Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.34 no.4 Pretoria 2004
The effects of storage condition and preservatives on maize-based diets for broiler chickens
P.B. Njobeh; P.A. Iji; I.V. Nsahlai; S.C. Slippers
Animal Science & Poultry Science, University of KwaZulu-Natal, Private Bag X01, Scottsville 3209, South Africa
ABSTRACT
A study was conducted to assess the effects of varying storage temperature and relative humidity (RH) conditions, and feed preservatives (a mixture of Mold-Zap (a fungal inhibitor) and Banox E (an antioxidant)) on performance, visceral organ weight, serum biochemistry and haematology of broiler chickens. Birds fed diets stored at low RH (50%) were heavier and had a better feed conversion efficiency (FCE) than those on diets stored at high RH (80%). Diet storage temperature had no significant effect on body weight of birds but FCE was improved when birds were maintained on diets stored at a low temperature (15 °C). Feed intake was unaffected by the main factors but the interactive effect of temperature x preservative influenced intake. Liver weight was lowest in birds that consumed feeds stored at a low temperature and low RH. The presence of a detoxifier (MTB 100) in the diet reduced the heart weight of birds by 11%. Diets stored at the low temperature or RH significantly decreased the weight of the gizzard in birds. Inclusion of the preservative in the diets also reduced gizzard weight by 4%. Similarly, gizzard weight was reduced by about 6% due to the presence of the detoxifier in the diets stored at low temperature. Inclusion of the detoxifier in the diet reduced spleen weight and the inorganic phosphorus concentration in serum. Further studies are required to test a wider range of storage conditions as well as the potential of some of the additives used in the present trial.
Keywords: Broiler chicks, performance, preservatives, relative humidity, spoilage, temperature
Introduction
For livestock feeds to be constantly available to the poultry industry, it is required that large amounts of feeds are produced and stored for a considerable period of time. Feed millers also buy ingredients during the season of production when prices are low, and such ingredients could be stored for a considerable length of time before they are used. Storage of feeds is generally associated with spoilage or loss in quality. The economic implication resulting from loss in feed quality cannot be overemphasised. Loss in feed quality depends on the nutrient composition as well as storage environmental conditions (Coker, 1979; Virginia, 1989; Blaha et al., 1990; Valarezo et al., 1997).
The use of preservatives in preventing spoilage and sorbent materials as toxin-binders has been examined but most of these studies involved the use of laboratory-produced toxins (Kubena et al., 1990; Bailey et al., 1998; Raju & Devegowda, 2000; Abdel-Wahhab et al., 2002). Studies in which the natural fungal growth pattern was monitored in diets during storage are limited. Furthermore, there is a dearth of research reports on the simultaneous assessment of the combined effects of factors involved in spoilage of feeds during storage. It is in this regard that this study was designed to determine the effect of climatic conditions and preservatives during storage on the productivity of broiler chickens.
Materials and Methods
This experiment was conducted with commercial broiler starter diets obtained from a commercial feed manufacturing company (Epol, Pietermaritzburg, KwaZulu-Natal, South Africa). Materials were received on the day of production. The composition of the diet is shown in Table 1. The apparent (AME) and true metabolisable energy (TME) values of the diets were determined using roosters (Fisher, 1982).
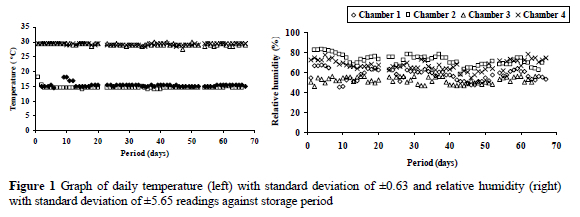
A preservative mixture composed of a mould inhibitor (Mold-Zap) and an antioxidant (Banox E), at a ratio of 1:3, i.e. 250 g/750 g per ton of feed, was obtained from Alltech Inc., and thoroughly mixed with one half of the diets. The diets were stored in 50 kg bags placed adjacent to one another (1 m apart) on wooden bases (10 cm high). Four environment-controlled chambers, each 12 x 4 x 8 m, at Ukulinga Research Farm, University of KwaZulu-Natal, Pietermaritzburg, South Africa, were used for storage. The four storage conditions were 15 °C and 50% RH; 15 °C and 80% RH; 30 °C and 50% RH, and 30°C and 80% RH. The environmental conditions were adjusted to meet the requirements of the research. Room temperature and RH were monitored and adjustments made on controlled heaters and humidifiers where necessary 10 days prior to commencement of storage. Throughout the storage period data on daily temperature and RH readings were recorded between 09:00 and 11:00 (Figure 1) with a hygro-thermometer suspended just above the bags.
At the end of the storage period (67 d), a detoxifier, MTB 100 (Alltech Inc.), an organic product derived from the cell wall of the yeast, Saccharomyces sp., was thoroughly mixed in one of the two portions of the stored feeds at a rate of 2 kg/ton of feed, according to the manufacturer's recommendation.
A 2 x 2 x 2 x 2 factorial design was used in which a commercial broiler starter diet (mash) with or without preservative mixture and with or without a detoxifier were stored in each of the four environment-controlled chambers to yield 16 main treatments. Four hundred and eighty day-old broiler chicks (Ross) were obtained from a commercial hatchery (National Chicks SA, Pty). Three replicate pens of male/female (1:1) each containing 10 chicks were randomly assigned to each treatment and fed from day-old to 21 days of age. The chicks were reared in single-tier electrically heated battery brooder cages. The chicks were weighed at the start of the trial and randomly assigned to the experimental diets and fed ad libitum until 21 days of age. Drinking water was provided ad libitum from two nipple drinkers per cage. Artificial lighting from normal fluorescent tubes was provided on a 24 hr basis throughout the study. Feed intake and body weight were measured at the end of each week. Mortality was recorded as it occurred. Room temperature was maintained in accordance with the requirements for chicks between day-old and 21 days of age.
Feed intake (FI) per week per bird was calculated as the difference between the feed-in and feed-out, all divided by the number of birds per cage per day within the week the feed was consumed. Total FI per bird was obtained by summation of the weekly FI. Body weight (Bwt) was calculated as the average weight per cage. Feed conversion efficiency (FCE) was calculated as the average weight gain divided by total FI. Other measurements were relative visceral organ weight (g/100 g body weight), inorganic phosphorus, protein, albumin and globulin concentrations in serum samples. At the end of the trial, four birds were randomly selected per pen and killed by CO2 asphyxiation. From each pen a bird was bled by cardiac puncture and a blood sample was obtained, put in a test tube and processed by centrifuge. The serum sample obtained, was put in a sealed mailing tube, snap-frozen in liquid nitrogen and immediately sent to Allerton Provincial Veterinary Laboratory (Pietermaritzburg, South Africa) for determination of inorganic phosphate (Pi), serum protein, albumin and globulin concentrations. The time taken from sacrifice to collection of serum was 10 -15 min. The birds were dissected and visceral organs, viz. liver, gizzard, spleen, pancreas and proventriculus were carefully removed, cleaned of excess fats and weighed. Serum biochemistry was analysed using UV-VIS spectrophotometer according to Heine et al. (1989), modified by the Biochemistry Laboratory, Allerton PVL, Pietermaritzburg, South Africa.
The experimental structure was a 2 x 2 x 2 x 2 factorial and data obtained in this study were analysed by the general linear model (GLM) of Minitab (Minitab Inc.). A one-way analysis of variance (ANOVA) was used to derive mean values, which were then compared by the least significant difference. Mean values were deemed to be different if level of probability was < 0.05.
Results
The effects of temperature, RH, preservative mixture and mycotoxin-binder on the performance of broiler chicks are presented in Table 2. Birds fed diets stored at the low RH had a higher (P < 0.01) Bwt than those receiving the diets stored at the high RH. This was most evident in diets that were stored at high temperature. However, storage temperature did not significantly affect Bwt of birds. It was also observed that the inclusion of the detoxifier in diets improved (P < 0.001) Bwt (Table 2). Inclusion of the preservative in the diet failed to influence the Bwt of the birds. The FCE of the birds fed diets stored at low RH was higher (P < 0.001) than that at the high RH, which was most noticeable at high storage temperature. Similarly, birds that consumed diets stored at the low temperature had higher (P < 0.05) FCE than those that were placed on diets stored at the high storage temperature. Unlike the preservative, the presence of a detoxifier in diets significantly (P < 0.01) improved the FCE of birds, regardless of storage temperature and RH. The FCE of birds fed diets containing the detoxifier was higher than that of birds on diets without this supplement. In contrast to Bwt and FCE, FI was not significantly affected by any of the factors. Among the interactions between factors tested, temperature x preservative was found to significantly (P < 0.05) influence FI. In addition, interaction between temperature and RH influenced (P < 0.01) Bwt (Table 2). The effects of other interactions on the production variables were not significant. Total mortality was less than 6% but there was no pattern with regard to treatment.
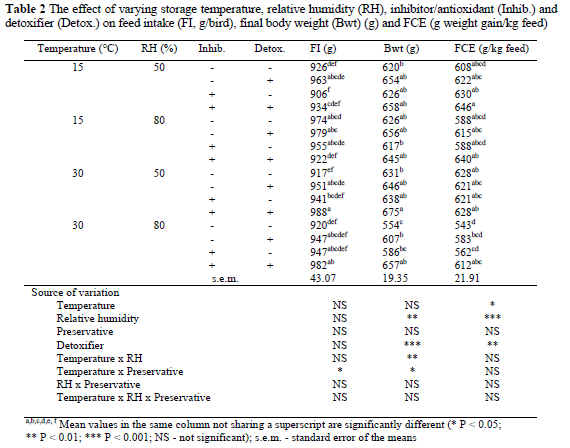
Liver weight was lowest (P < 0.001) in birds that consumed feeds stored at the low temperature (Table 3). Similarly, low storage RH reduced (P < 0.01) the liver weight of birds. Inclusion of the preservative in diets depressed (P < 0.001) liver weight. A similar reduction (P < 0.001) in the weight of liver was observed when the detoxifier was included in the diets. Interactions between the factors had no significant effect on liver weight. Storage temperature, RH and inclusion of preservative did not significantly influence heart weight. Furthermore, inclusion of the preservative in the diet did not significantly affect heart weight, although heart weight was slightly reduced. The presence of the detoxifier in the diet reduced (P < 0.001) the heart weight of the birds by 11%. Apart from a temperature x preservative interaction (P < 0.05), no other level of interaction studied, affected heart weight. Storage temperature did not significantly affect the weight of the proventriculus but at the high temperature, high storage RH increased (P < 0.001) the weight of the proventriculus weight by 4%. The RH x preservative interaction significantly (P < 0.01) affected proventriculus weight.
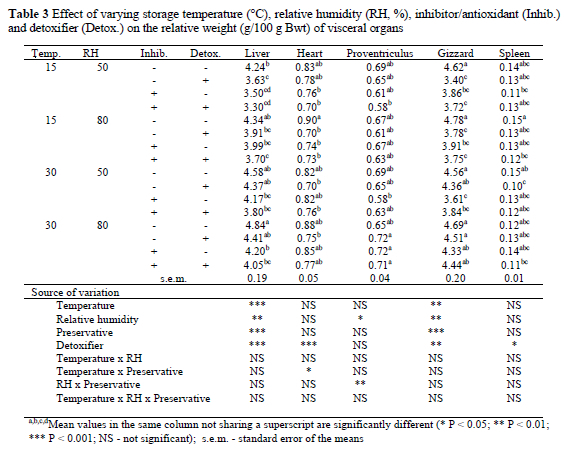
Diets stored at the low temperature or RH, decreased (P < 0.01) the weight of gizzard in birds. Inclusion of the preservative in the diets also reduced (P < 0.001) gizzard weight by 4%. Similarly, gizzard weight was reduced (P < 0.01) by about 6% due to the presence of the detoxifier in the diets stored at the low temperature. None of the levels of interactions tested, significantly influenced gizzard weight. Storage temperature, RH and the inclusion of the preservative had no significant effect on spleen weight. However, inclusion of the detoxifier in the diet reduced (P < 0.05) spleen weight.
Storage temperature and RH had no significant effect on the inorganic phosphorus concentration in serum (Table 4). The presence of the preservative also did not significantly increase serum Pi. However, inclusion of the detoxifier increased (P < 0.001) the concentration of serum Pi in birds by 3.5%. The temperature x RH x preservative interaction but not the other interactions, affected (P < 0.05) serum Pi concentration. Storage temperature and RH did not significantly affect serum protein concentration. Inclusion of the preservative in diets, however, resulted in a 6% increase (P < 0.05) in serum protein concentration. Temperature x RH x preservative also had a significant (P < 0.001) effect on serum protein.
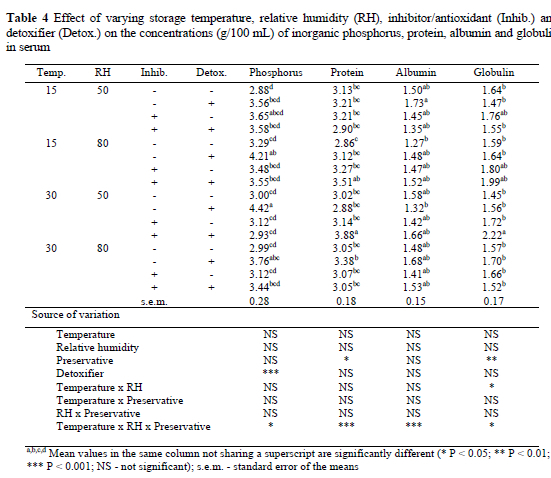
Serum albumin concentration was not affected by any of the factors studied. Furthermore, no level of interaction, except temperature x RH x preservative (P < 0.05) had any effect on the concentration of serum albumin. The concentrations of globulin in sera of birds followed a similar pattern to serum Pi, serum protein and albumin, with no significant effects of storage temperature or RH. Inclusion of the preservative in diets significantly (P < 0.01) improved globulin concentration. The detoxifier had no significant effect on globulin level in the sera of birds. Temperature x RH and temperature x RH x preservative had a significant effect (P < 0.05) on globulin level.
Discussion
The effects of temperature, RH, preservative and toxin-binder on feed intake were inconsistent and inconclusive. High temperature failed to cause off-flavour, malodour or reduced feed intake as observed in previous studies (Galliard, 1989; Lin et al., 1989; Sanders, 1989; Bautista, 1992). This may suggest that climatic conditions tested in the current study were not severe enough. In addition, supplementation of the diets with the preservative mixture to suppress oxidation and mould growth did not influence FI. It is probable that the concentration of peroxides in the diets with or without antioxidant did not attain critical levels to induce loss of appetite or feed refusal. However, at the high temperature it was found that the antioxidant significantly improved FI, which was not noticeable at the low temperature. This might be an indication that high temperature increases the rate of oxidation (Berger, 1989; Sander, 1989) as well as mould growth (Virginia, 1989; Blaha et al., 1990; Valarezo et al., 1997), which was suppressed in the presence of an antioxidant. The FI was markedly improved with detoxifier supplementation, as was previously observed by Kubena et al. (1998), in studies on toxin-binders.
Unlike FI, Bwt was significantly decreased by storage of diets at a high temperature. The low Bwt of birds fed diets stored at high temperature in high RH environment may be an indication of high peroxide levels which are known to negatively affect growth (Shermer & Calabotta, 1985). Results indicated that supplementation of Banox E or Mold-Zap did not significantly improve Bwt of birds, while supplementation with the detoxifier significantly improved body weight regardless of storage temperature and RH. A variety of sorbent materials have been widely used in preventing the occurrence of mycotoxicosis in birds. Previous reports indicated that the detoxifier (MTB 100) used in this study is quite effective, serving to bind toxins in the gastrointestinal tract to prevent their absorption (Valarezo et al., 1997; Devegowda et al., 2000).
High storage temperature and RH depressed FCE, indicating that diets stored at high temperature and high RH might have lost some quality. However, this condition was reversed when diets were supplemented with the preservative mixture. Further supplementation with the detoxifier significantly increased FCE (more evident at high temperature). The beneficial effect of the detoxifier in de-activating toxins (Bailey et al., 1998, McKenzie et al., 1998; Smith, 1999) has been well established. Increased body weight and FCE by supplemental detoxifier may have been mediated by efficient absorption and utilisation of nutrients (Van den Berghe et al., 1990). It is also possible that the mould preservative (partly composed of vitamin C) may have stimulated the immune system in preventing mycotoxicosis in chicks, leading to increased body weight and FCE.
This study demonstrated that high storage temperature and RH significantly increased liver and gizzard weights of birds, suggesting that there was probably a much higher production of toxins in diets stored under such conditions. In addition, increased liver weight may be caused by high concentrations of peroxides in diets stored at high temperatures (Sanders, 1989). Addition of the preservative mixture in diets was effective in reducing liver and gizzard weights through a reduction in toxin production. Results also demonstrated the ability of the detoxifier to decrease organ weight except for the weight of the proventriculus. How this will affect feed utilization is unknown.
Significant differences were obtained in the concentration of serum Pi when diets were supplemented with the detoxifier. Birds that were offered diets supplemented with the detoxifier had higher Pi concentrations than those without detoxifier, similar to results reported for ducklings and broilers exposed to aflatoxins (Khajarern & Khajarern, 1999). The concentration of serum protein was increased in birds fed diets supplemented with the preservative and the detoxifier. Similar results were reported by Raju & Devegowda (2000) and Abdel-Wahhab et al. (2002). Toxins impair protein synthesis (Bailey et al., 1998; Kubena et al, 1998).
Conclusion
There was some effect of storage conditions on feed quality, as measured by animal performance. There is also potential for the additives tested in the trial. Further studies are required to establish the impact of more severe climatic conditions on actual feed properties.
Acknowledgement
We acknowledge the assistance of Alltech Inc. for donating the feed preservative and detoxifier that were used in this study.
References
Abdel-Wahhab, M.A., Naba, S.A. & Khalil, F.A., 2002. Physiological and toxicological responses in rats fed aflatoxin-contaminated diet with or without sorbent materials. Anim. Feed Sci. Technol. 97, 209-219. [ Links ]
Bailey, R.H., Kubena, L.F., Harvey, R.B., Buckley, S.A. & Rottinghaus, G.E., 1998. Efficacy of various inorganic sorbents to reduce the toxicity of aflatoxin and T-2 toxin in broiler chickens. Environment and Health 77, 1623-1630. [ Links ]
Bautista, M.N., Subosa, P.F. & Celia, R.L., 1992. Effects of antioxidants on feed quality and growth of Penaeus monodon juveniles. J. Sci. Food Agric. 1, 55-60. [ Links ]
Berger, K.G., 1989. Practical measures to minimize rancidity in processing and storage. In: Rancidity in Foods. 2nd ed. Eds. Allen, J.C. & Hamilton, R.J., Elsevier Applied Science. London and New York. pp. 67-82. [ Links ]
Blàha, J., Tamchinova, J. & Reisnerova, H., 1990. The occurrence of moulds and aflatoxin B1 in Vietnamese feeds. J. Trop. Sci. 30, 21-31. [ Links ]
Cabel, M.C., Waldroup, P.W., Shermer, W.D. & Calabotta, D.F., 1989. Effect of ethoxiquin feed preservative and peroxide level on broiler performance. Poult. Sci. 67, 1725-1730. [ Links ]
Coker, R.D., 1979. Aflatoxin: Past, present and future. Trop. Sci. 21, 143-162. [ Links ]
Devegowda, G., Raju, M.V., Nazar, A. & Swamy, H.V., 1998. Mycotoxin picture worldwide: Novel solutions for their counteraction. In: Biotechnology in the feed industry. Proc. Alltech's 14th Annual Symp. Nottingham Univ. Press. pp. 241-255. [ Links ]
Fisher, C., 1982. Energy values of compound poultry feeds. Occasional Publication No. 2, Agricultural Research Council. Poultry Research Centre, Roslin, UK. [ Links ]
Galliard, T., 1989. Rancidity in cereal products. In: Rancidity in foods. (2nd ed.). Eds. Allen, J. & Hamilton, R.J., Elsevier Applied Science, London & New York. pp. 141-160. [ Links ]
Heine, E.P., Dekker, F.A. & Hudson, N., 1989. Methods in biochemistry, heamatology and toxicology. Veterinary Services, Directorate of Animal Health, USA. [ Links ]
Khajarern, J. & Khajarern, S., 1999. Protective effects on mycosorb against aflatoxicosis in ducklings and broilers. In: Mycosorb Technical Dossier, Alltech. Alltech Inc., Nicholasville, KY, USA. pp. 25. [ Links ]
Kubena, L.F., Harvey, R.B., Bailey, R.H., Buckley, S.A. & Rottinghaus, G.E., 1998. Effects of a hydrated sodium calcium aluminosilicate (T-BindTM) on mycotoxicosis in young broiler chickens. Poult. Sci. 77, 1502-1509. [ Links ]
Kubena, L.F., Harvey, R.B., Phillips, T.D., Corrier, D.E. & Huff, W.W., 1990. Diminution of aflatoxicosis in growing chickens by the dietary addition of a hydrated sodium calcium aluminosilicate. Poult. Sci. 69, 727-735. [ Links ]
Lin, C.F., Asghar, A., Gray, J.I., Buckley, D.J., Booren, A.M., Crackel, R.L. & Flegal, C.J., 1989. Effects of oxidized dietary oil and antioxidant supplementation on broiler growth and meat stability. Br. Poult. Sci. 30, 855-864. [ Links ]
Mckenzie, K.S., Kubena, F., Denvir, A.J., Rogers, T.D., Hitchens, G.D., Bailey, R.H., Harvey, R.B., Buckley, S.A. & Phillips, T.D., 1998. Aflatoxicosis in turkey poults is prevented by treatment of naturally contaminated corn with ozone generated by electrolyte1,2. Poult. Sci. 77, 1094-1102. [ Links ]
Minitab Inc., 1998. Minitab Release 12.2. Minitab Inc, State College, PA, 16801-3008, USA. [ Links ]
Raju, M.V. & Devegowda, G., 2000. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin, and t-2 toxin). Br. Poult. Sci. 41, 640-650. [ Links ]
Sanders, T.A., 1989. Nutritional aspects of rancidity. In: Rancidity in foods. (2nd ed.). Eds. Allen, J. & Hamilton, R., Elsevier Applied Science, London & New York. pp. 125-139. [ Links ]
Shermer, W.D. & Calabotta, D.F., 1985. Oxidation of feed: How much has occurred. Feedstuffs 4, 19-20. [ Links ]
Smith, T.K., 1999. Effect on feeding grains contaminated with mycotoxins with or without MTB-100 on performance and incidence of carcass bruising in broiler chickens. In: Mycosorb technical Dossier. Alltech. Alltech Inc., Nicholasville, KY, USA. pp. 25. [ Links ]
Valarezo, S., Jacques, K.A., Weir, J. & Obregon, H., 1997. Comparative effects of antibiotic, mannaoligosaccharide and mycotoxin absorbent on performance of commercial broilers fed pelleted diets. In: Mycosorb Technical Dossier, Alltech Inc., Nicholasville, KY, USA. pp. 25. [ Links ]
Van den Berghe, C.H., Ahouangninou, P.O. & Deka, E.K., 1990. The effect of antioxidant and mold inhibitor on feed quality and the performance of broilers under tropical conditions. Trop. Sci. 30, 5-13. [ Links ]
Virginia, N.S., 1989. Factors to control microbial spoilage of refrigerated foods. J. Food Protection 52 (6), 431-435. [ Links ]
# Corresponding author. E-mail: Ijipa@ukzn.ac.za
1 Present address: Meadow Feeds Natal, Ohrtmann Road, Willowton 3201, South Africa














