ecologists. Eds. Lawton, J.H. & Likens, G.E., Blackwell Science. pp. 314-331. [ Links ]
Fakae, B.B., Campbell, A.M., Barrett, J., Scott, I.M., Teesdale-Spittle, P.H., Liebau, E. & Brophy, P.M., 2000. Inhibition of glutathione S-transferases (GSTs) from parasitic nematodes by extracts from traditional Nigerian medicinal plants. Phytotherapy Res. 14, 630-634. [ Links ]
GenStat, 2002. GenStat Release 6.1 user's guide. Lawes Agricultural Trust, Rothamsted Experimental Station, United Kingdom. [ Links ]
Gill, H.S. & Le Jambre, L.F., 1996. Preface - Novel approaches to the control of helminth parasites of livestock. Intl. J. Parasit. 26, 797-798. [ Links ]
Hammond, J.A., Fielding, D. & Bishop, S.C., 1997. Prospects for plant anthelmintics in tropical veterinary medicine. Vet. Res. Comm. 21, 213-228. [ Links ]
Kotze, A.C., Stein, P.A. & Dobson, R.J., 1999. Investigation of intestinal nematode responses to naphthalophos and pyrantel using a larval development assay. Intl. J. Parasit. 29, 1093-1099. [ Links ]
Max, R.A., Wakelin, D., Dawson, J.M., Kimambo, A.E., Kassuku, A.A., Mtenga, L.A. & Buttery, P.J., 2005. Effect of quebracho tannin on faecal egg counts, worm burdens and performance of temperate sheep with experimental nematode infections. J. Agric. Sci., Camb. (in press). [ Links ]
Molan, A.L., Hoskin, S.O., Barry, T.N. & McNabb, W.C., 2000a. Effects of condensed tannins extracted from four forages on the viability of the larvae of deer lungworms and gastrointestinal nematodes. Vet. Rec. 147, 44-48. [ Links ]
Molan, A.L., Meagher, L.P., Spencer, P.A. & Sivakumaran, S., 2003. Effect of flavan-3-ols on in vitro egg hatching, larval development and viability of infective larvae of Trichostrongylus colubriformis. Intl. J. Parasit. 52, 209-218. [ Links ]
Molan, A.L., Waghorn, W.C., Min, B.R. & McNabb, W.C., 2000b. The effect of condensed tannins from seven herbages on Trichostrongylus colubriformis larval migration in vitro. Folia Parasitologica 47, 34-44. [ Links ]
Mori, T., Mohamed, A.S.A., Sato, M. & Yamasaki, T., 2000. Ellagitannin toxicity in the free-living soil-inhabiting nematode, Caenorhabditis elegans. J. Pestic. Sci. 25, 405-409. [ Links ]
Niezen, J.H., Robertson, H.A., Waghorn, G.C. & Charleston, W.A.G., 1998. Production, faecal egg counts and worm burdens of ewe lambs which grazed six contrasting forages. Vet. Parasit. 80, 15-27. [ Links ]
Niezen, J.H., Waghorn, T.S., Waghorn, G.C. & Charleston, W.A.G., 1993. Internal parasites and lamb production - a role for plants containing condensed tannins? Proc. N. Z. Soc. Anim. Prod. 53, 235-238. [ Links ]
Paolini, V., Fouraste, I. & Hoste, H., 2004. In vitro effects of three woody plant and sainfoin extracts on 3rd stage larvae and adult worms of three gastrointestinal nematodes. Parasitology 129, 69-77. [ Links ]
Prichard, R.K., 1994. Anthelmintic resistance. Vet. Parasit. 54, 259-268. [ Links ]
Waller, P.J., 1997. Nematode parasite control of livestock in the tropics/subtropics: the need for novel approaches. Intl. J. Parasit. 27, 1193-1201. [ Links ]
Wilson, I. & Gamble, M., 2002. The haematoxylins and eosin. In: Theory and Practice of Histological Techniques (5th ed.). Eds. Bancroft, J.D. & Gamble, M., Churchill Livingstone, London. pp. 125-138. [ Links ]
Yu, F., Mcnabb, W.C., Barry, T.N. & Waghorn, G.C., 1995. Effect of condensed tannin in cottonseed hulls upon the in vitro degradation of cottonseed kernel proteins by rumen micro-organisms. J. Sci. Food Agric. 69, 223-234. [ Links ]
# Corresponding author. E-mail: romso@yahoo.com
# Present address: Sokoine University of Agriculture, P.O. Box 3017, Morogoro, Tanzania














