Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.35 n.2 Pretoria 2005
Effect of dietary conjugated linoleic acid (CLA) on carcass quality, serum lipid variables and histopathological changes of broiler chickens infected with aflatoxin B1
M. DenliI, #; F. OkanI; F. DoranII; T.C. İnalIII
IDepartment of Animal Science, Faculty of Agriculture, Çukurova University, 01330 Adana, Turkey
IIDepartment of Pathology, Faculty of Medicine, Çukurova University, 01330 Adana, Turkey
IIIDepartment of Biochemistry, Faculty of Medicine, Çukurova University, 01330 Adana, Turkey
ABSTRACT
Three dietary inclusion rates of CLA (0, 2 and 4 g/kg feed) and aflatoxin B1 (0, 200 and 300 μg/kg feed) were tested in a 3 x 3 factorial experimental design on a total of 99 Ross-308 male broiler chickens from 1 to 42 days of age. The objective of this study was to determine the effect of dietary conjugated linoleic acid (CLA) on carcass characteristics, serum lipid variables and histopathological properties in broiler chickens receiving a diet containing aflatoxin B1 (AFB1). Carcass yield, abdominal fat weight and abdominal fat percentage were not significantly influenced by dietary CLA, AFB1 or CLA + AFB1. Altered serum lipid measurements induced by AFB1 treatments included increased serum cholesterol and triglyceride concentrations, and decreased serum concentration of high density lipoprotein (HDL). Serum HDL concentration was increased in birds supplemented with 2 and 4 g CLA/kg diet compared with the control group. However, CLA + AFB1 did not significantly affect these parameters compared to the groups that received AFB1 alone. Aflatoxin B1 administration induced degenerative changes in the liver tissue, but dietary CLA supplementation offered protection to the livers against these changes. Aflatoxin B1 residues were not detected in any breast tissues collected from the broiler carcasses. Our results suggest that CLA provided protection against the negative effects of liver damage induced by AFB1 in broiler chickens. Furthermore, dietary CLA supplementation increased serum HDL levels.
Keywords: Aflatoxin B1, conjugated linoleic acid, carcass quality, hepatotoxicity, serum lipid variables
Introduction
Aflatoxins are toxic metabolites produced by certain species of fungi, e.g. Aspergillus flavus and A. parasiticus. These fungi are present in soil and decaying plant material, promote the decay of stored grain and invade maize in the field. There are four different aflatoxins produced by these fungi, AFB1, B2, G1 and G2. Aflatoxin B1 is a very potent carcinogen in many species, including nonhuman primates, birds, fish and rodents (Jakhar & Sadana, 2004).
Liver damage, decreased milk yield, decreased egg production and overall performance, as well as immunosupression have been noted in animals consuming low dietary concentrations of aflatoxin (Robens & Richard, 1992). Aflatoxin B1 also showed potential immunotoxicity on peritoneal macrophages and splenic lymphocytes in certain animal species (Cusumano et al., 1990). Avoidance of contaminated feed is rarely possible and feeds that contain relatively low concentrations of AFB1 may have deleterious effects on sensitive species such as poultry (Giambrone et al., 1985). The residues of aflatoxin B1 and aflatoxin M1 in animal products destined for human consumption such as meat, eggs and milk have been reported for different species (Pattersen et al., 1980; Munksgaard et al., 1987).
Lately, several approaches to avoid contamination such as decontamination or remediation of feed and feedstuffs have been proposed (Bailey et al., 1998; Ledoux et al., 1999). A variety of adsorbents have been used for detoxifying AFB1 in contaminated feeds (Ramos & Hernandez, 1997) such as bentonite (Rosa et al., 2001), zeolite (Miazzo et al., 2000), hydrated sodium calcium aluminosilicate (Scheideler, 1993), clinoptilolite (Oguz et al., 2000), Saccharomyces cerevisiae (Celik et al., 2001) and charcoal (Jindal et al., 1994).
Conjugated linoleic acid (CLA) is a mixture of positional and geometric isomers of linoleic acid (c-9, cis-12 C18:2, linoleic acid) with two conjugated unsaturated double bounds at various carbon positions (Khanal & Olson, 2004). Conjugated linoleic acid has been shown to have anticarcinogenic effects in various cancer models such as chemically induced skin, stomach, colorectal cancer and mammary tumorigenesis (Aro et al., 2000). Ha et al. (1990) determined that mice dosed with CLA and treated with benzo(a)pyrene had about 50% of forestomach neoplasms and reduced tumour incidence. Recent evidence also demonstrated that CLA is effective in reducing the growth of human breast (Visonneau et al., 1997) and prostate cancer cells (Cesano et al., 1998). Hamsters that were fed different levels of CLA showed lower total circulating cholesterol concentrations (Nicolosi et al., 1997). Lee et al. (1994) reported that rabbits fed with an atherogenic diet providing 0.5 g CLA/d exhibited lower circulating low density lipoprotein (LDL) cholesterol and somewhat lower triglyceride concentrations. A recent study reported that mice fed with CLA had an increased serum high density lipoprotein (HDL)-cholesterol : total cholesterol ratio and lower serum triglycerides but increased development of aortic fatty streaks (Munday et al., 1999). Other beneficial effects of CLA include reduction in body fat, immuno-modulation and antioxidant properties (Cook et al., 1993). Due to these properties CLA was selected for use in this experiment.
The aim of this experiment was to determine the potential of CLA to reduce AFB1 toxicity in broiler chickens by observing their effects on serum lipid variables, histopathological changes, carcass characteristics and residues of AFB1 in breast tissues.
Materials and Methods
A total of 99 day-old male Ross-308 broiler chickens (42.8 g BW) was used. The nine treatments, arranged according to a 3 x 3 factorial experimental design, consisted of three levels of AFB1: 0, 200 and 300 μg/kg feed and three levels of CLA: 0, 2 and 4 g/kg feed. Pure crystalline AFB1 was obtained from Sigma-Makor Chemical Corp., Jerusalem, Israel. The AFB1 was weighed and dissolved under a hood in warm chloroform at 1 mg/10 mL. The AFB1 solution in chloroform was then sprayed on a thin layer (<1 cm) of the basal diet. The treated feed was left overnight at room temperature for the solvent to evaporate and was then mixed twice to provide the desired levels of AFB1/kg of diet. Conjugated linoleic acid was purchased from the Cognis Corporation, U.S.A. A starter diet was formulated according to the NRC (1994) recommendations to meet the nutrient requirements of broilers during their first 21 days of age and a grower diet for the following 21 days. The composition of the basal diets is presented in Table 1. The diets were analysed for aflatoxin content using thin layer chromatography (Howel, 1983), and there were no detectable levels present.
Each experimental group of birds received its specific diet ad libitum. Water was provided in continuous flow water troughs. The chicks were reared under a conventional temperature regimen, i.e. starting at 33 °C and reduced by 3 °C/week to 21 °C. The relative humidity was maintained at between 60 and 70%. The birds were exposed to continuous lighting. After 42 days all chickens were slaughtered by dislocation of the neck vertebrae and bleeding, and prepared for further analysis. The livers were removed and fixed in 10% neutral buffered formalin solution, dehydrated in graded alcohol and embedded in paraffin. Sections of 3-5 μm were obtained and stained with hematoxylin/eosin (H&E). Light microscopy was used to evaluate portal and periportal necrosis, congestion, fatty change, portal leucocytic infiltration, paranchymatous degeneration, dysplasia and neoplastic transformation.
At the end of the 42 day experimental period a sample of breast tissue was collected from three randomly selected chicks in each treatment. All samples were weighed and stored at -14 oC until further analysis. The samples of breast tissue were extracted with chloroform and acetone (60: 40). For this analysis a mobile phase (MeOH: ACN: H2O) + 120 mg KBr + 350 μL 4 M HNO3), and a HPLC (High Performance Liquid Chromatography), ACE5 C18 (4.6 x 250 mm) were used. The AFB1 was measured according to the method described by Şenyuva et al. (2004).
Blood samples were collected from all birds from the retroorbital venous plexus at the end of the experimental period for haematological and biochemical study. Within one hour of collection the serum was separated. Serum cholesterol and triglycerides concentrations were measured using the CHOD-PAP (cholesterol esterase, peroxidase) and the GPO-PAP (glycerol 3-phosphate oxidase, peroxidase) enzymatic colorimetric tests, respectively. Serum HDL-cholesterol concentration was measured using the no pretreatment, direct enzymatic colorimetric test while serum LDL-cholesterol concentration was calculated according to the Frieldwald formula, i.e. LDL-chol = Total chol - (HDL-chol + Triglyceride/5). Serum very low density lipoprotein (VLDL)-cholesterol was determined as triglyceride/5 (Tietz, 1995).
All data were subjected to analysis of variance using the statistical analyses software, SPSS (1993). If appropriate, post-hoc analyses were carried out using the Duncan's test for multiple comparisons. Statements effects of statistical significance are based on P < 0.05. The effects of AFB1 and CLA on histopathological changes were leucocytic estimated by the χ2 tests.
Results
The effects of dietary CLA, AFB1 and their combinations on the carcass characteristics of broilers are presented in Table 2. There were no significant differences in the carcass yield, abdominal fat weight and abdominal fat percentage between treatment groups. However, the results showed that feed containing AFB1 at 200 and 300 μg/kg feed caused decreases (P < 0.05) in carcass weight compared with the control group.
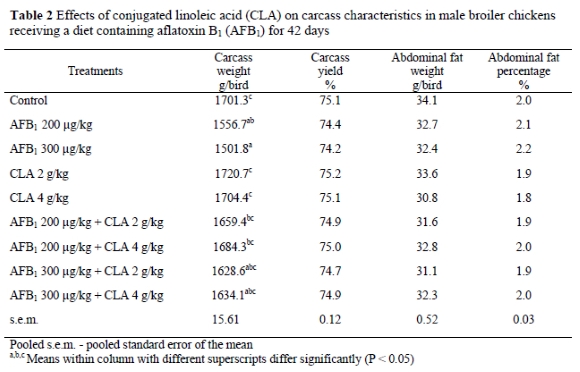
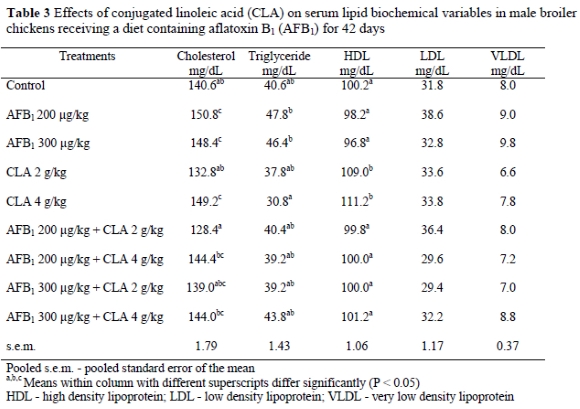
Data presented in Table 3 show the effects of dietary treatments on serum lipid variables (mg/dL). The diets containing 200 and 300 AFB1 ug/kg without CLA added, decreased HDL and increased cholesterol and triglyceride levels. However, addition of 4 g CLA/kg to the AFB1 contaminated diet prevented an increase in the level of serum triglyceride and cholesterol. The addition of 4 g CLA/kg alone to the diet increased HDL and decreased triglyceride, compared with the control group. Other serum variables such as LDL and VLDL were not affected by any treatments.
The control group presented normal hepatic histology. Livers of chickens fed the diets with 200 and 300 AFB1 μg/kg alone revealed lesions typical of aflatoxicosis. The liver showed portal leucocytic infiltration, congestion, periportal and multifocal fatty degeneration, necrosis and dysplasia of parenchymal cells with disorganization of the structure. However, these changes were reduced partly by addition of CLA (Table 4). Livers of chickens fed CLA alone showed no significant histological changes. In the CLA + AFB1 treated chicks, residues of AFB1 were not detected in breast tissues.
Discussions
Aflatoxins are a group of fungal toxins that have been associated with severe toxic effects in man and animals (Ramos & Hernandez, 1997). A variety of adsorbents such as zeolites, bentonites, synthetic aluminosilicates, dry yeast and glucomannan were capable of adsorbing aflatoxin from aqueous solutions. In contrast, CLA has been associated with the reduction of chemically induced cancers, in cancers of the skin and mammary carcinogenesis in animal studies (McGuire & McGuire, 1999). Conjugated linoleic acid is also thought to have antioxidant capacity (Parodi, 1994).
The main effects of aflatoxin are related to liver damage. The inhibition of protein synthesis in the liver (Jindal et al. , 1994) and changes of serum variables such as cholesterol and triglyceride concentrations during aflatoxicosis have been reported (Lindeman et al., 1993). In this study, the results showed that the inclusion of AFB1 at 200 and 300 ug/kg in the diet significantly increased the levels of serum triglyseride of broiler chickens. These results agree with the findings of Santurio et al. (1999). In contrast, Edrington et al. (1996) reported that aflatoxicosis in chickens induced strong reductions in serum triglyceride and cholesterol concentrations. The level of serum HDL was significantly increased by supplementation with 2 and 4 g CLA/kg as compared to those of the controls. Similarly, Munday et al. (1999) reported that mice fed CLA had an increased serum HDL-cholesterol : total cholesterol ratio and lower serum triglyceride concentrations. An addition of CLA to the diet can be beneficial to broiler chickens consuming AFB1 by preventing negative effects of AFB1 on serum lipid variables such as cholesterol and triglyceride. The beneficial effects of CLA might be related to the fact that CLA decreases the activity of lipoprotein lipase (Park et al., 1997) and also elicits an antiatherogenic effect (Lee et al., 1994).
The effects of aflatoxins on histopathological changes are directly correlated with the concentration of aflatoxin and the duration of the exposure (Boonyaratpalin et al., 2001). The liver is the principal target organ for aflatoxicosis. The histopathological changes of the liver induced by aflatoxins have been described previously (Rosa et al., 2001). In our study the microscopic appearance of the livers in the treatment with AFB1 showed portal and parenchymatous degeneration, leucocytic infiltration, multifocal dysplasia and disorganisation. Additionally, our results showed that fatty change, portal leucocytic infiltration and congestion in the liver are related to structural damage of the liver as a consequence AFB1 exposure.
The histopathological lesions of AFB1 treated groups are in agreement with reports of Miazzo et al. (2000). Supplementing CLA with the feed partly prevented liver lesions induced by AFB1. However, the liver lesions of chickens fed diets containing CLA + AFB1 did not completely return to a normal liver picture. The protective effects of CLA on liver may be attributed to the biological properties of CLA. Various studies have reported that CLA might possess antioxidant properties (Cook et al., 1993), be anticarcinogenic (Ha et al., 1990; Aro et al., 2000) and can reduce cell proliferative activity (Cunningham et al., 1997).
Residues of aflatoxin B1 have been found in the musculature and certain organs of poultry and pigs after they were given aflatoxins in their feed. When birds were fed diets contaminated with AFB1 the toxin was absorbed by the intestine and distributed by the bloodstream throughout the body; although approximately 90% of the AFB1 was removed through bile and renal excretion (Agacdelen & Acet, 1993). Gregory et al. (1983) reported that turkey poults fed with 500 μg AFB1/kg feed over 18 days had various levels (ranging from 0.01-1.19 ng/g tissue) of AFB1 residues in the liver tissue. In another study, Oliveira et al. (2003) reported that Japanese laying quails receiving diets containing 100 AFB1 (g/kg feed over 90 days showed residues of aflatoxins in their eggs at levels ranging from 0.01-0.03 μg/kg. In our experiment, no AFB1 residues were detected in breast tissues which were collected from treatment groups of broiler carcasses.
Conclusions
In conclusion, the observations from the current study showed that CLA intake decreased some of the toxic effects of AFB1. These antitoxic properties of CLA may be due to its effective immuno-modulation and antioxidant properties (Lee et al., 1994; Parodi, 1994). It is not clear how the protection was effected. This protective action was obviously manifested as microscopic changes in the liver. In addition, dietary CLA supplementation increased serum HDL levels in broiler chickens. However, further investigations are needed to verify the effects of different levels of CLA on the response of broiler chickens to aflatoxins.
Acknowledgements
The authors are grateful to Çukurova University Research Fund for their financial support. Project no: ZF2002BAP70.
References
Agacdelen, H.H. & Acet, H.A., 1993. Experimental studies on aflatoxin B1 and M1 deposition and clearance times in eggs. Veterinarium 4, 36-43. [ Links ]
Aro, A.S., Mannisto, I., Salminen, M.L., Onaskainen, V., Kataja, V. & Uustupa, M., 2000. Inverse association between dietary and serum conjugated linoleic acid and risk of breast cancer in postmenopausal women. Nutr. Cancer 38 (2), 151-157. [ Links ]
Bailey, R.H., Kubena, L.F., Harvey, R.B., Buckley, S.A. & Rottinghaus, G.E., 1998. Efficacy of various inorganic adsorbents to reduce the toxicity of aflatoxin and T-2 toxin in broiler chickens. Poult. Sci. 77, 1623-1630. [ Links ]
Boonyaratpalin, M., Supamattaya, K., Verakunpiriya, V. & Suprasert, D., 2001. Effects of aflatoxin B1 on growth performance, blood components, immune function and histopathological changes in black tiger shrimp (Penaeus monodon fabricius). Aquacult. Res. 32, 388-398. [ Links ]
Celik, K., Denli, M., Ertürk, M., Öztürkcan, O. & Doran, F., 2001. Evaluation of dry yeast Saccharomyces Cerevisiae in the feed to reduce aflatoxin B1 AFB1 residues and toxicity to Japonica Quails (Coturnix coturnix Japonica). J. Appl. Anim. Res. 20, 245-250. [ Links ]
Cesano, A., Visonneau, S.J.A., Kritchevsky, S.D. & Santoli, D., 1998. Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res. 18, 833-838. [ Links ]
Cook, M.E., Miller, C.C., Park, Y. & Pariza, M.W., 1993. Immune modulation by altered nutrient metabolism: Nutritional control of immune-induced growth depression. Poult. Sci. 72, 1301-1305. [ Links ]
Cunningham, D.C., Harrison, L.Y. & Shultz, T.D., 1997. Proliferative responses of normal human mammary and MCF-7 breast cancer cells to linoleic acid, conjugated linoleic acid and eicosanoid synthesis inhibitors in culture. Anticancer Res. 17, 197-204. [ Links ]
Cusumano, V., Costa, G.B. & Seminara, S., 1990. Effect of aflatoxins on rat peritoneal macrophages. Appl. Environ. Microb. 56, 3482-3484. [ Links ]
Edrington, T.S., Sarr, A.B., Kubena, L.F., Harvey, R.B. & Phillips, T.D., 1996. Hydrated sodium calcium aluminosilicate (HSCAS), acidic HSCAS, and activated charcoal reduce urinary excretion of aflatoxin M1 in turkey poults. Lack of effect by activated charcoal on aflatoxicosis. Toxicol. Lett. 89, 115-122. [ Links ]
Giambrone, J.J., Diener, U.L., Davis, N.D., Panangala, V.S. & Hoerr, F.J., 1985. Effects of aflatoxin on young turkeys and broiler chickens. Poult. Sci. 64, 1678-1684. [ Links ]
Gregory, J.F., Goldstein, S.L. & Edds, G.T., 1983. Metabolite distribution and rate of residue clearance in turkeys fed a diet containing aflatoxin B1. Food Chem. Toxicol. 21, 463-467. [ Links ]
Ha, Y.L., Storkson, J. & Pariza, M.W., 1990. Inhibition of benzofolpyrene-induced forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 50, 1097-1101. [ Links ]
Howel, M.V., 1983. Methods for determination of aflatoxins, ochratoxin A and zearalenone in mixed feeds with detection by thin layer chromatography or high performance liquid chromatography. Proc. Int. Symp. Mycotoxins. National Research Centre, Cairo, Egypt, 1983. pp. 293-296. [ Links ]
Jakhar, K.K. & Sadana, J.R., 2004. Sequential pathology of experimental aflatoxicosis in quail and the effect of selenium supplementation in modifying the disease process. Mycopathologia 157, 99-109. [ Links ]
Jindal, N., Mahipal, S.K. & Mahajan, N.K., 1994. Toxicity of aflatoxin B1 in broiler chickens and its reduction by activated charcoal. Res. Vet. Sci. 56, 37-40. [ Links ]
Khanal, R.C. & Olson, K.C., 2004. Factors affecting conjugated linoleic acid (CLA) content in milk, and egg: A review. Pak. J. Nutr. 3(2), 82-98. [ Links ]
Ledoux, D.R., Rottinghaus, G.E., Bermudez, A.J. & Alonso-Debolt, M., 1999. Efficacy of hydrated sodium calcium aluminosilicate to ameliorate the effects of aflatoxin in broiler chicks. Poult. Sci. 78, 204-210. [ Links ]
Lee, K.N., Kritchevsky, D. & Pariza, M.W., 1994. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 108, 19-25. [ Links ]
McGuire, M.A. & McGuire, M.K., 1999. Conjugated linoleic acid (CLA): A ruminant fatty acid with beneficial effects on human health. Proc. Am. Soc. Anim. Sci., 1999. pp. 1-8. [ Links ]
Miazzo, R., Rosa, C.A.R., De Queiroz Carvalho, E.C., Magnoli, C., Chiacchiera, S.M., Palacio, G., Saenz, M., Kikot, A., Basaldella, A. & Dalcero, A. 2000. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poult. Sci. 78, 1-6. [ Links ]
Munday, J.S., Thompson, K.G. & James, K.A.C., 1999. Dietary conjugated linoleic acids promote fatty steak formation in the C57BL/6 mouse atherosclerosis model. Br. J. Nutr. 81, 251-255. [ Links ]
Munksgaard, L., Larsen, J., Werner, H., Andersen, P.E. & Viuf, B.T., 1987. Carry over of aflatoxin from cows' feed to milk and milk products. Milchwissenschaft 42, 165-167. [ Links ]
Nicolosi, R.J., Rogers, E.J., Kritchevsky, D., Scimeca, J.A. & Huth, P.J., 1997. Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atherosclerosis in hyperscholesterolemic hamsters. Artery 22, 266-277. th [ Links ]
NRC, 1994. Nutrient Requirements of Poultry. 9th rev. ed. National Academy Press, Washington, D.C., USA. [ Links ]
Oguz, H. & Kurtoglu, V., 2000. Effect of clinoptiloite on performance of broiler chickens during experimental aflatoxicosis. Br. Poult. Sci. 41, 512-517. [ Links ]
Oliveira, C.A., Rosmaninho, J.F., Castro, A.L., Butkeratis, P., Alves-Reis, T. & Correa, B., 2003. Aflatoxin residues in eggs of laying Japanese quail after long term administration of rations containing levels of aflatoxin B1. Food Addit. Contam. 20, 648-653. [ Links ]
Park, Y., Albright, K.J., Cook, M.E. & Pariza, M.W., 1997. Effect of conjugated linoleic acid on body composition in mice. Lipids 32, 853-858. [ Links ]
Parodi, P., 1994. CLA: An anticarcinogenic fatty acid present in milk fat. Aust. J. Dairy Technol. 49, 93-94. [ Links ]
Patterson, D.S.P., Glancy, E.M. & Roberts, B.A., 1980. The carry-over of aflatoxin M1 into the milk of cows fed rations containing a low concentration of aflatoxin B1. Food Cosmet. Toxicol. 18, 35-37. [ Links ]
Ramos, A.J. & Hernandez, E., 1997. Prevention of aflatoxicosis in farm animals by means of hydrated sodium calcium aluminosilicate addition to feedstuff: A review. Anim. Feed Sci. Technol. 65, 197-206. [ Links ]
Robens, J.F. & Richard, J.L., 1992. Aflatoxins in animal and human health. Rev. Environ. Contam. T. 127, 69-94. [ Links ]
Rosa, C.A.R., Miazzo, R., Magnoli, C., Salvano, M., Chiacchiera, S.M., Ferrero, S., Saenz, M., Carvalho, E.C.Q. & Dalcero, A., 2001. Evaluation of the efficacy bentonite from the south of Argentina to ameliorate the toxic effects of aflatoxin in Broilers. Poult. Sci. 80, 139-144. [ Links ]
Santurio, J.M., Mallmann, C.A., Rosa, A.P., Appel, G., Heer, A., Dageforde, S. & Bpttcher, M., 1999. Effect of sodium bentonite on the performance and blood variables of broiler chickens intoxicated with aflatoxin. Br. Poult. Sci. 40, 115-119. [ Links ]
Scheideler, S.E., 1993. Effects of various types of aluminosilicates and aflatoxin B1 on aflatoxin toxicity, chick performance and mineral status. Poult. Sci. 72, 282-288. [ Links ]
Şenyuva, H.Z., Özcan, S. & Kabasakal, B.V., 2004. Tavuk Etinde Toplam Aflatoksin Analizi. Metot: MT001. [ Links ]
SPSS, 1993. SPSSx for Windows. Release, 6.0, Copright SPSS inc., 1989-1993, New York, USA. [ Links ]
Tietz, N.W., 1995. Clinical guide to laboratory tests, 3rd ed., Philadelphia, Pa:WB Saunders Comp. pp. 130-135. [ Links ]
# Corresponding author. E-mail: denli_m@yahoo.com














