Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Animal Science
versión On-line ISSN 2221-4062
versión impresa ISSN 0375-1589
S. Afr. j. anim. sci. vol.35 no.1 Pretoria 2005
Model comparisons and genetic and environmental parameter estimates of growth and the Kleiber ratio in Horro sheep
S. AbegazI, #; J.B. van WykI; J.J. OlivierI, II
IDepartment of Animal, Wildlife and Grassland Sciences, University of the Free State, P.O. Box 339, Bloemfontein 9300, South Africa
IIARC, Animal Improvement Institute, Private Bag X5013, Stellenbosch 7599, South Africa
ABSTRACT
Genetic and environmental parameters were estimated for pre- and post-weaning average daily gain (ADG1, ADG2) and Kleiber ratio (KR1, KR2) using the ASREML program. Twelve models, formed with inclusion or exclusion of the maternal genetic, permanent environmental and common (litter) environmental variance components and the covariance between the direct and maternal additive effect on the basic direct additive genetic model, were used. The same models were applied to birth weight (BWT), weaning weight (WWT) and bi-monthly weights to 12 months of age (WT2 to WT12), and weight at 18 months of age (WT18). Two-trait analyses were done among all traits. Maternal genetic and common environmental components were found to be important for ADG1, KR1 and weights up to six-months of age, while the common environmental component was found to be important for ADG2 and KR2. The maternal permanent environmental component was important for WT2 and WWT. Total heritability estimates for ADG1, ADG2, KR1 and KR2 were 0.13, 0.04, 0.13, and 0.01, respectively. Direct genetic correlations of ADG1 with BWT, WWT and WT6 were 0.01, 0.96 and 0.84 while with KR1 they were -0.40, 0.75 and 0.66, respectively. The relatively higher heritability in weight traits and the presence of positive and high correlations of weight traits with daily gain and Kleiber ratio tend to suggest that it would be more practical to select on the weight traits to improve gain and efficiency.
Keywords: Average daily gain, Kleiber ratio, common environmental effect, genetic parameters
Introduction
A slow growth rate, resulting in a low market weight of sheep, has been identified to be one of the factors limiting profitability in the highlands of Ethiopia, where about 75% of the country's sheep population is found. (Mukasa-Mugerwa & Lahlou-Kassi, 1995). Genetic improvement can be one of the avenues to improve growth rate. The Kleiber ratio (KR) has been suggested to be a useful indicator of efficiency of growth and an important selection criterion for efficiency of growth (Bergh, 1990; Köster et al., 1994). In a recent study Arthur et al. (2001) showed that the KR is highly correlated (r = -0.81) with feed conversion efficiency in beef cattle. In addition the possibility also exists to select for weight per age traits in order to improve the marketable weight of sheep. Studies elsewhere (Tosh & Kemp, 1994; Saatci et al., 1999; Maniatis & Pollott, 2002; Van Wyk et al., 2003) indicated that the maternal environmental effects have sizeable contributions to the overall variance. Incorporation of this component in the analytical models will thus contribute to the accuracy of estimates of parameters while exclusion may lead to biased estimates (Van Wyk et al., 1993; Saatci et al., 1999; Satoh et al., 2002). Genetic parameters may vary because of genotype, breed, location or herd. Hence, appropriate parameter estimates for growth traits are important for adequate breeding strategies and for accurate breeding value estimation.
The objectives of this study were to compare different models and estimate genetic and environmental parameters for average daily gain, Kleiber ratio, bi-monthly weights from birth to 12-months, 12-month weight weaning weights and 18 month weights of Horro sheep. The information generated would be useful in designing breed improvement programs in Ethiopia.
Material and Methods
The Horro sheep breed is one of the dominant sheep breeds in Ethiopia and has been adequately described by Galal (1983). On-station performance data on the breed have been collected from 1978 to 1997 at Bako Research Centre, Ethiopia. A detailed description of the environment, flock management and data collection procedures has been reported by Abegaz et al. (2002). After preliminary editing for outliers, 4031 lambs born from 3014 parturitions of 904 ewes and 184 sires were used. Further editing for missing or doubtful values with respect to fixed effects and pedigree has resulted in the data described in Table 1. Traits considered were pre- and post-weaning average daily gain (ADG1 & ADG2, respectively) and pre and post-weaning Kleiber ratio (KR1 & KR2, respectively). Bi-monthly weights from birth to one-year of age (BWT, WT2 to WT12), weaning weight (WWT) at about three months of age and eighteen-month weight (WT18) were also considered. The ADG1 and ADG2 were calculated as total gain divided by the number of days in the period while KR1 and KR2 were calculated as a ratio of ADG to metabolic weight at weaning and six months of age, respectively.
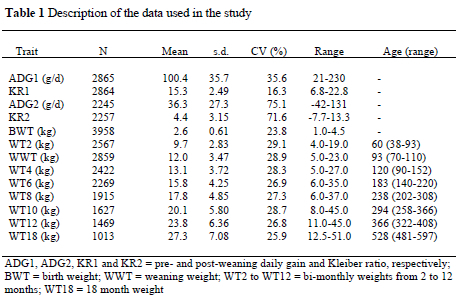
Important fixed effects and interactions for all traits were identified from preliminary analysis using the GLM procedure of SAS (1994). Year of birth, sex, type of rearing (type of birth for BWT and WT2) and age at measurement were found to be significant (P < 0.05) in all cases. Age of dam was also found to have a significant effect (P < 0.05) on pre-weaning gain and KR1 as well as weights to the age of 12 months. Year of birth and sex were combined into a class to account for the interaction after weaning due to animals of different sexes being raised separately. This interaction was found to be important for 12 and 18-month weights.
(Co)variance components were estimated for each trait under an animal model in a univariate analysis using the ASREML program (Gilmour et al., 1999). Twelve different models were used (Table 2). Tests of significance of each random effect were performed using log likelihood ratio tests after including each random effect (excluding residual) to the fixed effects model. An effect was considered significant when its inclusion in the model caused a significant increase in the log likelihood. A Chi-square distribution for α = 0.05 and one degree of freedom was used as the critical test statistic (3.841). When -2 times the difference between log likelihoods was greater than the critical value the inclusion of the effect was considered significant. Correlations and cross-correlations between the different components of the different traits were estimated from bivariate analyses using the appropriate model for each of the traits. In some cases convergence was not possible to achieve in the bivariate analysis and thus a 'reduced' model where one or more random components were removed, was used in the analysis. When differences between log likelihoods were not significant the model with the fewest random effects was chosen. The following univariate animal models (in matrix notation) were fitted:
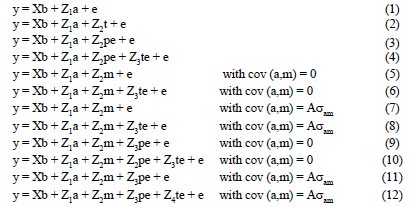
where y was a vector of observations for the different traits, b, a, m, pe and te were vectors of fixed effects, direct and maternal genetic effects, permanent and temporary (litter) environmental effects due to the dam, respectively. Matrices X, Z1, Z2, Z3 and Z4 were the corresponding incidence matrices relating observations to the respective fixed and random effects and e was the vector of residuals. It was assumed that: V(a) = Aσ2a; V(pe) = Iσ2pe; V(te) = Iσ2te; V(e) = Iσ2e, with A being the numerator relationship matrix, I identity matrices of order equal to the number of dams, number of litters and number of records respectively, σ2a, σ2m σ2pe,, σ2te and σ2e direct and maternal genetic variance, dam permanent environmental variance (half sibs across years), temporary environmental variance (full sibs within year) and environmental (residual) variance respectively.
Results and Discussion
Log likelihood values for the different models on all traits are presented in Table 2. Maternal genetic components were significant (P < 0.05) for ADG1 and KR1 and for weights to eight months of age. In the presence of the other components, with the exception of WT2 and WWT, the permanent environmental component was found to have no significant (P > 0.05) contribution to ADG1, ADG2, KR1, KR2 and weights to the different ages. Structure of data (i.e. number of records per dam, the proportion of dams with their own record) has been reported to affect the accuracy of partitioning of maternal genetic and environmental effects (Maniatis & Pollott, 2003). In the current study, the data structure was acceptable since most of the dams have their own records and, on average, each dam has had more than three lambing records. The temporary environment was found to be important for ADG1, ADG2, KR1, KR2 and weights to the age of six months. The importance of the temporary environmental effect was highest for BWT and it declined with age, as expected. The covariance between direct and maternal genetic effects was found to be significant (P < 0.05) for ADG1 and weights to weaning (BWT, WT2, WWT). In the literature carry-over effect of the maternal genetic effect was shown to persist for longer periods, namely to the age of 18 months (Snyman et al., 1996) and 22 months (Vaez Torshizi et al., 1996) and the permanent environmental effect to the age of 12 months (Matika et al., 2003). Lewis & Beatson (1999) observed that the permanent environmental effect was important for hogget weight, which was taken between 8 and 12 months of age.
Numerous reports have been published on the contribution and importance of the maternal genetic variance, permanent environmental variance and direct-maternal genetic covariance in improving the fit of models for growth performance in sheep (e.g. Van Wyk et al., 1993; Maria et al., 1993; Snyman et al., 1996; Okut et al., 1999; Cloete et al., 2001, Maniatis & Pollott, 2002) and goats (e.g. Van Niekerk et al., 1996).
Due to low rates of multiple births in some sheep breeds and also due to the analytical problem that might arise when maternal genetic, permanent environmental and temporary environmental (litter) effects are fitted simultaneously, there are few reports that considered the importance of the litter variance in model choice. Improved fit of analytical models by the inclusion of a temporary environmental component (fitted with different other components) has been reported for weaning and hogget live weight of New Zealand Coopworth sheep (Lewis & Beatson, 1999) for weights at weaning (about 65 days), 90 and 120 days of crosses involving three breeds (Al-Shorepy & Notter, 1996) for birth and weaning weight of Dormer sheep (Van Wyk et al., 2003) and for 12-week weight of Welsh mountain lambs (Saatci et al., 1999). Schoeman et al. (1997) and Hagger (1998) have also reported significant litter effects for birth and weaning weight, ADG and Kleiber ratio of Boer goat and for ADG in the first 30 days of two breeds of sheep, respectively. However, the twinning rate (35%) of sheep reported in the study of Saatci et al. (1999) is similar to that in the current study (34%). This implies the temporary environmental effect can have a significant effect even in situations where the incidence of twinning is as low as slightly above 30%.
Genetic and environmental parameter estimates for all traits are presented in Table 3. Estimates of total heritability for ADG1 and KR1 were 0.13 and 0.13. Total heritability (h2t) estimates are useful in estimating response for selection based on phenotypic values. For comparison, h2t was calculated from studies in the literature that reported direct and maternal variance and covariance. Corresponding estimates for ADG1 ranging from 0.08 to 0.27 in sheep (Van Wyk et al., 1993; Analla et al., 1995; Yazdi et al., 1997; Hagger, 1998; Larsgard & Olesen, 1998; Matika et al., 2003) and goats (Van Niekerk et al., 1996; Schoeman et al., 1997) have been reported. The current estimate falls on the lower end of this range. For KR1 literature estimates for total heritability ranged from 0.09 for Sabi sheep (Matika et al., 2003) to 0.15 for Dormer sheep (Van Wyk et al., 1993) and to 0.16 in the Boer goat (Van Niekerk et al., 1996; Schoeman et al., 1997). These values are consistent with the estimate of 0.13 in this study.
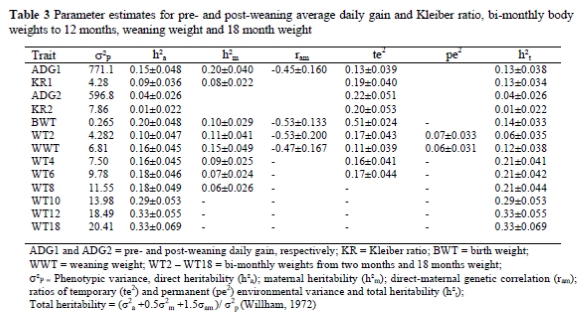
For weights from birth to six months of age, the temporary environmental effect accounted for 11 to 51% of the total variation while the maternal genetic component accounted for 5 to 17% for weight until about 8 months of age (Table 4). The permanent environmental variance component accounted for 7 and 6% of the variation in WT2 and WWT, respectively. From models with varying components fitted simultaneously, proportions of temporary environmental variance ranging from 0.04 to 0.44 were reported for birth weight and weaning weight (Al-Shorepy & Notter, 1996; Larsgrad & Olesen, 1998; Lewis & Beatson, 1999; Nagy et al., 1999; Saatci et al., 1999). Tosh & Kemp (1994) also reported that litter effect accounted for 0.12 to 0.30 of the variance in weights recorded at birth, 50 days and 100 days of age. The estimate of 0.51 in the current study for BWT is higher than estimates in the literature. This may be the result of rounding of birth weights to the nearest quarter kilogram, a procedure followed in the recording of the birth weights. Usually twin born lambs have birth weights close to each other, which become identical when rounded. According to log likelihoods for BWT the permanent environmental effect should not be included in the model. The inclusion or exclusion (Model 8 vs. 12) of this effect was, however, marginal. Therefore, the magnitude of this effect (0.51) should be interpreted with caution since it could be biased.
Estimates of total heritability for BWT, WWT, WT6 and WT12 were 0.14, 0.12, 0.21 and 0.33, respectively. These values are slightly lower for BWT and WWT and higher for WT12 than estimates reported from the same data set fitting other models (model 4 vs. 7 current) by Abegaz et al. (2002). Exclusion of important components (in this case litter) obviously has the effect of inflating the remaining parameter estimates. The difference in the heritability estimate of WT12 is the result of difference in the data edit criteria and in the fixed part of the model used in the previous and the current study. For bi-monthly weights from two to 10 months and for WT18, heritability estimates were 0.06, 0.21, 0.21, 0.21, 0.29 and 0.33, respectively. For weight at 18 months of age, Lee et al. (2000) reported a direct heritability of 0.43 from a simple animal model, while from a sire model Groenewald et al. (1999) have estimated a heritability of 0.34 for weight of Merino sheep recorded between 15 and 18 months of age. The latter value is close to current estimates. The heritability reaches a maximum at the ages of 10 to 12 months and these traits may be used for selection to improve growth given favourable relationships with the other economically important traits.
Genetic and phenotypic correlations and cross-correlations among ADG1, KR1, ADG2, KR2, BWT, WWT, WT6, WT12 and WT18 are presented in Tables 4 and 5. Phenotypic correlations of ADG1 with KR1, ADG2, and KR2 were 0.98, -0.11 and -0.27, while genetic correlations were 0.96, 0.63 and 0.89, in the respective order. It appears that lambs with higher gain in the pre-weaning period gain less and are also less efficient during the post-weaning period on the 'phenotypic level and vice versa. Positive genetic correlations between ADG1 and ADG2 in the presence of negative phenotypic correlations might have arisen as a result of compensatory growth mediated through environmental effects in lambs that were gaining at lower rates during the pre-weaning period. Similar negative phenotypic correlations between pre- and post-weaning ADG have been reported for Muzaffarnagri sheep (Sinha & Singh, 1997) and for Baluchi sheep (Yazdi et al., 1997) while María et al. (1993) have reported high positive phenotypic correlations for Romanov sheep. With respect to genetic correlations, Maria et al. (1993) and Yazdi et al. (1997) have reported negative genetic correlations between pre- and post-weaning ADG. Phenotypic and genetic correlations between ADG1 and KR1 were reported to be 0.93 and 0.94 in Dormer sheep by Van Wyk et al. (1993). Van Niekerk et al. (1996) estimated a corresponding genetic correlation of 0.97 on Boer goats using a sire model. These estimates are in accordance with the values obtained in the current study (0.98 phenotypic and 0.96 genetic).
Except for maternal additive correlations with ADG1 and KR1, all correlations and cross correlations between BWT and ADG1, KR1, ADG2 and KR2 were low and in some cases negative. This indicates that maternal genetic (additive) effects, which favour the growth of the foetus, could also have some favourable effect on postnatal growth and efficiency. The absence of any sizeable direct additive correlation between ADG1 and BWT (and medium maternal genetic correlations) indicates that these traits are not antagonistic to each other. Bromley et al. (2000) have reported direct correlations ranging from 0.18 to 0.57, maternal correlations ranging from -0.03 to 0.40, and cross correlations of -0.12 to 0.21 between BWT and ADG in four breeds of sheep. The maternal genetic correlation estimate of 0.68, though slightly higher, agrees with the estimate of Bromley et al. (2000).
Cross correlations between the direct and maternal additive effects of ADG1 with WWT, were negative (-0.36 to -0.39), while the phenotypic, direct additive, maternal additive and residual correlations were positive and high (0.72 to 1.00). Similarly, Analla et al. (1995) have reported negative cross correlations for all direct and maternal variances of WWT, ADG, and weight at 90 days of age. High correlations between pre-weaning daily gain and weaning and subsequent weights are expected, as this is governed by a part-whole relationship.
All direct genetic correlations between the weights were lower than values reported from the same data set using only direct animal models for all traits (Abegaz et al., 2002). Similar overestimation of the direct genetic covariance when models do not include maternal effects, has been reported by Analla et al. (1995) for sheep and by Meyer (1994) for beef cattle. Cross correlations between direct and maternal effects of the weight traits were low to medium and in some cases negative.
Conclusions
Genetic variation in early growth traits in the Horro sheep is sufficient to warrant inclusion in the breeding objectives. A number of findings from the current and a previous study (Abegaz et al., 2002) indicate that weight at about one year of age is the most important trait to consider in improving productivity in Horro sheep. In Ethiopia the great majority of sheep for slaughter are unfinished milk tooth lambs weighing 10 to 20 kg (Galal et al., 1979; Kassahun, 2000). This weight is achieved from about the age of six months to one year of age. The existence of high correlations between body weight at one year of age and earlier ages allows earlier weights to respond to improvement protocols based on 12-month weight, and it also permits some initial culling on performance at an earlier age. As reported in numerous studies in other breeds, the results of this study confirm that for accurate parameter estimation of growth performance and efficiency during the early life of Horro sheep, models should consider the maternal genetic, permanent and temporary environmental components.
References
Abegaz, S., 2002. Genetic evaluation of production, reproduction and survival in a flock of Ethiopian Horro sheep. Ph.D. dissertation, University of the Free State, Bloemfontein, South Africa. pp. 107. [ Links ]
Abegaz, S., Negussie, E., Duguma, G. & Rege, J.E.O., 2002. Genetic parameter estimates for growth traits in Horro sheep. J. Anim. Breed. Genet. 119, 35-45. [ Links ]
Al-Shorepy, S.A. & Notter, D.R., 1996. Genetic variation and covariation for ewe reproduction, lamb growth, and lamb scrotal circumference in a fall-lambing sheep flock. J. Anim. Sci. 74, 1490-1498. [ Links ]
Analla, M., Muñoz-Serrano, A., Cruz, J.M. & Serradilla, J.M., 1995. Estimation of genetic parameters of growth traits in Segureña lambs. J. Anim. Breed. Genet. 112, 183-190. [ Links ]
Arthur, P.F., Renand, G. & Krauss, D., 2001. Genetic and phenotypic relationships among different measures of growth and feed efficiency in young Charolais bulls. Livest. Prod. Sci. 68, 131-139. [ Links ]
Badenhorst, M.A., Olivier, J.J., Schoeman, S.J. & Delport, G.J., 1991. Ondersoek na seleksiemaatstawwe by Afrinoskape. Genetiese parmeters van groei- en woleienskappe. S. Afr. J. Anim. Sci. 21, 162-165. [ Links ]
Bergh, L., 1990. Die gebruik van die Kleiberverhouding in vleisbeesteelt. M.Sc. (Agric.) thesis. University of the Orange Free State, Bloemfontein, South Africa. pp. 130. [ Links ]
Bromley, C.M., Snowder, G.D. & Van Vleck, L.D., 2000. Genetic parameters among weight, prolificacy, and wool traits of Columbia, Polypay, Rambouillet, and Targhee sheep. J. Anim. Sci. 78, 846-858. [ Links ]
Cloete, S.W.P., Greeff, J.C. & Lewer, R.P., 2001. Environmental and genetic aspects of survival and early live weight in Western Australian Merino sheep. S. Afr. J. Anim. Sci. 31, 123-130. [ Links ]
Galal, E.S.E., 1983. Sheep germplasm of Ethiopia. Animal Genetic Resources Information 1/83. FAO, Rome. pp. 4-12. [ Links ]
Gilmour, A.R., Cullis, B.R., Welham, S.J. & Thompson, R., 1999. ASREML Reference Manual. NSW Agriculture. NSW, Australia. [ Links ]
Greeff, J.C., Scholtz, M.M. & Roux, C.Z., 1993. Preliminary genetic parameters of growth during different growth phases in sheep. S. Afr. J. Anim. Sci. 23, 57-60. [ Links ]
Groenewald, P.G.J., Olivier, J.J. & Olivier, W.J., 1999. Heritability estimates for Merino sheep obtained from a national progeny test. S. Afr. J. Anim. Sci. 29, 174-178. [ Links ]
Hagger, C., 1998. Litter, permanent environmental, ram-flock, and genetic effects on early weight gain of lambs. J. Anim. Sci. 76, 452-457. [ Links ]
Köster, E., Van der Westhuizen, J. & Erasmus, G.J., 1994. Heritability estimates for different Kleiber ratios obtained from growth performance data in a Hereford herd. S. Afr. J. Anim. Sci. 24, 71-72. [ Links ]
Larsgard, A.G. & Olesen, I., 1998. Genetic parameters for direct and maternal effects on weights and ultrasonic muscle and fat depth of lambs. Livest. Prod. Sci. 55, 273-278. [ Links ]
Lee, J.W., Waldron, D.F. & Van Vleck, L.D., 2000. Parameter estimates for number of lambs born at different ages and 18-month body weight of Rambouillet sheep. J. Anim. Sci. 78, 2086-2090. [ Links ]
Lewis, R.M. & Beatson, P.R., 1999. Choosing maternal-effect models to estimate (co)variances for live and fleece weight in New Zealand Coopworth sheep. Livest. Prod. Sci. 58, 137-150. [ Links ]
Maniatis, N. & Pollott, G.E., 2002. Nuclear, cytoplasm and environmental effects on growth, fat, and muscle traits in Suffolk lambs from a sire referencing scheme. J. Anim. Sci. 80, 57-67. [ Links ]
Maniatis, N. & Pollott, G.E., 2003. The impact of data structure on genetic (co)variance components of early growth in sheep, estimated using an animal model with maternal effects. J. Anim. Sci. 81, 101-108. [ Links ]
María, G.A., Boldman, K.G. & Van Vleck, L.D., 1993. Estimates of variances due to direct and maternal effects for growth traits of Romanov sheep. J. Anim. Sci. 71, 845-849. [ Links ]
Matika, O., Van Wyk, J.B., Erasmus, G.J. & Baker, R.L., 2003. Genetic parameter estimates in Sabi sheep. Livest. Prod. Sci. 79, 17-28. [ Links ]
Meyer, K., 1994. Estimates of direct and maternal correlations among growth traits in Australian beef cattle. Livest. Prod. Sci. 38, 91-105. [ Links ]
Mousa, E., Van Vleck, L.D. & Leymaster, K.A., 1999. Genetic parameters for growth traits for a composite terminal sire breed of sheep. J. Anim. Sci. 77, 1659-1665. [ Links ]
Mukasa-Mugerwa, E. & Lahlou-Kassi, A., 1995. Reproductive performance and productivity of Menz sheep in the Ethiopian highlands. Small Rumin. Res. 17, 167-177. [ Links ]
Nagy, I., Sölkner, J., Komlósi, I. & Sáfár, L., 1999. Genetic parameters of production and fertility traits in Hungarian Merino sheep. J. Anim. Breed. Genet. 116, 399-413. [ Links ]
Notter, D.R., 1998. Genetic parameters for growth traits in Suffolk and Polypay sheep. Livest. Prod. Sci. 55, 205-213. [ Links ]
Notter, D.R. & Hough, J.D., 1997. Genetic parameter estimates for growth and fleece characteristics in Targhee Sheep. J. Anim. Sci. 75, 1729-1737. [ Links ]
Okut, H., Bromley, C.M., Van Vleck, L.D. & Snowder, G.D., 1999. Genotypic expression with different ages of dams , III. Weight traits of sheep. J. Anim. Sci. 77, 2372-2378. [ Links ]
Saatci, M., Ap Dewi, I. & Ulutas, Z., 1999. Variance components due to direct and maternal effects and estimation of breeding values for 12-week weight of Welsh Mountain lambs. Anim. Sci. 69, 345-352. [ Links ]
SAS. 1994. Statistical Analysis Systems user's guide. SAS Institute Inc., Cary, North Carolina, USA. [ Links ]
Schoeman, S.J., Els, J.F. & Van Niekerk, M.M., 1997. Variance components of early growth traits in the Boer goat. Small Rumin. Res. 26, 15-20. [ Links ]
Sinha, N.K. & Singh, S.K., 1997. Genetic and phenotypic parameters of body weights, average daily gains and first shearing wool yield in Muzaffarnagri sheep. Small Rumin. Res. 26, 21-29. [ Links ]
Snyman, M.A., Olivier, J.J. & Olivier, W.J., 1996. Variance components and genetic parameters for body weight and fleece traits of Merino sheep in an arid environment. S. Afr. J. Anim. Sci. 26, 11-14. [ Links ]
Satoh, M., Hicks, C., Ishii, K. & Furukawa, T., 2002. Choice of statistical model for estimating genetic parameters using restricted maximum likelihood in swine. J. Anim. Breed. Genet. 119, 285-296. [ Links ]
Tosh, J.J. & Kemp, R.A., 1994. Estimation of variance components for lamb weights in three sheep populations. J. Anim. Sci. 72, 1184-1190. [ Links ]
Vaez Torshizi, R., Nicholas, F.W. & Raadsma, H.W., 1996. REML estimates of variance and covariance components for production traits in Australian Merino sheep, using an animal model 1. Body weight from birth to 22 months. Aust. J. Agric. Res. 47, 1235-1249. [ Links ]
Van Niekerk, M.M., Schoeman, S.J., Botha, M.E. & Casey, N.H., 1996. Heritability estimates for pre-weaning growth traits in the Adelaide Boer goat flock. S. Afr. J. Anim. Sci. 26, 6-10. [ Links ]
Van Wyk, J.B., Erasmus, G.J. & Konstantinov, K.V., 1993. Variance component and heritability estimates of early growth traits in the Elsenburg Dormer sheep stud. S. Afr. J. Anim. Sci. 23, 72-76. [ Links ]
Van Wyk, J.B., Fair, M.D. & Cloete, S.W.P., 2003. Revised Models and genetic parameter estimates for production and reproduction traits in the Elsenburg Dormer sheep stud. S. Afr. J. Anim. Sci.33, 213-222. [ Links ]
Willham, R.L., 1972. The Role of Maternal Effects in Animal Breeding, III. Biometrical aspects of maternal effects in animals. J. Anim. Sci. 35, 1288-1293. [ Links ]
Yazdi, M.H., Engström, G., Näsholm, A., Johansson, K., Jorjani, H. & Liljedahl, L.-E., 1997. Genetic parameters for lamb weight at different ages and wool production in Baluchi sheep. Anim. Sci. 65, 247-255. [ Links ]
# Corresponding author. E-mail: solo_abegaz@yahoo.co.uk
Current address. P.O. Box 110284, Addis Ababa, Ethiopia














