Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.34 no.5 Pretoria 2004
Genetic assay of Caprine arthritis encephalitis in the Hungarian goat herd
Sz. KuszaI, #; Zs. BöszeII; S. KukovicsIII; A. JávorI
IUniversity of Debrecen, Centre of Agricultural Sciences, Department of Animal Breeding & Nutrition 4032, Debrecen, Böszörményi st. 138., Hungary
IIAgricultural Biotechnology Center, Dept. for Animal Biology 2100 Gödöllö, P.O. Box 411, Hungary
IIIResearch Institute for Animal Breeding and Nutrition 2053 Herceghalom, Gesztenyés st. 1., Hungary
ABSTRACT
Caprine arthritis encephalitis (CAE) is a retroviral infection of goats. CAEV is closely related to the virus which causes Maedi- Visna in sheep and AIDS in humans. The first survey of the seroprevalence of CAEV in Hungarian goats was performed. Parallel with it we tried to ascertain whether there is any association between either the susceptibility of CAE or the appearance of clinical symptoms in infected individuals and MHC II DRBP1 microsatellite polymorphism. Our aim was to encourage control measures before the infection can spread rapidly. The experiments were launched in 2003. Hungarian Milk Goats (White, Brown, Multicolour) and Saanen were selected for this initial study. Blood samples were taken for DNA extraction. Serological data showed that 30% of the Hungarian goat population was infected with Caprine Arthritis Encephalitis Virus (CAEV). Microsatellite polymorphism located within the DRBP1 MHC class II candidate gene was analysed. The genotypes of 130 animals were determined out of the 2000 animals which are planned to be examined until the end of 2004. A significant association between serological results and DRBP1 genotypes was not detected using a Chi-square test (at level 5%).
Keywords: CAE susceptibility, DRBP1, goat
Introduction
The nervous disease first reported in 1974 was named Viral Leukoencephalitis of Goats (VLG). When it was found that arthritis could also result from the same virus infection, the name of the disease was changed to Caprine Arthritis Encephalitis Syndrome (Sherman, 1992). Body excretions, which contain white blood cells are potential sources of the virus infection to other goats in the herd. CAEV may lead to chronic disease of the joints and in rare cases encephalitis in goat kids younger than six months of age (Sherman, 1992). Some infected animals which can transmit the disease may never show clinical symptoms. The best protection against disease is the prevention. This disease is widely distributed in the dairy goat populations (Contreras et al., 1998) in those countries where modern dairy technologies have been introduced for several years (Adams et al., 1982). Presence of the virus is not detectable in goat kids younger than six months of age. Therefore, linkage studies were initiated in Switzerland, Spain and France to reveal the background of the disease. Goats imported from other European countries (France, Switzerland, Netherlands) have a high prevalence of the disease and they might have introduced the disease into the Hungarian goat population.
CAEV infections are detected in two ways:
- by demonstrating the presence of antibodies to CAEV in goat serum (Adams, 1982; 1986)
- by using a polymerase chain reaction (PCR), which detects the virus's genome in the white blood cells (Vander Schale, 1994).
Dolf & Ruff (1994) demonstrated that a DNA fingerprint band is associated with susceptibility of CAE in Saanen goats. Another DNA fingerprint band was present in clinically healthy animals of the Toggenburg breed. However, DNA fingerprint experiments were not continued because the low reproducibility of the technique.
The MHC II molecules are dimeric glycoproteins which are expressed on the surface of B lymphocytes, macrophages and other antigen presenting cells (Andersson, 1990). There are different kinds of MHC class II molecules, but DQ and DR subtypes are the most polymorhic in human and domestic animals, and probably play a major role in the development of MHC restricted immune responses (Groenen et al., 1990, Van de Poel et al., 1990). In goats there are 22 different alleles of the DRB gene (Schwaiger et al., 1993).
Association studies were performed by Ruff et al. (Personal communication, 2003) using candidate microsatellite loci of the MHC class II DRBP1 gene to reveal possible association with CAE susceptibility. The Be1 serologically defined MHC class I allele segregated with disease development and a higher viral load in Swiss Saanen breed and it was associated with a 213bp allele of the DRBP1 microsatellite locus. The MHC I allele Be7 associated with another II DRBP1 allele (196bp) in which case the clinical symptoms did not appear in CAEV infected Swiss Saanen and Alpine breed.
According to our recent survey (Kukovics et al., 2003a; b; c) 30% of the Hungarian goats was infected by CAEV. The first infected animals could be traced back to the end of the 1990s and the infection supposedly arrived with imported goats and spread with their offspring. The extent of infection is different in the Hungarian breeds. It is most frequently observed in purebred and crossbred Saanen as well as in crossbred Alpine flocks. Generally the presence of the infection has a negative influence on production traits. However, its effect depends on the breed. Our results revealed that the semen quality of seropositive individuals was weaker than that of seronegative bucks (Kukovics et al., 2003a, b and c). The ratio of infected animals has increased with the size of herds in Hungary. Moreover, significant differences were found between the countries regarding the herds contracting the CAEV infection.
Materials and Methods
Three imported (Alpine, Saanen, Boer) and four local goat breeds (Hungarian Improved Goat, Hungarian Milk White Goat, Hungarian Milk Brown Goat, Hungarian Milk Multicolour Goat) are represented on Hungarian goat farms. Up to date a total of 56 Saanen and 74 Hungarian Milk Goats was examined with both a serological analysis and a microsatellite analysis. Out of them 50% was seropositive.
CAEV specific ELISA and AGID tests were performed at the Central Veterinary Institute based on the method published by Heckert et al. (1992). A DRBP1 microsatellite analysis was performed as published by Ruff et al. (1988). Genomic DNA was extracted from blood samples by standard protocol as published earlier (Bösze et al., 2000). PCR was performed in a 25 µL reaction mixture consisting of 100 ng goat genomic DNA, 0.38 µM Ready Mix (Sigma), 0.04 µM of each primer (MWG-Biotech AG). The following primers were used:
F: 5' -GGA-CAC-GTT-CTT-GCA-GAT-ACA-ACT-AC-3'
R: 5'-GAA-CTC-TCC-TTA-AGC-ATA-CTT-GCToC-3'
Thermal cycling conditions were: a denaturation step of 95 oC for 5 min., 34 cycles of 95 oC for 40 sec, 58 oC for 40 sec and 72 oC for 40 sec, followed by a final extension at 72 oC for 4 sec. Genotype analysis was carried out on an automated DNA sequencer (Alf II). Internal standards were 142, 164, 252, 275 bp, while external standards were 142, 164, 252, 275, 206 and 309 bp long. Detailed serological data of the CAEV ELISA and AGID tests from 2000 sampled goats have been presented by Kukovics et al. (2003 b). The frequency of genotypes was calculated using the following formula:
Pi= Ni/n
Where: Pi = frequency of the ith genotype
Ni = number of the „i" genotype animals
n = total number of animals
Statistical analysis were performed using SPSS for Windows 11.0 and Microsoft Excell (SPSS InC., 2001; Microsoft Corporation, 2002).
Results and Discussion
The DRBP1 MHCII microsatellite analysis revealed eight bp intervallums which were the following: 197-201 bp- A allele, 202-208 bp- B allele, 209-213 bp- C allele, 214-215 bp- D allele, 216-219 bp- E allele, 220-222 bp- F allele, 226-229 bp- G allele, 230- bp- H allele. In the Saanen breed most of the individuals was heterozygous (54%). However, in the case of the Hungarian Milk breed the rate of homozygous was higher (homozygous: 62%; heterozygous: 38%). Among Saanen goats the CC genotype had the highest frequency. The FF genotype was found only in the Hungarian Milk breed and the GG genotype only in the Saanen breed.
In the Hungarian Milk breed the frequency of the E allele was the highest. The DNA samples from Swiss reference animals were homozygous for the MHC II DRBP1 213 and 196 bp allelic variants and (kindly donated by G. Ruff) were included in the microsatellite analysis in order to establish which are the corresponding variants in the Hungarian samples. The results are presented in Table 2.
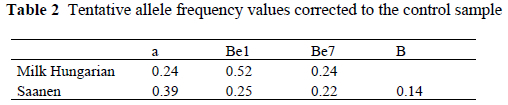
In the Saanen breed the 205-211 bp long interval microsatellite's lengths were the most frequent, while in the Hungarian Milk breed the 212- 222 bp long interval were microsatellite's lengths were the most frequent. A significant difference was not detected in the allele frequency between seropositive and seronegative goats. A significant association was not detected in the examined populations between serological results (virus infected/non infected) and MHC II DRBP1 genotypes (P < 0.05).
Conclusions
The microsatellite polymorhism is a powerful tool to differentiate between goat breeds and was successfully adapted in association studies with infectious caprine diseases. Unfortunately a reliable assay has not yet been developed for determining the susceptibility to CAE at birth. Based on our results the following further experiments will be necessary and are planned:
• To confirm the serological results with CAEV specific PCR, since serological results are strongly influenced by age and physiological status of goats;
• To reveal if there is any correlation between clinical symptoms and DRBP1 genotypes;
• To use Swiss DNA samples to determine whether Be1 MHC I cosegregates with the 213bp allele of DRBP1 in Hungarian breeds. MHC haplotypes can change between breeds and populations and it is not known how close the Hungarian Saanen is to the Swiss Saanen;
• To evaluate the correlation between MHC II DRBP1 genotypes and the development of clinical symptoms with the help of a pyrosequencing approach.
Acknowledgements
We wish to thank G. Obexer-Ruff at University of Bern, Switzerland for her valuable help and discussions. This research is financially supported by a fellowship from the Ministry of Education. The authors express their sincere thanks to the farmers for their co-operation.
References
Adams, D.S., 1982. The meaning of the agar gel immunodiffusion test (AGID) for antibody against caprine arthritis encephalitis virus (CAEV). Dairy J. 60, 17. [ Links ]
Adams, D.S. & Gorham, J.R., 1986. The gp 135 of caprine arthritis encephalitis virus affords greater sensitivity than the p28 in immunodiffusion serology. Res.Vet. Sci. 40, 157. [ Links ]
Andersson, L., 1990. Major histocompatibility genes in cattle and their significante for immune response and disease susceptibility. In: Genome analysis in domestic animals. Eds. Geldermann, H. & Ellendorff. F., VCH, Weinheim, Germany. pp. 213-223. [ Links ]
Bösze, Z., Hiripi, L., Viràg, G., Toth. S., Makovic, S., Fontaine, M.L. & Devinoy, E., 2000. Polymorphism of the rabbit kappa casein gene and its influence on performance traits. Pflugers Arch. 439 (3 Suppl), R2-3. [ Links ]
Contreras, A., Corrales, J.C., Sanchez. A., Aduriz, J.J., Gonzalez, L. & Marco, J., 1998. Caprine Arthritis -encephalitis in an indigenous Spanish breed of dairy goat. Vet. Rec. 142, 140-142. [ Links ]
Dolf, G. & Ruff, G., 1994. A DNA fingerprinting band associated with the susceptibility to CAE virus-induced arthritis in goats. Br. Vet. J. 150, 349-353. [ Links ]
Groenen, M.A.M., Van der Poel, J.J., Dijkhof, R.J.M. & Giphart, M.J., 1990. The nucleotide sequence of bovine MHC class II DQB and DRB genes. Immunogenetics 31, 37-44. [ Links ]
Heckert, R., McNab, W.B., Richardson, S.M. & Briscoe, M.R., 1992. Evaluation of an enzyme linked Immunosorbent assay for the detection of antibodies to Caprine Arthritis Encephalitis virus in goat serum. Can. J. Vet. Res. 56, 237-241. [ Links ]
Kukovics, S., Molnár, A., Abraham, M., Dani, Z., Kusza, Sz. & Fülöp, Gy., 2003a. The presence of CAE in the Hungarian goat breeds. EU konform mezogazdaság és élelmiszerbiztonság. Gödöllö. pp. 219-228. [ Links ]
Kukovics, S., Molnár, A., Abraham, M., Dani, Z., Kusza, Sz. & Fülöp, Gy., 2003b. Presence of CAEV infection in Hungarian goat industry as an effect of livestock import. In: Book of Abstracts; 54th Annual Meeting of EAAP, Rome, Italy, 31 August-3 Sept. 2003; No. 9. 320 p. [ Links ]
Kukovics, S., Molnár, A., Abrahám, M., Dani, Z., Kusza, Sz. & Fülöp, Gy., 2003c. Presence of CAEV infection in the Hungarian goat industry as an effect of livestock import. In: Buletinul, Universitatii de Stiinte Agricole Si Medicina Veterinara, Seria Zootechnie si Biotechnologii, ISSN 1454-2382, Vol. 59, 42-48 p. [ Links ]
Ruff, G. & Lazary, S., 1988. Evidence for linkage between the caprine leucocyte antigen (CLA) system and susceptibility to CAE virus induced arthritis in goats. Immunogenetics 28, 303-309. [ Links ]
Schwaiger, F.W., Buitkamp, J., Weyers, E. & Epplen, J.T., 1993. Typing of MHC-DRB genes with the help of intronic simple repeated DNA sequences. Mol. Ecol. 2, 55-59. [ Links ]
Sherman, D.M., 1992. Goat handbook [ Links ]
Tyler, J.W. & Cullors, J.S., 1989. Titers, tests and truisms: Rational interpretation of diagnostic serologic testing. J. Am. Vet. Med. Assoc. 194, 1550-1558. [ Links ]
Van de Poel, J.J., Groenen, M.A.M., Dijkhof, R.J.M., De Ruyter, D. & Giphart, M.J., 1990. The nucleotide sequence of the bovine MHC class II alpha genes: DRA, DQA and DYA. Immunogenetics 31, 29-36. [ Links ]
Vander Schale, J., 1994. Evaluation of a kinetic enzyme linked immunosorbent assay for detection of caprine arthritis encephalitis virus antibodies. J. Vet. Diagn. Invest. 6, 30-33. [ Links ]
# Corresponding author. E-mail: kusza@helios.date.hu














