Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Animal Science
versión On-line ISSN 2221-4062
versión impresa ISSN 0375-1589
S. Afr. j. anim. sci. vol.34 no.4 Pretoria 2004
Comparison of the thermostability and efficacy of a solid-substrate culture and a liquid culture phytase in broiler chickens
J.P. DriverI, ; J.L. PierceII; J.H. Harter-DennisIII; J. TimmonsIII; N.H. CaseyI
IDepartment of Animal & Wildlife Sciences, University of Pretoria, Pretoria 0002, South Africa
IIAlltech Inc., Nichollasville, KY, USA
IIIUniversity of Maryland Eastern Shore, Princess Anne, MD, USA
ABSTRACT
A 14-day battery trial using 336 four-day old male broilers was undertaken to compare the thermostability and efficacy of phytase produced in solid-substrate culture, a Solid Substrate Enzyme Product (SSEP), with phytase produced in liquid culture, a Liquid Enzyme Product (LEP). Five maize-soyabean meal based diets were formulated; three contained increasing levels of non-phytate phosphorus (nPP) (2.5, 3.0 and 3.5 g nPP/kg) and another two contained 2.5 g nPP/kg with 300 phytase activity (PU)/kg of either SSEP or LEP. Half of each diet was pelleted at 80 °C using a California Pelleting Mill Co. Master Model (30 hp) pelleter and conditioner. Both pelleted and mash diets were passed through a hammer mill before feeding so that a consistent particle density was achieved for all treatments. Chicks fed the diets that were first pelleted had lower weight gain, feed intake, percentage tibia and toe ash accumulation and bone breaking strength values compared to chicks fed the mash diets, although differences were not significant. Only feed efficiency was improved by pelleting the diet first. There was a significant improvement in all parameters, with the exception of feed efficiency, as the level of nPP was increased from 2.5 g/kg to 3.5 g/kg. Either variety of phytase on the unpelleted mash diet did not improve performance significantly over the unsupplemented diets with different nPP levels, although the SSEP did appear to be better than the LEP. When the same diets were pelleted, chicks that received the SSEP did not perform better than those on the 2.5 g/kg diets with no added phytase, while chicks fed the LEP performed similarly to those receiving 3.0 g/kg and 3.5 g nPP/kg. Both phytases improved percentage tibia and toe ash accumulation as well as bone breaking strength over the control (2.5 g nPP/kg) on the unpelleted mash. Pelleting completely destroyed the SSEP while the LEP was still able to show results similar to the 3.5 g nPP/kg. Treatment for tibia ash and the 3.0 g nPP/kg. Treatment for percentage toe ash and bone breaking strength. Although the SSEP was superior to the LEP on the unpelleted mash for all variables, the results of this experiment did not show any significant differences between the two phytase varieties with the exception of feed efficiency. Pelleting at 80 °C appeared to completely destroy the SSEP while a certain degree of enzyme activity was retained after pelleting the LEP.
Keywords: Broiler, phytase, non-phytate phosphorus, thermostability
Introduction
Environmental concerns and a well-informed public have advanced the search for biological alternatives to the traditional practice of supplying pigs and poultry with hefty safety margins for phosphorus. The search has so far yielded a number of fungi able to produce the enzyme phytase. This enzyme is capable of liberating phosphorus from phytic acid, an otherwise inaccessible source of phosphorus for monogastric animals. Unfortunately several phytase products, while performing well when fed in mash feed, are unable to withstand the high temperatures of pelleting. This is extremely significant in view of the fact that poultry feeds must typically endure high pelleting temperatures. Simoes Nunes (1993) reported that steam-pelleting feed at temperatures in excess of 60 °C strongly reduces the activity of phytase produced by the fungus Aspergillus niger. It was further demonstrated that this reduction increased as temperatures increased with only 50% of the original activity being detected at 80 °C, a common commercial pelleting temperature.
Fungal phytase is manufactured through two distinct processes, liquid culture fermentation and solid substrate culture fermentation. Liquid culture fermentation is the fermentation technique traditionally used for the production of microbially derived enzymes and involves the submersion of the microorganism in an aqueous solution containing all the nutrients needed for growth. Two important criteria of liquid culture fermentation include the ability of the system to operate aseptically for a number of days and provide adequate aeration and agitation to meet the metabolic requirements of the microorganism. The most common designs are based on a stirred upright cylinder with sparger aeration (Filer, 2000). Organisms used in this type of production system are typically genetically altered to enhance production of the desired product, in this case the phytase enzyme.
Solid substrate culture fermentation is an ancient technology based on the growth of microorganisms on water-insoluble substrates in the presence of varying amounts of free water (Mitchell & Losane, 1992). The single most important feature of solid substrate culture fermentation is the low water content of the medium, which favours the growth of filamentous fungi. These grow well at water activities of between 0.93 and 0.98 while bacteria and yeasts grow at a water activity of above 0.99 (Filer, 2000). The substrates used for this type of fermentation are composite and heterogeneous products from agriculture or by-products of agro-industries (Filer, 2000). Organisms used in solid substrate culture fermentation are selected for increased phytase production but are not genetically modified.
This study was carried out to investigate the effectiveness with which fungal phytase, manufactured through the two distinct fermentation processes described, is able to withstand the high temperatures associated with typical steam-pelleting conditions by means of a chick performance experiment. It was also the intention of the study to ascertain whether the solid substrate culture phytase product results in superior performance due to the detectable levels of side activities present, including cellulase, protease, and xylanase, which are not present in the liquid culture product.
Materials and Methods
Four hundred male day-old Ross x Ross broilers were placed on a standard maize-soyabean based starter diet containing 5.0 g non-phytate phosphorus (nPP)/kg from zero to three days of age (Table 1). On day four, after an overnight fast, 336 of these chicks were then wing banded, weighed and fed the treatment diets to day 18. A randomised complete block design was used with five replicates per treatment with the exception of the 3.5 g nPP/kg treatments where only four replicates were used due to the limitations of the facilities. Seven chicks were allocated to each replicate. The study consisted of a 2 x 5 factorial arrangement with two feed types (unpelleted mash vs. pelleted) and three levels of dietary nPP (2.5 g/kg, 3.0 g/kg, 3.5 g/kg). Furthermore, diets containing 2.5 g nPP/kg were supplemented with 300 PU/kg SSEP or LEP (2.5 g/kg + 300 PU SSEP/kg and 2.5 g/kg + 300 PU LEP/kg).
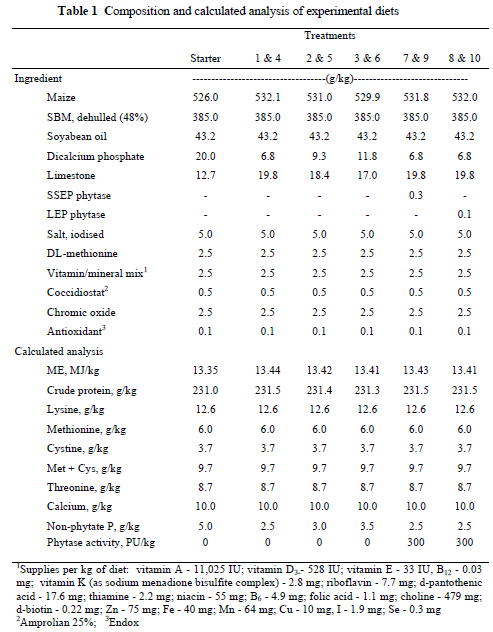
The experimental diets were maize-soyabean based (Table 1). Dicalcium phosphate and limestone were added to the diets to supply nPP and calcium. All experimental diets were formulated to contain 10.0 g calcium/kg and NRC (1994) recommendations for crude protein and ME were met. Treatments 1-3 and 4-6 contained 2.5, 3.0 and 3.5 g nPP/kg respectively (Table 1). These diets were formulated to provide standard response curves. Treatments 7 and 9 contained 2.5 g nPP/kg with the addition of 300 PU/kg SSEP. Treatments 8 and 10 also contained 2.5 g nPP/kg but with the addition of 300 PU/kg LEP. The inclusion rates for the enzymes were determined on an equivalency basis using an in vitro enzyme assay. It was found that either 0.3 g SSEP/kg diet or 0.1 g LEP/kg diet was equivalent to 300 PU/kg of phytase activity.
Treatments 1, 2, 3, 7, and 8 were unpelleted mash diets while the same diets were pelleted to make up Treatments 4, 5, 6, 9, and 10. Diets were steam-pelleted using a California Pelleting Mill Co. Master Model (30 hp) pelleter and conditioner. A 3.2 mm pelleting die was employed with a width of 50.8 mm and a relief of 12.7 mm. A pelleting temperature of 80 °C was selected. Both pelleted and mash diets were passed through a hammer mill before feeding so that a consistent particle density was achieved for all treatments. The chicks were allowed ad libitum access to their respective diets and water. The chicks were housed in stainless steel batteries in an environmentally controlled room. Fluorescent lighting was used to illuminate the facility 24 hours a day. The chicks were fed from feed troughs placed in each battery.
Mortalities were monitored on a daily basis and the feed intake of each pen was recorded. All chicks were slaughtered and weighed on day 18 and the right tibia of each chick was removed and frozen. The tibias were then thawed and the flesh and tibial caps removed. The bone breaking strength was subsequently determined by placing each individual bone across a set of rollers and applying a constant pressure to each using an Instron machine. The force in kilograms required to break through the centre of each bone was recorded. All pieces of bone were collected after breaking and pooled by pen for percentage tibia ash determination, measured according to AOAC (1995) procedures. The third toe of each foot was severed between the third and fourth phalangeal joint, pooled according to pen and dried at 100 °C. The toes were then weighed, ashed and reweighed to determine percentage toe ash.
The statistical analysis of the data was performed using the PC-SAS Version 8.01 commercial software. Duncan's Multiple Range test was used to determine the significance of differences between treatment means at a P < 0.05 level.
Results and Discussion
There was no significant interaction between feed type and phosphorus level or feed type and phytase supplementation for average weight gain, average feed intake or feed efficiency over the course of the trial. Therefore only main effects were analysed for these variables (Table 2).
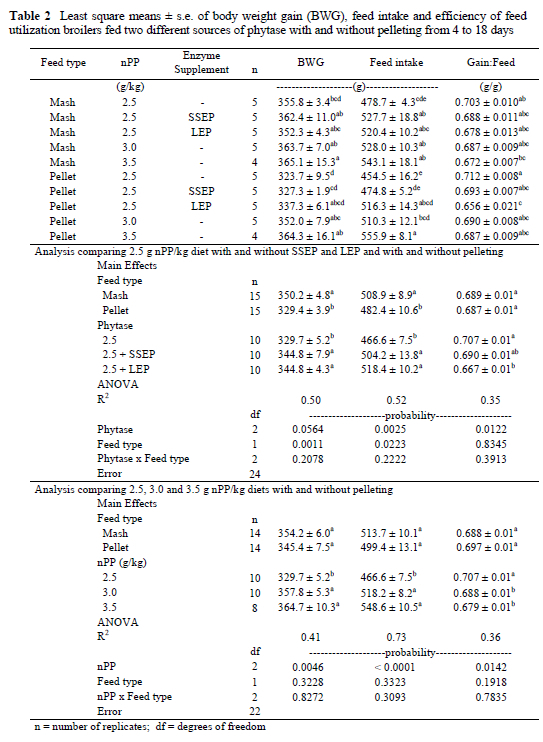
Increasing the nPP concentration in the diet from 2.5 to 3.5 g/kg with inorganic phosphorus, resulted in an increase in the least squares means for average body weight gain and average feed intake and reduced feed efficiency. However, the difference between the chicks fed the 3.0 g nPP/kg and 3.5 g nPP/kg was not significant for any of these three variables. No significant difference in weight gain and feed intake was observed between the 2.5 g nPP/kg + SSEP or the 2.5 g nPP/kg + LEP treatments, or the other levels of nPP when the unpelleted mash diets were compared. However, this changed when diets were pelleted. Chicks that received the SSEP did not perform better than those on the 2.5 g/kg diets with no added phytase, while chicks fed the LEP, grew and consumed similarly to those receiving 3.0 g/kg and 3.5 g nPP/kg (Table 2). This suggests that a proportion of the LEP phytase survived pelleting and increased the availability of phosphorus in the low nPP diet, while the SEPP appeared to be all but destroyed.
The positive body weight gain and feed intake responses to phytase supplementation on unpelleted mash diets observed in this experiment were similar to responses reported in the literature (Simons et al., 1990; Broz et al., 1994; Sebastian et al., 1996; Yi et al., 1996). This may have been, in addition to increasing phosphorus availability, the consequence of improved energy, protein and amino acid utilization.
Chicks fed the lowest level of nPP were consistently the most feed efficient and increasing dietary phosphorus by increasing levels of inorganic phosphorus or improving phosphorus availability by adding phytase reduced efficiency (Table 2.). Significant interaction effects were found between feed type and phytase supplementation for percentage tibia ash and percentage toe ash variables as well as bone breaking strength. Therefore individual treatment means were compared (Table 3).
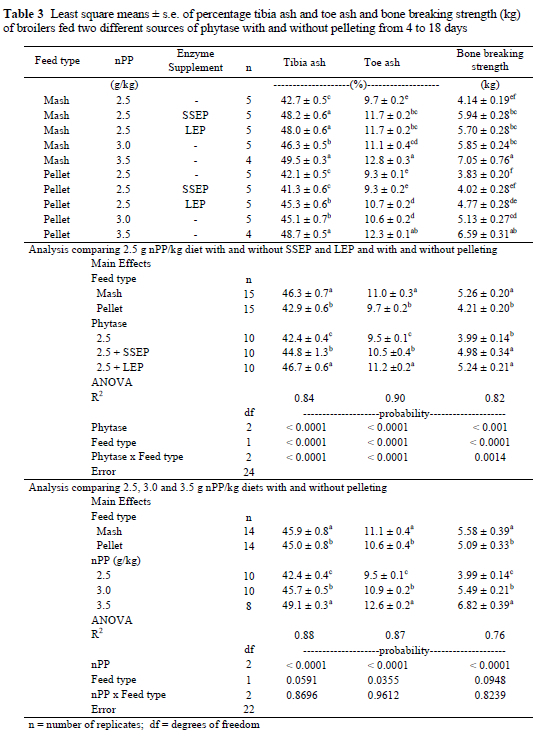
Increasing the percentage nPP in the mash and the pelleted treatments produced a significant linear improvement in percentage tibia ash, percentage toe ash and bone breaking strength (Table 3 and Figure 1). Although, neither pelleted nor mash treatments demonstrated significantly superior results over the other at similar nPP levels, quantitatively, the mash diets performed better than the pelleted diets for all three variables. Pelleting in itself may or may not affect the absorbability or availability of phosphorus and considerable disparity on this point exists in the literature. Summers et al. (1967), Bayley & Thomson (1969), Bayley (1975) and Joengbloed (1987) all reported improved absorption of phosphorus with steam pelleting of mixed feeds, including maize-soyabean meal diets. This phenomenon might have been as a result of the increase in crushed cells, which could enhance the hydrolysis of phytic acid by native phytases (Joengbloed & Kemme, 1990). Ross et al. (1983), on the other hand, found the nPP of maize unaffected by pelleting while Harrold et al. (1982) showed a reduction in nPP when pelleting barley, oats, maize or soyabean meal separately.
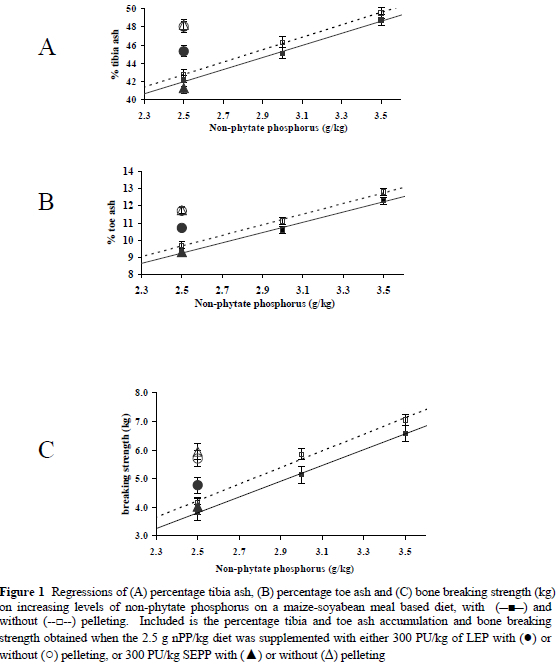
No significant differences in percentage tibia and toe ash or bone breaking strength were detected between the SSEP and LEP supplemented mash treatments. Furthermore, chicks fed either of the two enzyme treatments were not significantly different from those on the 3.5 g nPP/kg mash diet for the percentage tibia ash variable. Interestingly, percentage toe ash and bone breaking strength values for these same chicks were similar to those fed 3.0 g nPP/kg and significantly lower than chicks on 3.5 g nPP/kg (Table 3 and Figure 1).
When comparing tibia ash, toe ash and breaking strength for the pelleted diets, responses imitated those already reported for weight gain and feed intake, i.e. it was found that the SSEP treatment (2.5 g nPP/kg + SSEP) produced similar values to the 2.5 g nPP/kg treatment (Table 3 and Figure 1) while the 2.5 g nPP/kg + LEP pelleted diet produced values similar to the 3.0 g nPP/kg pelleted diet for all three variables (Table 3 and Figure 1). The percentage tibia ash and toe ash accumulation and bone breaking strength were improved substantially on the mash supplemented with either variety of phytase. This clearly illustrated the ability of the phytase enzyme to liberate phosphorus from phytic acid (Table 3). Although both enzyme supplements behaved similarly in the mash, the susceptibility of the SSEP and LEP phytases to the extreme temperatures and pressures of pelleting, as described under the materials and methods, differed when measured using the three bone variables as criteria even though both phytase products were produced from the same Aspergillus niger gene (A. niger 3135). The improvement in thermostability was probably as a result of downstream processing. However, due to the proprietary nature of the information regarding the manufacturing process of each enzyme, the exact reason behind the dissimilarity could not be established. The most common methods of increasing thermostability by way of processing includes granulation, absorption of the enzyme to a carrier, coating of the enzyme, or a combination of these methods (Cowan & Rasmussen, 1993).
Conclusion
Of the two phytases tested, the enzyme produced under liquid culture conditions, LEP, seemed to be better able to withstand the pelleting conditions described under the materials and methods. The enzyme produced under solid substrate culture conditions, SSEP, appeared to be completely destroyed at 80 °C. This contrasted with the performance of the two enzymes on the mash, where the SSEP appeared to generate slightly better results, possibly due to the addition enzymes in the product including cellulose, protease and xylanase. The difference in thermostability was probably due to differences in downstream processing.
The results of this study underline the importance of establishing the degree of enzyme deactivation that is likely to occur during feed processing before substituting a microbial phytase for inorganic phosphorus. The amount of deactivation that occurs is particular to the pelleting conditions as well as to the enzyme.
Acknowledgements
The authors would like to thank Alltech Inc., who funded this study and H. Borrain and R. Owen of the University of Pretoria Statistics Department who helped with the analysis of the data.
References
AOAC, 1995. Official methods of analysis (16th ed.). Association of Official Analytical Chemists, Inc. Gaithersburg, Maryland, USA. [ Links ]
Bayley, H.S., 1975. Influence of steam pelleting and dietary calcium level on the utilization of phosphorus by the pig. J. Anim. Sci. 40, 857-863. [ Links ]
Bayley, H.S. & Thomson, R.G., 1969. Phosphorus requirements of growing pigs and effects of steam pelleting on phosphorus availability. J. Anim. Sci. 28, 484-491. [ Links ]
Broz, J., Oldale, P., Perrin-Voltz, A.H., Rychen, G., Schulze, J. & Simoes Nunes, C., 1994. Effect of supplemental phytase on performance and phosphorus utilization in broiler chickens fed a low phosphorus diet without addition of inorganic phosphates. Br. Poult. Sci. 35, 273-280. [ Links ]
Cowan, W.D. & Rasmussen, P.B., 1993. Thermostability of microbial expander and pelleting processes and application systems for post pelleting addition. In: Proc. First Symp. Enzymes in Animal Nutrition. Kartause Ittingen, Switzerland. pp. 263-267. [ Links ]
Filer, K., 2000. Production of enzymes for the feed industry using solid substrate fermentation. In: Biotechnology in the Feed Industry. Proc. Alltech's 16th Annual Symp. Eds. Lyons, T.P. & Jacques, K., Nottingham University Press. pp. 131-152. [ Links ]
Harrold, R.L., Johnson, J.N., Slanger, W.D. & Haugse, C.N., 1982. The bioavailable phosphorus content of some common North Dakota feedstuffs. N. D. Farm. Res. 40, 22-24. [ Links ]
Joengbloed, A.W., 1987. Phosphorus in the feeding of pigs; effect of diet on the absorption and retention of phosphorus by growing pigs. I.V.V.O. Rep. No. 179, Leyland, The Netherlands. [ Links ]
Joengbloed, A.W. & Kemme, P.A., 1990. Effect of pelleting mixed feeds on phytase activity and the apparent absorbability of phosphorus and calcium in pigs. Anim. Feed Sci. Technol. 28, 233-242. [ Links ]
Mitchell, D.A. & Losane, B.K., 1992. Definition, Characteristics and Potential. Ch 1 in: Solid Substrate Cultivation. Eds. Doelle, H., Mitchell, D.A. & Rolz, C.E., Elsevier Applied Science, London. pp. 1-13. [ Links ]
NRC, 1994. Nutrient Requirements of Poultry (9th ed.). National Academy Press, Washington D.C., USA. [ Links ]
Ross, R.D., Cromwell, G.L. & Stahly, T.S., 1983. Biological availability of the phosphorus in high moisture and pelleted corn. J. Anim. Sci. 57, 96 (Abstract). [ Links ]
Sebastian, S., Touchburn, S.P., Chavez, E.R. & Lague, P.C., 1996. The effects of supplemental microbial phytase on the performance and utilization of dietary calcium, phosphorus, copper, and zinc in broiler chickens fed corn-soybean meal diets. Poult. Sci. 75, 729-736. [ Links ]
Simoes Nunes, C., 1993. Evaluation of phytase resistance in swine diets to different pelleting temperatures. In: Proc. First Symp. Enzymes in Animal Nutrition, Kartause Ittingen, Switzerland. pp. 269-271. [ Links ]
Simons, P.C.M., Versteegh, H.A.J., Joengbloed, A.W., Kemme, P.A., Slump, P., Bos, K.D., Wolters, M.G.E., Beudeker, R.F. & Verschoor, G.J., 1990. Improvement of phosphorus availability by microbial phytase in broilers and pigs. Br. J. Nutr. 64, 525-540. [ Links ]
Summers, J.D., Slinger, S.J. & Cisneros, G., 1967. Some factors affecting the biological availability of phosphorus in wheat by-products. Cereal Chem. 44, 318-323. [ Links ]
Yi, Z., Kornegay, E.T., Ravindran, V. & Denbow, D.M., 1996. Improving phytate phosphorus availability in corn and soybean meal for broilers using microbial phytase and calculation of phosphorus equivalency values of phytase. Poult. Sci. 75, 240-249. [ Links ]
# Corresponding author. E-mail: jdriver@uga.edu














