Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.35 n.2 Pretoria 2005
Effect of method of sample preparation on ruminai in situ disappearance of dry matter and nitrogen in annual ryegrass in dairy cows
B.J. van der MerweI, *; T.J. DugmoreI, #; J.B.J. van RyssenII; L.M. ThurtellI; S.J. MorningI
IKwaZulu-Natal Department of Agriculture and Environmental Affairs, Private Bag X9059, Pietermaritzburg 3200, South Africa
IIDepartment of Animal & Wildlife Sciences, University of Pretoria, Pretoria 0002, South Africa
ABSTRACT
The objective of this study was to investigate the effect of method of sample preparation on the degradation kinetics of herbage when applying the in situ technique. Ryegrass (Lolium multiflorum cv. Midmar) was harvested at three and four weeks after cutting and fertilizing with 200 kg nitrogen (N)/ha. Freshly cut herbage was used to investigate the following four sample preparation methods. In trial 1, herbage was (1) chopped with a paper-cutting guillotine into 5-10 mm lengths, representing fresh (FR) herbage; (2) chopped and stored frozen at -20 °C in a chest freezer, representing frozen (FN) herbage; (3) chopped, stored frozen at -20 °C for 12 h followed by freeze drying for 48 h and thereafter milled through a 2 mm screen, representing freeze dried (FD) herbage and (4) chopped and oven dried at 65 °C for 48 h and thereafter milled through a 2 mm screen, representing oven dried (OD) herbage. Prepared samples were incubated in three rumen cannulated, lactating Holstein-Friesian cows. The dry matter (DM) and N loss (%) of the FR, FN, FD and OD herbage differed significantly at 0 and 5 hours incubation but not at 24 or 48 hours. Sample characteristics (particle size, wet or dry sample) clearly influenced the degradability of herbage DM and N. Frozen herbage showed similar degradation characteristics to fresh herbage. In trial 2, ryegrass samples were prepared by the same four methods, but at a standardized particle size, namely, chopped into 5-10 mm lengths. The treatments were: chopped fresh, chopped frozen, chopped freeze dried and chopped oven dried. The N losses differed significantly between treatments for the 0, 5 and 10 hour incubations, but not at the 22 and 46 hour incubation periods. Although the a, b and c fractions differed significantly between treatments, the effective degradability did not differ for fresh, frozen and freeze dried chopped samples. Oven dried chopped samples had reduced effective degradability and are not recommended for determining effective degradability. It is proposed that fresh chopped herbage be used in degradability studies.
Keywords: Ruminal disappearance, degradability, nitrogen, annual ryegrass, dairy cows
Introduction
The in situ nylon bag technique, in which feed samples in nylon bags are suspended in the rumen, is widely used to estimate the rate and extent of degradation and digestion of feed in the rumen (Marinucci et al., 1992; NRC, 2001). At present this technique is used as the standard method for the estimation of ruminal degradation of protein in the protein evaluation systems of the USA, UK, Nordic countries and Australia (Van der Honing & Alderman, 1988; AAC, 1990; NRC, 2001), and is also used locally to estimate the protein degradability of various feed sources (Erasmus et al., 1990). The methodology used to generate degradability values has a major influence on degradability values published (Michalet-Doreau & Cerneau, 1991). Standardization of the method is related to a large number of factors causing variation, e.g. the bag (sample dry matter, bag surface area to sample weight ratio and cloth pore size), sample preparation (particle size of sample), pre-incubation treatment, washing procedure, diet of fistulated animals, correcting for microbial contamination of residues, etc. (Lindberg, 1985; Nocek, 1988; Vanzant et al., 1998). In various reports recommendations have been made for the standardization of the technique (Huntington & Givens, 1997; Vanzant et al., 1998; NRC, 2001). These recommendations focus mainly on dry feeds.
There exists considerable variation in the methods of preparation of fresh herbage by different researchers studying rumen degradation kinetics. Although a masticated sample would be the most accepted form, representing selected and consumed herbage, the oesophageal sampling technique is very demanding in terms of animal care, maintenance and extrusa processing (Coffey et al., 1991). Elizalde et al. (1999) chopped fresh frozen samples, after thawing, in a food chopper to simulate forage disruption due to chewing. However, the AFRC (1992) warned that care must be taken to avoid losses of juices. The use of chopped herbage samples with particle sizes ranging from 5 mm to 20 mm in length has been widely accepted. Herbage samples are either freshly chopped (AAC, 1990; Halliday, 1990; Phillips et al., 1991); stored frozen and chopped when frozen (Filmer, 1982; Petit & Tremblay, 1992; Valk et al., 1996) or chopped fresh, frozen and then thawed (Van Vuuren et al., 1990; 1991; Aumont et al., 1994) prior to rumen incubation. Other preparation methods commonly used are freezing of fresh herbage followed by freeze drying and milling through a 1 to 2 mm screen (Lindberg, 1985; Erasmus et al., 1990; Lopez et al., 1990; Wales et al., 1999) or oven drying of fresh herbage (40 to 100 °C) followed by milling (Ould-Bah & Michalet-Doreau, 1990; Aumont et al., 1994; Coblentz et al., 1998) prior to rumen incubation.
Recommendations differ regarding the preparation of fresh forage. The AFRC (1992), for instance, recommended the incubation of fresh or frozen samples, while Michalet-Doreau & Ould-Bah (1992) suggested that wet samples should be oven-dried at 60 °C or freeze dried. The objectives of this study were to investigate the effect of sample preparation method on the degradation kinetics of herbage and to propose a method, relevant to the herbage in question, as a means of standardizing sample preparation among research centres applying the in situ technique. It became apparent that the different sample lengths advocated in the methods tested in the first study were a confounding issue. Consequently, the study was repeated using a standardized chop length for the material under test for fresh, frozen, freeze dried and oven dried material.
Material & Methods
Three multiparous Holstein-Friesian cows were each fitted with a rumen cannula with an internal diameter of 80 mm (Beruc Equipment, P.O. Box 17260, Benoni 1500, RSA). The cows were artificially inseminated to obtain synchronized calving dates near the start of the ryegrass grazing season. The cows had an average post calving weight of 640 ± 34.2 kg and a body condition score (scale 0 to 5) of 2.5. The cannulated cows were kept within the dairy herd structure, and managed accordingly. They were separated from the herd only when nylon bags were inserted or withdrawn during rumen incubations. Cows were milked at 05:00 and at 14:00 and returned to pasture within two hours after the start of milking. The cows received a maize based concentrate mixture, comprising 80% maize and 20% groundnut oilcake, at 2% of body weight, plus 130 g of a mineral mixture per day as a top dressing. The concentrates were split into two feedings and offered individually after morning and afternoon milking to each cow in a post-parlour feeding system.
Herbage was collected from a small plot experiment carried out on a uniform sward of annual Italian ryegrass (Lolium multiflorum cv. Midmar) under irrigation to investigate the effect of N level and growth stage on the degradability of ryegrass. The present study utilized material from the high N treatment to determine the effect of sample preparation on N degradability as an addendum to the main study. The high N plots received fertilizer dressings of 200 kg N/ha per dressing and the low N treatment 50 kg N/ha per dressing, applied one, two, three and four weeks prior to harvesting, conducted six times over the growing season for the main study. In the middle of the growing season (August) the plot was mowed and fertilized with 200 kg N/ha and subsequently the three and four week regrowths were harvested by cutting the herbage at between 30 and 40 mm above ground level, using a hand-operated sickle bar mower for the present study. Ruminal degradation of the herbage dry matter (DM) and N was estimated through application of the in situ nylon bag technique, as described by AFRC (1992) with the following modification, namely that the bags were attached to a stainless steel disc as described by Erasmus et al. (1988).
Harvesting of the ryegrass took place between 07:30 and 08:00 on a Monday morning. The cut herbage was immediately taken to the laboratory, mixed and representative samples were prepared for rumen incubation. The herbage was chopped into 5 to 10 mm lengths with a paper-cutting guillotine. Four sample preparation methods (representing treatments) were followed: (1) chopped with a paper-cutting guillotine into 5 to 10 mm lengths, representing fresh (FR) herbage; (2) chopped and stored frozen at -20 °C in a chest freezer, representing frozen (FN) herbage; (3) chopped, stored frozen at -20 °C for 12 h followed by freeze drying for 48 h and thereafter milled through a 2 mm screen, representing freeze dried (FD) herbage and (4) chopped and oven dried at 65 °C for 48 h and thereafter milled through a 2 mm screen, representing oven dried (OD) herbage. The bags containing the FR herbage were suspended in the rumens at 09:00 on the day of harvesting, following preparation in the laboratory, while the FN, FD and OD herbage were incubated one week later. Frozen herbage was thawed for 1 h prior to rumen incubation.
A further study was undertaken in a subsequent season to investigate the effect of preservation method when using a standardised particle chop size. Ryegrass samples were prepared by the same four methods, but chopped into 5 to 10 mm lengths. The treatments were: chopped fresh (CFR), chopped frozen (CFN), chopped freeze dried (CFD) and chopped oven dried (COD).
Nylon cloth (ASTM 270-53, Rhologan Engineering, P.O. Box 125, Brakpan 1540, RSA) with an average pore size of 53 was used in the manufacture of the incubation bags. The bags were made as described by 0rskov et al. (1980) and had internal dimensions of 200 x 100 mm, with rounded corners and an effective surface area of ca. 400 cm2. In both studies, bags were filled with prepared herbage, equivalent to approximately 5 g herbage DM, representing a sample size of approximately 12.5 mg herbage DM/cm2 of bag surface area. Milled lucerne hay was used as a standard sample in both studies to provide a between assay control (Filmer, 1982; AAC, 1990). Runs were rejected if the standard sample values deviated by 10% from the mean determined for the standard. The standard lucerne samples had an average DM of 92%. Thus, approximately 5.5 g of standard sample were placed in bags to give ca. 12.5 mg DM/cm2 of bag surface area. The bags were tied to a stainless steel disc (ca. 90 g), as described by Erasmus et al. (1988), to suspend the bags in the rumen. Two bags containing standard sample were tied at the bottom of the disc and removed after 24 and 72 h, respectively. Samples of the differently prepared herbage samples were incubated for 5, 24 and 48 h in the first study and for 5, 10, 22 and 46 h in the second study, as it was found that there was insufficient residue left after 48 hours for chemical analyses. To ensure that sufficient residue would be available for chemical analyses up to 48 h, duplicate bags of FR and FN samples were incubated for the 48 h period, while, for the FD and OD samples, duplicate bags were incubated for the 24 h period and triplicate bags for the 48 h period. Two 700 mm nylon lines (Polyarn, 0.80 mm diameter with 27 kg breaking strength) were tied to each disc and secured to the cannula spacer ring at the other end.
In both studies, four bags per treatment were not incubated in the rumen, but were washed after filling to represent the zero hour incubation period. Bags were removed from the rumen after the respective incubation times, placed in a bucket of cold water to arrest microbial activity (AAC, 1990) and taken to the laboratory for further treatment. All the bags were washed and squeezed by hand under running cold water for 5 min (Lindberg, 1985). After excess water had been squeezed from the bags, they were oven dried at 65 °C for 48 h, followed by recording of the bag and residue weights. Where appropriate, multiple bags were pooled (within cow, incubation period and preparation method). All samples were milled through a 2 mm screen and placed in glass bottles, pending chemical analyses. Dried grass and residue samples were subjected to total Kjeldahl N analysis (AOAC, 1975).
The data from the different sample preparation methods in the first study is presented as percentage DM or N loss from the bags after incubation at 0, 5, 24 and 48 hours. The data from the second study comparing the preparation methods at a standardized chop length is presented as percentage N loss from the bags and the associated N degradabilities following incubation at 0, 5, 10, 22 and 46 hours. Although it is acknowledged that the amount of data points were limited, the N-disappearance percentages (indicative of the herbage degradation kinetics) with time were fitted to the following exponential model (0rskov & McDonald, 1979): degradability (p) = a+b [1 - exp(-ct)]. In this equation, p is the percentage herbage DM or N degraded after time t (hours), a is the soluble or rapidly degradable DM or N fraction and b is the insoluble but potentially degradable DM or N fraction, while c is the fractional rate constant, indicating the degradation rate (/h) of the b fraction. The maximum extent of degradation (total potentially degradable fraction) would be indicated by a + b.
The parameters, a, b and c, were obtained by fitting the data using a non-linear regression procedure, based on Marquardt's method, performed by the NONLIN procedure of Statgraphics 4.0 (Statsgraphics, 1988). The effective DM degradability (DM dg) and effective N degradability (N dg) values were then calculated with alternative fractional rumen outflow rates (k) according to the equation (0rskov & McDonald, 1979) dg = a + [(bc) + (c+k). Two alternative fractional outflow rates (0.05 and 0.08/h) were used in the calculations.
Analysis of variance was performed on disappearance data and model derived a, b, c and dg values using the ANOVA procedure of Statgraphics 4.0 (Statsgraphics, 1988). Differences between means were determined by the least significant difference (LSD) range test method. The LSD method was protected by F test (Snedecor & Cochran, 1980) and differences were only accepted as being significant when the overall F test was significant (P < 0.05). One-way analysis of variance was used to study the effect of sample preparation method on the mean parameter values and effective degradabilities (Genstat, 1993).
Results
The collected grass samples from the first study contained an average N concentration of 56 g/kg DM (350 g crude protein/kg DM). The DM disappearance data of the differently prepared three- and four-week regrowth herbage are presented in Table 1.
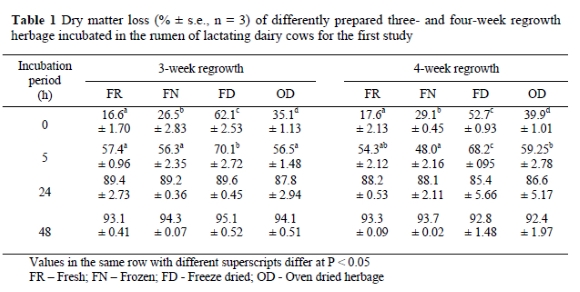
Herbage preparation method had a significant effect on the rumen disappearance of DM incubated for 0 and 5 h at both stages of regrowth (Table 1). The soluble DM fractions (0 h) were all significantly different from each other within three and four week regrowth herbage. Fresh herbage showed the lowest and FD herbage the highest a values. After 5 hours incubation the FD herbage had a significantly higher DM loss than the other methods for 3- and 4-week regrowth. Frozen and oven dried also differed significantly at the 5 h incubation at 4 week regrowth stage, but not at the 3-week regrowth stage. No significant differences were observed in the DM losses at the 24 and 48 h incubation periods.
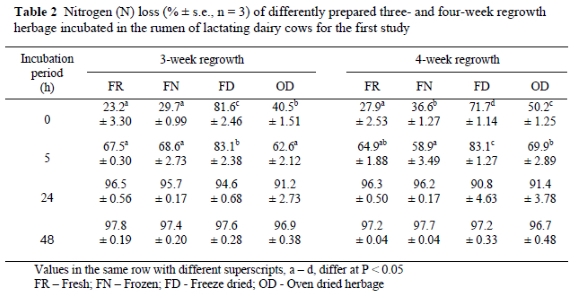
Herbage preparation method had a significant effect on the rumen disappearance of N incubated for 0 and 5 h at both stages of regrowth (Table 2). Although soluble N fractions were higher in FN compared to FR herbage, this difference was not significant for 3-week regrowth. For the 4-week regrowth samples, however, all the preparation treatments differed significantly from one another. The soluble N content of FD herbage was significantly higher than that of the other preparation methods within both three and four week regrowth stages. The N losses for the FD treatment were significantly higher than the other treatments after 5 hours incubation. Oven dried N losses were also higher than FN after 5 hours incubation for 4-week regrowth. No significant differences in N loss were recorded for the preparation methods at the 24 and 48 h incubations.
In the second study, the N content of the herbage collected was 49.5 g N/kg DM (309.4 g crude protein/kg DM). The N loss for the treatments (Table 3) differed significantly between all treatments at 0 h, while only the oven dried differed significantly at 5 hours. At the10 h incubation the frozen and freeze dried values did not differ significantly, but these differed from both the fresh and oven dried samples, with the fresh and oven dried also differing significantly. Differences were not observed in the 22 (P = 0.252) and 46 (P = 0.431) h incubations.
The non-linear parameters and effective N dg of four week regrowth herbage, prepared to represent chopped fresh (CFR), chopped frozen (CFN), chopped freeze dried (CFD), and chopped oven dried (COD) herbage are presented in Table 3. When standardizing sample length within the different preparation methods, preservation method still had a significant effect on the rumen parameters of herbage N. All four preservation methods had different (P < 0.05) soluble N fractions and insoluble but potentially degradable N fractions. The COD herbage displayed the lowest (P < 0.05) soluble N fraction and CFR herbage the highest soluble N fraction. Both the b fraction degradation rates of CFN and CFD herbage were similar but higher (P < 0.05) compared to that of the CFR and COD herbage. The N dg of the CFR herbage was similar to that of CFN and CFD herbage within rumen outflow rates. Chopped oven dried herbage displayed lower (P < 0.01) N dg within both outflow rates compared to that of CFR, CFN and CFD herbage.
Discussion
In general, the soluble DM and N fractions showed similar differences between methods of preparation within stage of regrowth (Tables 1 and 2). Soluble N fractions were consistently higher than corresponding soluble DM fractions, in agreement with the results of Petit & Tremblay (1992) and Elizalde et al. (1999). The high N values in the herbage resulting from the high N application rate would be indicative of substantial amounts of soluble N present in the herbage (Eckard, 1989; Valk et al., 1996). The soluble N fractions in the FR and FN treated herbage did not differ significantly at the 3-week regrowth stage, but at the 4-week regrowth stage, FN values were higher than FR values. Freezing of herbage is associated with a breakdown of the vacuolar membrane, which leads to a mixing of vacuolar and cytoplasmic content and a resultant precipitation of protein (MacRae, 1970). From a chemical analysis point of view, frozen herbage displayed reduced soluble N concentrations (MacRae, 1970; MacRae et al., 1975). This effect was also observed by Kohn & Allen (1992) who reported a 40% increase in neutral detergent insoluble N as a result of freezing bromegrass. Filmer (1982) cautioned the use of frozen herbage in degradability studies, as extensive cellular damage may occur. This author found that the rates of DM and N degradation of FR and FN herbage were significantly different after eight hours of rumen incubation. In the present study, frozen samples were darker in colour and did not display the structural integrity on commencement of wilting associated with FR samples.
The exceptionally high soluble N fractions observed for FD herbage could be attributed to the high N content of the herbage and the contribution of the milling process towards enhancing the availability of the potentially soluble N fraction. Visually the latter effect was observed as the formation of a frothy mass during the washing process. Milling possibly also contributed to increased wash out losses of material from the bags. The soluble DM and N fractions of FD herbage were significantly higher than those of the OD herbage, in agreement with the results of Vik-Mo (1989). Abdalla et al. (1988) also showed, through laboratory estimates, that the N solubility of FD herbage was significantly higher than that in OD herbage. Oven drying clearly influenced the DM and N solubility as significantly higher OD values were obtained compared to FR and FN values. However, the OD samples were milled. The effect of heating would therefore be confounded by particle losses through the washing out of the finer plant material, as observed by Aumont et al. (1994).
The insoluble but potentially degradable DM and N fractions reflected the inverse of the soluble fraction, in agreement with the results of Nocek & Grant (1987), Petit & Tremblay (1992), Aumont et al. (1994) and Wales et al. (1999). The high values observed for maximum extent of DM and N degradation at 48 h, and even 24 h incubation, indicate the highly degradable nature of this herbage, given sufficient rumen exposure, irrespective of preparation method. Similar DM degradation of fresh herbage was reported by Halliday (1990), Phillips et al. (1991) and Wales et al. (1999). The maximum extent of N disappearance did not differ between FR and FD herbage, which agrees with the findings of Du Preez & Meissner (1992) and Wales et al. (1999). Erasmus et al. (1990) observed very high maximum extent of N degradation values in FD herbage, which are similar to those reported in our study. The maximum extent of DM and N degradation of FD and OD samples was similar, with DM values slightly lower than N values, in agreement with the results of Vik-Mo (1989).
In the follow-up study, when particle size was standardized within each of the four preparation methods the soluble N fractions were different between methods, with chopped frozen herbage values the closest to chopped fresh herbage values. Although a direct comparison cannot be made between the studies, it is worthwhile noting the large difference in the soluble N fractions of milled freeze dried and milled oven dried herbage compared, respectively, to chopped freeze dried and chopped oven dried herbage. Clearly, particle size played a major role in determining the recorded amounts of soluble N when utilizing the in situ technique (Aumont et al., 1994). In a milled state more of the potentially soluble N fractions are exposed to the washing procedure and one cannot disregard the potential which exists for small particles (smaller than the bag pore size) to be washed out. When working with a chopped sample the heating effect of oven drying reduced the soluble N fraction, degradation rate of the b fraction and effective N degradability as opposed to that of fresh, frozen and freeze dried herbage. Milling of an oven dried sample masked this heating effect to such an extent that the milled oven dried herbage had a N disappearance similar to that of chopped fresh and chopped frozen herbage within 24 hours of incubation (Table 1).
Van Vuuren et al. (1991) reported that the degradation rate of the potentially degradable N fraction increased with increasing N fertilization rate and their values obtained at a high N rate are similar to those obtained in the present study. The high rumen degradability of green herbage observed by Van Vuuren et al (1991) and Elizalde et al. (1999) is of a similar magnitude to the estimated effective rumen degradability values obtained in the present study.
Correcting for bacterial N contamination of the residues in the bags is a serious consideration, which will affect N degradation estimates (Vanzant et al., 1998). However, Gonzales et al. (1998) showed that the relative contribution of microbial N contamination becomes smaller the higher the N concentration of the substrate. Ryegrass, especially when well-fertilized contains a high concentration of N which is rapidly degraded in the rumen. The contribution of microbial contamination to estimated N degradability values should therefore be relatively small.
Conclusion
The study on the effect of sample preparation method showed that sample characteristics (particle size, wet or dry sample) clearly influenced the estimated N degradability of ryegrass. A chopped fresh herbage sample appears to be the preferred preparation method used in nylon bag studies to represent a masticated herbage sample as ingested by the cow. This is in agreement with the recommendations by the AAC (1990) and AFRC (1992). Contrary to this, the other methods are also commonly used, and recommended (Michalet-Doreau & Ould-Bah, 1992). The results of the present study indicate that the process of freeze drying combined with milling influenced the soluble N fractions and potentially the effective N degradability. The effects of sample preparation were notable in the initial stages of rumen incubation, however, these effects on DM and N losses were not observed beyond 24 hour incubation. When particle size was standardized at 5 to 10 mm, there were no differences in the effective degradability of fresh, frozen and freeze dried samples, although there were differences in the values of the a, b and c fractions. Oven drying reduced the effective degradability and is not recommended.
Acknowledgements
The authors thank B. Zondi and the staff in the Metabolism Building; C. Ngcobo and the Dairy Staff at Cedara for the willing manner in which they assisted with the execution of the trial, and C. Stevens and M. Whitwell of the Biometry Section at Cedara for assistance with statistical matters and statistical analyses.
References
AAC, 1990. Feeding standards for Australian livestock. Ruminants. Australian Agricultural Council, Ruminants Subcommittee. CSIRO Publications, Melbourne, Australia. [ Links ]
Abdalla, H.O., Fox, D.G. & Van Soest, P.J., 1988. An evaluation of methods for preserving fresh forage samples before protein fraction determinations. J. Anim. Sci. 66, 2646-2649. [ Links ]
AFRC, 1992. Nutritive requirements of ruminant animals: Protein. AFRC Technical Committee on response to nutrients. Report No.9. Nutr. Abstr. Rev. (Series B) 62, 787-835. [ Links ]
AOAC, 1975. Official methods of analysis (12th ed.). Association of Official Analytical Chemists, Inc., Washington D.C., USA. [ Links ]
Aumont, G., Saminadin, G., Cerneau, P. & Xandé, A., 1994. Effects of sample preparartion on nitrogen degradability of pangola grass (Digitaria decumbens) and tropical tree legumes. J. Agric. Sci., Camb. 123, 47-54. [ Links ]
Coblentz, W.K., Fritz, J.O., Fick, W.H., Cochran, R.C. & Shirley, J.E., 1998. In situ dry matter, nitrogen, and fiber degradation of alfalfa, red clover, and eastern gamagrass at four maturities. J. Dairy Sci. 81, 150-161. [ Links ]
Coffey, K.P., Moyer, J.L., Lomas, L.W. & Turner, K.E., 1991. Technical note: Sampling technique and drying method effects on chemical composition of Tall Fescue or Fescue-Ladino clover pasture samples. J. Anim. Sci. 69, 423-428. [ Links ]
Du Preez, M.M. & Meissner, H.H., 1992. Utilization of Lolium multiflorum by sheep. 2. The effect of drying the herbage on soluble nitrogen content and partial digestion. J. Grassl. Soc. Sth Afr. 9, 18-23. [ Links ]
Eckard, R.J., 1989. The response of Italian ryegrass to applied nitrogen in the Natal Midlands. J. Grassl. Soc. Sth Afr. 6, 19-22. [ Links ]
Elizalde, J.C., Merchen, N.R. & Faulkner, D.B., 1999. In situ dry matter and crude protein degradation of fresh forages during the spring growth. J. Dairy Sci. 82, 1978-1990. [ Links ]
Erasmus, L.J., Prinsloo, J., Botha, P.M. & Meissner, H.H., 1990. Establishment of a ruminal protein degradation data base for dairy cattle using the in situ polyester bag technique. 3. Roughages. S. Afr. J. Anim. Sci. 20, 130-135. [ Links ]
Erasmus, L.J., Prinsloo, J. & Meissner, H.H., 1988. The establishment of a protein degradability data base for dairy cattle using the nylon bag technique. 1. Protein sources. S. Afr. J. Anim. Sci. 18, 23-29. [ Links ]
Filmer, D.G., 1982. Assessment of protein degradability of forage. In: Forage protein in ruminant animal production. Eds. Thompson, D.J., Beever, D.E. & Gunn, R.G., BSAP Occ. Publ. No. 6, D & J Croal Ltd., Haddington. p. 129. [ Links ]
Genstat, 1993. Genstat 5. Committee of the Statistical Department, Lawes Agricultural Trust, Rothamsted Experimental Station, Reference Manual, Oxford, Claredon Press, UK. [ Links ]
Gonzales, J., Rodriguez, C.A., Andres, S.G. & Alvir, M.R., 1998. Rumen degradability and microbial contamination of fish meal and meat meal measured by the in situ technique. Anim. Feed Sci. Technol. 73, 71-84. [ Links ]
Halliday, L.J., 1990. Rumen degradation of various grass species at different stages of growth. In: Proc. 16th Int. Grassl. Congr., 4-11 October 1989, INRA, Route de Saint Cyr, Nice, France. p. 933. [ Links ]
Huntington, J.A. & Givens, D.I., 1997. Studies on in situ degradation of feeds in the rumen: 1. Effect of species, bag mobility and incubation sequence on dry matter disappearance. Anim. Feed Sci. Technol. 64, 227-241. [ Links ]
Kohn, R. A. & Allen, M.S., 1992. Storage of fresh and ensiled forages by freezing affects fibre and crude protein fractions. J. Sci. Food Agric. 58, 215-220. [ Links ]
Lindberg, J.E., 1985. Estimation of rumen degradability of feed proteins with the in sacco technique and various in vitro methods: A review. Acta Agric. Scand. 25, Suppl., 64-97. [ Links ]
Lopez, S., Carro, D., Gonzales, J.S. & Carpintero, C., 1990. Effect of chemical composition of roughages on their degradation characteristics in the rumen and their relationship with the in vitro organic matter digestibility. In: Proc. 16th Int. Grassl. Congr., 4-11 October 1989, INRA, Route de Saint Cyr, Nice, France. p. 935. [ Links ]
MacRae, J.C., 1970. Changes in chemical composition of freeze-stored herbage. N. Z. J. Agric. Res. 13, 45. [ Links ]
MacRae, J.C., Campbell, D.R. & Eadie, J., 1975. Changes in the biochemical composition of herbage upon freezing and thawing. J. Agric. Sci., Camb. 84, 125-131. [ Links ]
Marinucci, M.T., Dehority, B.A. & Loerch, S.C., 1992. In vitro and in vivo studies of factors affecting digestion of feeds in synthetic fiber bags. J. Anim. Sci. 70, 296-307. [ Links ]
Michalet-Doreau, B. & Cerneau, P., 1991. Influence of foodstuff particle size on in situ degradation of nitrogen in the rumen. Anim. Feed Sci. Technol. 35, 69-81. [ Links ]
Michalet-Doreau, B. & Ould-Bah, M.Y., 1992. In vitro and in sacco methods for the estimation of dietary nitrogen degradability in the rumen: a review. Anim. Feed Sci. Technol. 40, 57-86. [ Links ]
Nocek, J.E., 1988. In situ and other methods to estimate ruminal protein and energy digestibility: A review. J. Dairy Sci. 71, 2051-2069. [ Links ]
Nocek, J.E. & Grant, A.L., 1987. Characterization of in situ nitrogen and fiber digestion and bacterial nitrogen contamination of hay crop forages preserved at different dry matter percentages. J. Anim. Sci. 64, 552-564. [ Links ]
NRC, 2001. Nutrient requirements of dairy cattle. (7th ed.). National Academy Press, Washington D.C., USA. [ Links ]
Ørskov, E.R. & McDonald, I., 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci., Camb. 92, 499-503. [ Links ]
Ørskov, E.R., Hovell, F.D. De B. & Mould, F., 1980. The use of the nylon bag technique in the evaluation of feedstuffs. Trop. Anim. Prod. 5, 195-213. [ Links ]
Ould-Bah, M.Y. & Michalet-Doreau, B. 1990. Effect of forage conservation methods on in sacco nitrogen degradability in the rumen. In: Proc. 16th Int. Grassl. Congr., 4 - 11 October 1989, Nice, France. INRA, Route de Saint Cyr, Nice, France. p. 907. [ Links ]
Petit, H.V. & Tremblay, G.F., 1992. In situ degradability of fresh grass and grass conserved under different harvesting methods. J. Dairy Sci. 75, 774-781. [ Links ]
Phillips, C.J.C., Margerison, J.K., Azazi, S., Chamberlain, A.G. & Omed, H., 1991. The effect of adding surface water to herbage on its digestion by ruminants. Grass For. Sci. 46, 333-338. [ Links ]
Snedecor, G.W. & Cochran, W.G., 1980. Statistical Methods (7th ed.). Iowa State University Press, Iowa, USA. p. 234. [ Links ]
Statgraphics, 1988. Statistical Graphic System. Statistical Graphics Corporation Inc., Rockville, Maryland, USA. [ Links ]
Valk, H., Kappers, I.E. & Tamminga, S., 1996. In sacco degradation characteristics of organic matter, neutral detergent fibre and crude protein of fresh grass fertilized with different amounts of nitrogen. Anim. Feed Sci. Technol. 63, 63-87. [ Links ]
Van der Honing, Y. & Alderman, G., 1988. Feed evaluation and nutritional requirements: Ruminants. Livest. Prod. Sci. 19, 217-278. [ Links ]
Van Vuuren, A.M., Tamminga, S. & Ketelaar, R.S., 1990. Ruminal availability of nitrogen and carbohydrates from fresh and preserved herbage in dairy cows. Neth. J. Agric. Sci. 38, 499-512. [ Links ]
Van Vuuren, A.M., Tamminga, S. & Ketelaar, R.S., 1991. In sacco degradation of organic matter and crude protein of fresh grass (Lolium perenne) in the rumen of grazing dairy cows. J. Agric. Sci., Camb. 116, 429-436. [ Links ]
Vanzant, E.S., Cochran, R.C. & Titgemeyer, E.C., 1998. Standardization of in situ techniques for ruminant feedstuff evaluation. J. Anim. Sci. 76, 2717-2729. [ Links ]
Vik-Mo, L., 1989. Degradability of forages in sacco. 1. Grass crops and silages after oven and freeze drying. Acta Agric. Scand. 39, 43-52. [ Links ]
Wales, W.J., Dellow, D.W. & Doyle, P.T., 1999. Degradabilities of the dry matter and crude protein from perennial herbage and supplements used in dairy production systems in Victoria. Aust. J. Exper. Agric. 39, 645-656. [ Links ]
# Corresponding author. E-mail: dugmoret@dae.kzntl.gov.za
* Present address: Senwesko Feeds, P.O. Box 52, Viljoenskroon 9520, South Africa














