Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.34 no.4 Pretoria 2004
Effects of different hatcher temperatures on hatching traits of broiler embryos during the last five days of incubation
I. Yildirim; R. Yetisir
Department of Animal Science, Faculty of Agriculture, Selcuk University, 42031, Campus, Konya, Turkey
ABSTRACT
This study deals with the effects of different hatcher temperatures on hatching traits in modern commercial broiler eggs during the last five days of incubation. The hatching eggs were obtained from a 52-wk old (Ross 308) flock. All eggs were distributed randomly into one incubator and incubated for 17 d using uniform conditions (37.6 ± 0.5 °C and 58% relative humidity). At the start of 18th days of incubation, the eggs were randomly distributed to four experimental hatching cabinets. The temperatures were set in the cabinets at 36.1, 37.2, 38.3 and 39.4 °C from 17 d of incubation until hatch. Hatching time, hatchability, age of mortality and the incidence of embryo malpositions were recorded as percentage of fertile eggs. The highest mean embryonic heat production or eggshell surface temperature occurred in the hatching cabinets operated at 39.9 °C and lowest at 36.1 °C. Eggs incubated at 37.2 °C and 38.3 °C had a significantly higher hatchability than the other treatment groups. High embryo mortality at the late term stage of development was recorded at low (36.1 °C) and very high temperatures (39.9 °C). No significant difference in the incidence of malpositions was observed among the groups. These findings revealed that hatchability might be improved if incubation temperatures of 37.2 °C to 38.3 °C are used during last five days of incubation. The results indicate that the modern hatching broiler egg shows almost similar pattern as past generations for heat production and temperature in hatchers during the last five days of incubation. In other words, in spite of genetic improvements in the modern broilers, the incubation conditions and techniques remained largely unchanged.
Keywords: Incubation, temperature, last five days, Ross broilers
Introduction
The development of the avian embryo is temperature-dependent (Al-Thani & Simkiss, 1992; French, 1994) and a change in temperature of only 1 °C from the optimum can have a major impact on hatch results (French, 1994). Temperature dictates the rate of embryonic growth and the successive proportional development of the different organs and body structures of the embryo in time, and because of this, also the final result of the hatching process, both in quantity and quality (Meijerhof, 1999). The optimum temperature for successful hatch of eggs in a forced draft incubator is between 37 and 38 °C (Lundy, 1969). Variations in incubation temperature could have profound effects on embryonic growth and metabolism (Zhang & Whittow, 1992). The most apparent changes in the development of the embryo occur during the third week of incubation. During this period the needs of the embryo are greatest and the ability of incubator environment to provide respiratory gas at the right levels prove to be most difficult (Wineland, 1996). In all species the increase in metabolic rate of eggs during the incubation period reaches a plateau after about 80% of the incubation period has been completed. The length of this plateau period is about two days (days 17-18) in chickens (Dietz et al., 1998). An important physiological process occurs around the time of transfer from the setter to the hatcher is the achievement of plateau metabolism and high oxygen demand, which coincides with internal pipping (Swann & Brake, 1990). A lower incubation temperature during the last third of incubation would allow normal hatch and reduce water loss but increase the incubation period (Wilson, 1991). Increased incubator temperatures are known to accelerate growth rates of avian embryos (Romanoff, 1960; Wilson, 1991). The effects of high incubation temperature have also been shown to depend on the stage of chick embryo development, although there are differences between studies as to when during the incubation period, embryos are most susceptible to high temperatures (French, 2000). In the early 1950s and 1960s numerous studies have been conducted to quantify the metabolic heat production of eggs, and these data are still used to a large extend. The question is whether the incubating eggs of modern breeds produce the same amount of heat as 40 years ago. Due to the increased genetic growth potential of the birds, the heat production might have increased (Meijerhof, 1999).
The objective of the present study was to determine the effects of incubator temperatures during the last five days of incubation upon eggshell temperature, hatching time and other hatching traits of modern broiler eggs.
Materials and Methods
Eggs were obtained from a 52-wk-old flock (Ross 308) and held at 18 °C and 70% relative humidity (RH) for five days before setting. Experimental incubation setter and cabinets simulating commercial incubators were manufactured and used in the study. Each incubator cabinet (IC) contained four incubator trays with a capacity for 400 eggs each. Digital thermostats connected to microprocessors with temperature sensitivity of ± 0.1 °C controlled the wet and dry bulb temperatures. Digital thermometers were used in each cabinet to verify set point temperatures. Initially all eggs were distributed randomly into one experimental incubator and were incubated for 17 d under uniform conditions (dry bulb temperature 37.6 ± 0.5 °C and 58% RH). The experimental setter was monitored twice daily to ensure proper operation. After day 17 (408 h), the eggs were transferred at random to four hatchers which operated at different temperatures. The first group of eggs was placed in an IC operating at a temperature of 36.1 °C (Low-L). The second group was placed in an identical IC operating at 37.2 °C (Control-CON), the third group in an IC at 38.3 °C (High-H) and the fourth in an IC at 39.4 °C (Very High-VH). During the study RH was kept at around 75% in all IC's. Four replicates per treatment were conducted with about 240 eggs per group (960 eggs in all groups). At the time of transfer, all eggs were candled for evidence of live embryos. Eggs showing no evidence of a live embryo were removed from the trial. Eggs with viable embryos were transferred to wire pedigree baskets where they were allowed to hatch.
Measuring internal egg temperature is problematic, though there is only small difference between internal and eggshell surface temperatures (French, 1997). Therefore, the embryo temperatures were taken indirectly on the eggshell surface. The eggshell surface temperature (Tes) was monitored every six hours from 411 h to end of the 20.4th (489 h) day of incubation, using an ear thermometer (Braun ear thermometer, Thermoscan type; IRT 3020, Germany). Time of hatch (HT) was monitored every 6 h from days 19.8 (475.2 h) to 21.5 (516 h). At pull time all unhatched eggs were opened and examined macroscopically to determine percentage of late term mortality. Any gross pathology was classified as described by French (1994). Infertile, cracked or contaminated eggs containing early and mid dead embryos were excluded from the data analysis. All results (except for Tes measurement) were expressed as a percentage of fertile eggs incubated, unless otherwise stated.
Two-way analyses of variance were conducted for Tes. The results for the incubation variables (hatching time, hatchability of fertile eggs, mortality stages and embryo malpositions) were analysed by ANOVA (one-way) with the Minitab (1998) statistical program. The differences between means were obtained by Mstat - Range program (1989), using a Duncan Multiple Range Test.
Results
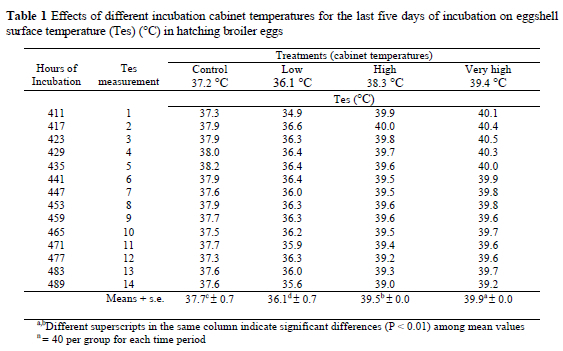
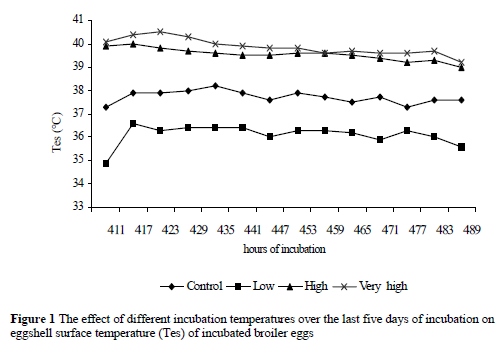
The temperatures of the eggshell surfaces in the different treatments increased (P < 0.01) in correspondence with the temperatures of the incubation cabinets (Table 1 and Figure 1)
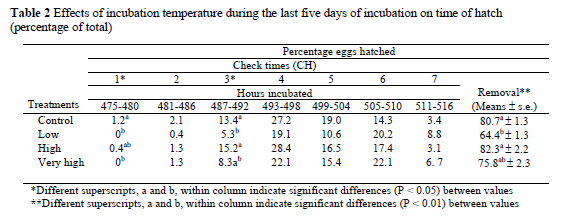
Hatchability was affected by the treatments in some check hours of incubation (CH) period. In the first CH period (between 475-480 hours) the highest (P < 0.05) hatchability was obtained in the CON group compared to other treatment groups. In contrast, there were no differences between CON and H treatment groups in this period. A second critical CH period occurred between 487 and 492 hours of incubation. The highest (P < 0.05) hatchability scores were obtained from CON at 13.5% and the H groups at 15.2%, respectively in this period. The lowest total mean hatchability value was obtained from the L group whereas the highest (P < 0.01) values occurred in the CON and H groups. No significant differences in hatchability were noted between the VH and the other groups at hatch. The hatchability of fertile eggs with different hatching times is presented in Table 2.
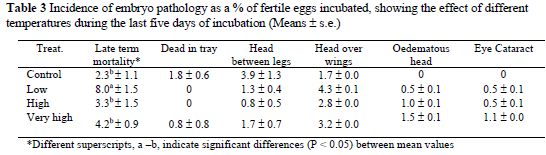
Late term embryonic mortality was affected (P < 0.05) by temperature treatment. The highest mortality of 8.01% was observed in the L treatment group. No significant treatment effects were observed in the "dead in tray" category. Where eggs were incubated at high and low temperatures in the last stages of incubation, only two malpositions - "head between legs" and "head over wings" and two abnormalities "oedematous head" and "eye cataract" occurred. Most of the increased incidence of "head between legs" among groups occurred in the CON group. In contrast, a high incidence of "head over wings" occurred in L group compared to the others. The abnormalities, "oedematous head" and "eye cataract" occurred when eggs were incubated at 38.3 °C in the last stages of incubation. No significant differences were observed for pathologic (head between leg, "head over wing, oedematous head and eye cataract) problems in the study. The effects of treatments on mortality stages and embryo pathology are shown in Table 3.
Discussion
The results from the current study show that the effects of temperature in the last five days of incubation on broiler embryos were mainly dependent on the IC temperature. In other words, there is a linear relationship between IC temperature and mean eggshell temperature. As previously stated, the experimental IC temperature of the VH group had the highest value in the treatment groups. Similarly, the highest mean Tes value occurred in the eggs of the same group at the end of the measuring period. Moreover, the other groups have exhibited a similar trend. These findings partly agree with those of Romijn & Lokhoest (1955) and Tazawa & Rahn (1987) who stated that there is 1- 2 °C difference just prior to hatching between Tes and incubation temperature. There was no linear relationship between Tes and increasing embryonic age. The results of the current study are in agreement with Meijerhof (1999) and Tazawa et al. (2001) who stated that the embryo is not able to regulate its own body temperature. It therefore reacts as a cold-blooded animal on temperature fluctuations during incubation. This is also in agreement with Romijn & Lokhoest (1955) and Tazawa &Rahn (1987) who stated that before hatching the chicken embryos have no or poorly developed chemical heat regulation and that the embryo is poikilothermic and relying on external heat sources (parent or incubator) for maintenance of its body temperature. The present results might probably be a confirmation of Meijerhof s (1999) observation that eggs of modern breeds produced the same amount of heat as eggs of 40 years ago, especially in the case of broiler chickens. The modern broiler breeder's eggs are still exhibiting similar patterns for heat production with past generation in hatchers. In spite of genetic advancements in broiler performance their eggs should still be incubated at the temperatures outlined 40 years ago in the last stages of development.
Hatching time was influenced by the treatments. The results from this study imply that the best hatchability might be obtained from CON and H treatment groups in current conditions. The result agrees with that of Wilson (1991) who stated that the optimum incubation temperature is between 37 and 38 °C. The temperature tolerance limits of embryos may be expanded from 38 until 38.3 °C in the last five days of incubation. The embryonic mortality at the end of incubation increased in the L treatment group. The high mortality rate in L group might be due to low metabolic activity in the eggs. As stated by Landauer (1967), Lancaster & Jones (1988) and Wilson (1991) lower incubation temperatures during the third week of incubation allow for a normal hatch but an increase in the incubation period. It is correct to speculate that if the trial had been continued for an additional 10 or more hours the incidence of mortalities at the late stage of incubation would have decreased in the L treatment group. This is in accordance with Wilson (1991) who stated that lower incubation temperatures during the last third of the incubation period would allow for a normal hatch, reduce the water loss, but increase the incubation period.
Wilson (1991) believed that high or low temperatures in incubators could be a cause of malpositions. In contrast, there were no differences among the groups with regard to malpositions. The difference in results might partly be explained by the exposure time. Freeman & Vince (1974) indicated that the embryo moves its head towards the air cell end and its feet towards the small end of the egg on day 15 of incubation. Lesley (1995) stated that prehatching behaviour begins on day 16 or 17 days of incubation with the appearance of new behaviour pattern and on day 18 the chick orients into the tucking position. It would appear that the temperatures in the treatment groups in the last five days of incubation could not disrupt the embryo's ability to correctly position itself within the egg over the current temperature limits in this study.
Conclusion
The results of current study suggest that the temperature experienced by the developing embryo is dependent on the incubator environment temperature and the metabolic heat production of embryo. Prior to hatching, the chicken embryo has no or poorly developed chemical heat regulation. Therefore, the egg temperature is a reflection of the environmental temperature. Embryo temperatures higher than 39.5 °C (or 38.3 °C incubator environment temperature) or less than 37.7 °C (or 37.2 °C incubator environment temperature) during the last five days of incubation appear to negatively affect embryo development. This may cause a decrease in hatching success of broiler eggs under the conditions of the experiment. Further studies are required to determine the effects of incubation temperature on the hatchability of broiler embryos and their subsequent performance.
Acknowledgments
This research was supported by a Faculty Research and Development (BAP) Grant from Selcuk University, Konya, Turkey.
References
Al-Thani, R. & Simkiss, K., 1992. Effects of temperature on the migration of primordial germ cells in the chick embryo. Br. Poult. Sci. 33, 735-739. [ Links ]
Dietz, M.W., Kampen, M.P., Griensven, J.M.M. & Mourik, S., 1998. Daily energy budgets of avian embryos: the paradox of the plateau phase in egg metabolic rate. Physiol. Zool. 71, 147-156. [ Links ]
Freeman, B.M. & Vince, M.A., 1974. Development of the avian embryo. Chapman and Hall, London. [ Links ]
French, N.A., 1994. Effect of incubation temperature on the gross pathology of turkey embryos. Br. Poult. Sci. 35, 363-371. [ Links ]
French, N.A., 1997. Modelling incubation temperature: The effects of incubator design, embryonic development, and egg size. Poult. Sci. 76, 124-133. [ Links ]
French, N.A., 2000. Effect of short periods of high incubation temperature on hatchability and incidence of embryo pathology of turkey eggs. Br. Poult. Sci. 41, 377-382. [ Links ]
Lancaster, F.M. & Jones, T.R., 1988. Cooling of broiler hatching eggs during incubation. Br. Poult. Sci. 27, 157. [ Links ]
Landauer, W., 1967. The hatchability of chicken eggs as influenced by environment and heredity. Storrs Agricultural Experiment Station Monogram 1 (Revised). [ Links ]
Lesley, J.R., 1995. The development of brain and behaviour in the chicken. CAB International, Oxon, UK. [ Links ]
Lundy, H., 1969. A review of the effects of temperature, humidity, turning and gaseous environment in the incubator on the hatchability of hen's egg. In: the fertility and hatchability of the hen's egg. Edinburgh. pp. 143-176. [ Links ]
Meijerhof, R., 1999. Embryo temperature is the key factor in incubation. World Poultry - Elsevier. 15, 42-43. [ Links ]
Minitab, 1998. Minitab for Windows. Release 10 Xtra, Minitab Inc., USA. [ Links ]
Mstat-C, 1989. A Microcomputer program for the Design, Management, and Analysis of Agronomic Research Experiments (Distribution April, 1989, After Version I in 1983). Michigan State University, USA [ Links ]
Romanoff, A.L., 1960.The Avian Embryo. New York: Macmillan. pp. 200-207. [ Links ]
Romijn, C. & Lokhoest, W., 1955. Chemical heat regulation in the chick embryo. Poult. Sci. 34, 649-654. [ Links ]
Swann, G.S. & Brake, J., 1990. Effect of Incubation dry-bulb and wet-bulb temperatures on time of hatch and chick weight at hatch. Poult. Sci. 69, 887-897. [ Links ]
Tazawa, H., Moriya, K., Tamura, A., Komoro, T. & Akiyama, R., 2001. Ontogenetic study of thermoregulation in birds. J. Therm. Biol. 26, 281-286. [ Links ]
Tazawa, H. & Rahn, H., 1987. Temperature and metabolism of chick embryos and hatchlings after prolonged cooling. J. Exp. Zool. Suppl. 1, 105-109. [ Links ]
Wilson, H.R., 1991. Physiological requirements of the developing embryo: temperature and turning. In: Avian Incubation. Ed. Tullet, S.G., Butterworth- Heinemann Ltd. pp. 145-156. [ Links ]
Wineland, M.J., 1996. Factors influencing embryo respiration. Poultry Digest, September. pp. 16-20. [ Links ]
Zhang, Q. & Whittow, G.C., 1992. The effect of incubation temperature on oxygen consumption and organ growth in domestic fowl embryos. J. Therm. Biol. 17 (6), 339-345. [ Links ]
# Corresponding author. E-mail: iyildir@selcuk.edu.tr














