Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Animal Science
On-line version ISSN 2221-4062
Print version ISSN 0375-1589
S. Afr. j. anim. sci. vol.34 n.3 Pretoria 2004
Sperm storage and duration of fertility in female ostriches (Struthio camelus)
I.A. MaleckiI, ; S.W.P. CloeteII, III; W.D. GertenbachIII; G.B. MartinI
ISchool of Animal Biology M085, Faculty of Natural and Agricultural Sciences, University of Western Australia, Crawley 6009, Western Australia
IIDepartment of Animal Sciences, University of Stellenbosch, Private Bag X1, Matieland 7602, South Africa
IIIElsenburg Agricultural Development Institute, Private Bag X1, Elsenburg 7607, South Africa
ABSTRACT
In two experiments, one carried in South Africa and the other in Western Australia, the duration of sperm storage and the fertile period following separation of sexes were investigated by egg break-out and by counting the sperm in the perivitelline membrane (spermOPVL) above the germinal disc (GD) region. Fertilisation status was determined by the appearance of the GD. The perivitelline membrane above the GD was collected to count sperm under fluorescence following staining with 4',6'-diamidino-2-phenyindole (DAPI). In both experiments, after the males were removed, the rate of lay declined by about 50% within a week. Following separation, spermOPVL were detected for up to four weeks and fertilised eggs were laid for nearly the same duration. The loss of sperm followed an approximately logarithmic function and sperm numbers declined at rates similar to that of the turkey. Since the duration of sperm storage was longer than indicated by clutch duration, ostrich clutch size might be larger than reported or female ostriches might store sperm for up to two clutches. Fertilisation rate in ostrich eggs is high because most eggs contain excessive numbers of sperm and very low numbers of sperm appear sufficient to achieve fertilisation.
Keywords: Sperm storage, fertile period, sperm loss, vitelline membrane spermatozoa, fertilisation
Introduction
Fertility of ostriches (Struthio camelus) is a very important measure of their reproductive efficiency. On commercial farms, it directly affects profitability because it determines the proportion of fertile eggs laid by each female (Cloete et al., 1998). Fertility of ostrich eggs varies considerably between and within farms (Badley, 1997; Bunter & Graser, 2000) despite a reproductive strategy that should guarantee high fertility through high rates of sperm supply and the presence of sperm storage tubules in the female (Bezuidenhout et al., 1995; Holm et al., 2000; Malecki & Martin, 2003b).
The ostrich breeding system is complex and a "breeding unit" can range from a pair on a farm to a male with multiple females in the wild (Sauer & Sauer, 1966; Handford, & Mares, 1985). A territory-holding male and a "dominant female" are responsible for the nest, incubation of eggs and care of the chicks. Eggs are laid by the dominant female and a number of "subordinate females", some of which appear to come from other breeding units (Kimwelle & Graves, 2003). In such a system the male ostrich is likely to copulate frequently to protect his parental investment because paternity in birds can best be assured by frequent copulations (Birkhead, 1988; Møller & Birkhead, 1991). As a result birds supply more sperm than is required to fertilise the eggs produced by their companions (Birkhead et al., 1987; 1989). Ostriches are no exception.
On commercial farms ostriches are usually kept in pairs, trios, quartets or small colonies. The pair-breeding system is generally adopted to facilitate pedigree recording for genetic evaluation in South Africa, although it leads to the confounding of direct and maternal genetic effects if few generations are available in the data (Cloete et al., 1998). In the other systems the recommended male : female ratio usually requires one male to copulate with two and more females. Fertilisation rates are high and female ostriches are generally oversupplied with sperm under such conditions (Malecki & Martin, 2003b). When infertility is detected, it appears to be associated with the lack of sperm transfer due to either inadequate mating or poor sperm production (Malecki & Martin, 2003b). While those problems can be caused by a male that deposits low numbers of sperm or none at all (Birkhead et al., 1987) they can also be caused by a female that retains few sperm following copulation or retains sperm for fertilisation for only a short period (Bakst et al., 1994; Birkhead & Fletcher, 1994; Wishart, 1997; Malecki & Martin, 2002b). Female ostriches store sperm in their sperm storage tubules following copulation, so they would be expected to have a period of fertility during which a number of fertilised eggs are laid (Lake, 1975; Wishart, 1987; Birkhead, 1988; Malecki & Martin, 2002a,b). However, no studies have so far been undertaken to determine how long ostrich females can maintain their fertility following the last copulation.
The fertile period of female ostriches could be determined indirectly from clutch duration, since the number of days it takes to lay a clutch should be proportional to the duration of sperm storage (Birkhead & Møller, 1992). However, nesting of ostriches is communal so it is difficult to reliably determine the number of eggs laid by a single female. Several estimates of ostrich clutch size have been made and they suggest that a single female lays between seven and 11 eggs at 2-day intervals (Jarvis et al., 1985; Bunter et al., 2001; Davies, 2002). If these estimates are reliable, the fertile period ([clutch size - 1] x egg interval - (Birkhead & Fletcher, 1994) of the female ostrich should last between 12 and 20 days.
We carried out this study to test the hypothesis that, after the last copulation, female ostriches will lay fertilised eggs for the period of clutch duration.
Material and Methods
Experiment 1 was carried out in October 2002 at the Klein Karoo Agricultural Research Centre near Oudtshoorn in South Africa. The experimental site is situated in the arid Klein Karoo region of South Africa (33°38'S and 22°15'E). Seven pairs were used to determine the duration of sperm storage and the fertile period following separation, and to test the effect of separation on the rate of lay. The other seven pairs and two nests (one from each colony of 32 birds) were used to determine egg fertilisation rate and variation in sperm supply under natural mating. Ostriches consisted mainly of Zimbabwe Blue males and South African Black females and they had been laying since July.
For the study of the rate of lay and duration of sperm storage eggs were collected for eight days after which the males were removed and placed together in one enclosure located about one km away from the females. After eight days of egg collection from the isolated females, the males were returned to their females wearing "aprons" to prevent copulations. Feed bags with both sides open were fitted over the tails of individual males and stitched to the stomach girdle mounted over the back, abdomen and the breast of the male (Swart et al., 1993). These "aprons" allowed the passing of urine and faeces, but prevented copulation. Eggs were collected for a further period of eight days. Eggs from three pairs were not fertilised, therefore, they could only be used to estimate the rate of lay. To study fertilization rate and sperm supply, eggs were collected for 16 days from seven unseparated pairs and from two breeding units in colonies, one from each colony. The colony nests appeared to be attended by a single male and four to six females.
Experiment 2 was carried out between July and December 2003 to supplement Experiment 1. A longer duration of separation from a male was needed to determine the maximum sperm storage and fertile period duration. Because the absence of a male can lead to an extended oviposition interval or the termination of laying (as observed in Experiment 1) a male was needed in close proximity to the experimental females. We used four females and two males that were part of the flock held at the Shenton Park Field Station of the University of Western Australia (32°13'S and 115°38'E). These birds were hatched in November 2000 on a commercial ostrich farm in Western Australia and brought to the Field Station at the age of three months. Ostriches were of mixed descent, being derived from African Black, Reds and Blues, and also from Australian stock. The birds were kept as one group until 16 months of age when they were penned as two pairs and two single females in 9x15 m pens adjacent to each other. The females were labelled A-D. Male 1 paired to Female B and had Female A on one side and Male 2 paired to Female C on the other. Female D was in the adjacent pen to Male 2. The birds were maintained in this arrangement throughout their first breeding season (2002/2003). The mated females (B and C) laid 23 and 20 eggs, respectively, while non-mated females (A and D) laid 10 and 9 eggs, respectively. In May and June of the 2003/2004 breeding season, Males 1 and 2 were occasionally joined with Females A and D, respectively, to determine whether they would accept another female, and to familiarize them with the separation procedure. The experiment was conducted as follows. First, males were removed from their companion females (Male 1 from Female B and Male 2 from Female C) for two weeks and placed in the empty pens adjacent to the females. These pens were at 90° angle to the female pens and equalled the female pens for width. Male 1 was then joined with Female B and Male 2 with Female C, while females A and D were on their own. After eight weeks, Male 1 was moved to Female A and Male 2 to Female D. Females B and C were left on their own for the next eight weeks. Separated females always had visual contact with the males being separated only by a wire fence. In this way, 8-week mating and separation periods were created for four females during which eggs were collected for estimation of the rate of lay, the duration of sperm storage and the fertile period. In both experiments, the ostriches were fed ad libitum with the commercial ostrich breeder ration.
To determine egg fertilisation status and to quantify sperm in the outer perivitelline layer, each egg was opened and the fertilization status of the germinal disc was determined with an unaided eye (Exp 1 and 2) or under the stereomicroscope (Exp 2). The egg was assumed unfertilised if it contained a blastodisc or fertilised when a blastoderm was present (Malecki & Martin, 2003b). A piece of the vitelline membrane (2 cm in diameter) directly overlying the germinal disc (GD) region was collected onto a filter ring, cleaned of yolk and stored in 1% PBS at 5°C. Sperm trapped in the outer perivitelline layer (spermOPVL) were visualized under fluorescence following staining with 1 μg/mL of DAPI in PBS (4',6-diamidino-2-phenylindole; Sigma Chemical Co., St Louis, USA) and counted in six successive fields starting from the centre of the GD where most sperm are concentrated (Malecki & Martin, 2003a). Each field was defined by a perimeter of a 40X objective (0.55 mm in diameter).
When copulations were not observed, the time of the last copulation was determined indirectly from the last time of the number of spermOPvL increased markedly near separation (Malecki & Martin, 2002b). A 2-day correction for the ostrich egg cycle was made, because sperm detected in the perivitelline membrane would need to be supplied to the female and/or released from the sperm storage tubules approximately two days prior to oviposition (Malecki & Martin, 2002b). To determine duration of sperm storage and the fertile period, data were aligned to the time of last copulation. The duration of sperm on the vitelline membrane was defined as the number of days from the last copulation to the day of the last egg containing spermOPVL. The fertile period was defined as the number of days over which sequentially laid eggs were fertilised following the last copulation. Linear regression of log10-transformed spermOPvL counts was used to determine the relationship between the number of spermOPVL and time from the last copulation. The slope of this relationship (Exp 2) was used to determine the rate of sperm loss (Wishart, 1987; Birkhead & Fletcher, 1994; Malecki & Martin, 2002b). Eggs with zero spermOPvL were excluded. The maximum duration of sperm storage (Exp 1) and duration of the fertile period (Exp 1 and 2) could not be determined because the rate of lay declined or laying ceased after removal of the male. Therefore, they were expressed as median duration.
The total sperm count per mm2 of GD was used for all analyses that, unless otherwise specified, were performed using SuperANOVATM software (Abacus Concepts, 1989). Data are presented as means ± s.e.m. and P < 0.05 is considered significant.
Results
In Experiment 1, the rate of lay declined in the first week after removal of the males (P < 0.05, n = 7) and remained at that level after the males were returned for the second week (Figure 1a). The rate of lay for the seven unseparated pairs was 0.40 ± 0.04 eggs per day throughout. Following separation, spermOPVL were detected in eggs for 17.5 days (range 12-20 days) and all eggs were fertilised.
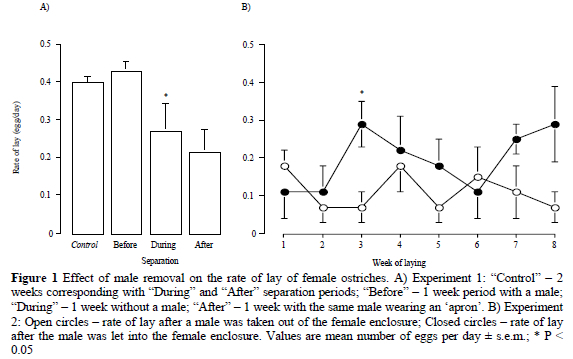
In Experiment 2, females laid at the rate of 0.26 ± 0.04 eggs per day when with males. In the absence of the males, that rate was reduced (P < 0.01) to 0.11 ± 0.02 (n = 4). Following introduction of the male, the rate of lay appeared to increase by Week 3, while following removal of the male it appeared to decrease by Week 2 and remained low for the remainder of the 8-week separation period (Figure 1b). SpermOPVL was detected in eggs for 27.8 ± 0.5 days (25 - 29). Fertilised eggs were laid for 17 days (4 - 28).
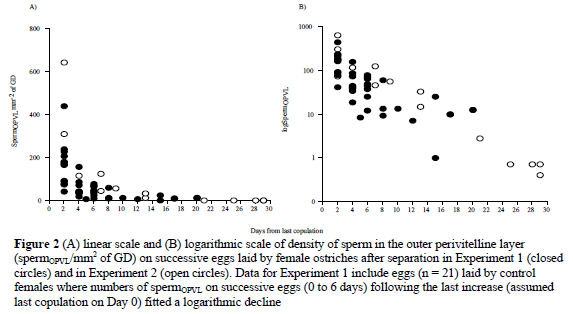
After separation (Experiment 1), the numbers of spermOPVL declined from 118 ± 22 per mm2 to 14 ± 3 per mm2 of GD (P < 0.01). The decline (an instantaneous loss of sperm) was described by the function logSpermOPVL = -0.04x(day) + 1.72 (r2 = 0.38, P < 0.01, n = 19 eggs). Since the derived correlation was relatively low, spermOPVL data from the control group that also showed an exponential decline in successively laid eggs were pooled with that of the treatment group. The relationship at the initial part of the curve (0 to 6 days) could only be improved by this procedure. For the pooled data (Fig. 2), a decline in the numbers of spermOPVL was described by logSpermOPVL = -0.07x(day) + 2.11 (r2 = 0.51, P < 0.01, n = 40 eggs). During Experiment 2, the numbers of spermOPVL declined from 140 ± 24 to 8 ± 4 per mm2 of GD after separation (P < 0.001). This decline (Figure 2) was described by logSpermOPVL = -0.09x(day) + 2.53 (r2 = 0.94, P < 0.001, n = 16). The mean loss of sperm per day (mean slope of n = 4 females) was -0.093 ± 0.008, or -0.004 ± 0.000 per hour. When data from both experiments were combined (including those from the control group in Experiment 1) the relationship between the number of spermOPVL and days after copulation was logSpermOPVL = -0.08x(day) + 2.19 (r2 = 0.76, P < 0.001, n = 56).
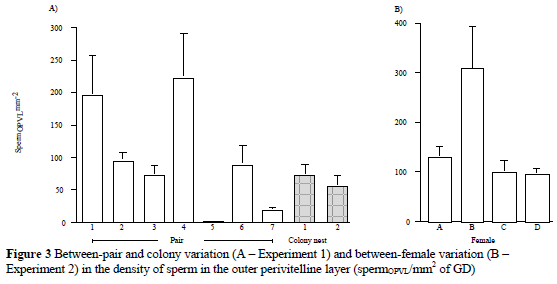
The number of spermOPVL in Experiment 1 varied between pairs (P < 0.001), but not between colony nests (Fig. 3a). There were 90 ± 12 SpermOPVL/mm2 of GD ranging from 0 to 626 (CV = 126%, n = 84 eggs). Mean fertilisation rate was 96 ± 3%. The lowest fertilisation rates were found in a nest from Colony 2 (80%, n = 15 eggs) and in the pair supplying lowest numbers of sperm (83%, n = 6 eggs). In Experiment 2 (Fig. 3b), the number of spermOPVL varied between females during the mating period (P < 0.05). There were 140 ± 24 SpermOPVL/mm2 of GD ranging from 11 to 640 (CV = 91%, n = 29 eggs). All eggs were fertilised. Following separation, three out of four eggs laid at the end of the fertile period that contained 0.6 ± 0 spermOPVL per mm2 of GD were not fertilised while eggs containing greater than that number of sperm were fertilised.
Discussion
After separation of sexes, sperm numbers in the eggs of female ostriches decline rapidly over the first week but then persist at low numbers on the perivitelline membrane for a further three weeks, and fertilised eggs are laid for approximately four weeks. Thus, the fertile period of the female ostrich appears longer than estimated from their clutch duration of 12 to 20 days, suggesting clutches larger than reported could be a feature of ostrich reproduction. Fertilisation of eggs in the ostrich is associated with highly variable numbers of detectable sperm in the perivitelline membrane but the probability of fertilisation is high because most eggs contained excessive numbers of sperm.
The pattern of decline in numbers of sperm in the perivitelline membrane and the relationship between numbers of sperm detected on eggs and the number of days over which sperm are detected are in accordance with that observed in other avian species. However, the number of days over which sperm are detected suggests that the ostrich clutch size may be larger than values previously reported, or female ostriches store sperm for up to two clutches. Their long sperm storage duration could have a basis in a relatively large utero-vaginal region containing long sperm storage tubules, a storage system resembling that of the turkey (Holm et al., 2000), a species that stores sperm for up to 72 days (Birkhead, 1988). Similarities in the utero-vaginal region between the two species could explain why the rate of sperm loss in the ostrich is similar to that of the turkey (-0.003 per hour log; Wishart, 1988). Therefore, if female ostriches have a large sperm storing capacity they would be receiving large sperm numbers, as the numbers of the sperm storage tubules and the numbers of spermatozoa per ejaculate are positively correlated (Birkhead & Møller, 1992). This is supported by the large numbers of spermatozoa observed in the perivitelline membrane of ostrich eggs in the present and in our previous study (Malecki & Martin, 2003b). From the literature, it seems that: a) twice as many sperm are detected in ostrich eggs as in emu eggs (Malecki & Martin, 2003a), b) that the loss of sperm in female ostriches is nearly 50% lower than in female emus (Malecki & Martin, 2002b) and, c) sperm storage duration and the fertile period in the emu are about half of that in the ostrich. Supplies of large numbers of spermatozoa, their residence in the utero-vaginal junction and subsequent transfer into eggs thus clearly determine for how long fertilised eggs will be laid (Wishart, 1987; Birkhead et al., 1993; Birkhead & Fletcher, 1994; Malecki & Martin, 2002b). The fact that the sperm storage duration in the ostrich appears longer than their clutch duration and that, in the emu (Malecki & Martin, 2002a; b) and the bearded tit (Sax et al., 1998), more than one copulation is needed to fertilise the clutch, suggests that studies into the ostrich clutch size are needed to determine whether female ostriches retain spermatozoa for up to two clutches or if their clutch size is larger than reported.
Supplies of large numbers of sperm by males and the rate of sperm use by females provide some insight into the mating strategy that the ostrich has evolved. In nature, the breeding unit generally consists of one male and a few females, so male ostriches need to employ an effective strategy in order to protect their paternity. In birds, either copulation frequency or mate guarding is the most common means of paternity protection (Birkhead, 1988; Møller & Birkhead, 1992). Male ostriches are very territorial and highly vigilant. Their vigilance appears to be divided between predators, competing males and receptive females (Bertram, 1980; Burger & Gochfeld, 1988) so they may use both mate guarding and copulation frequency for paternity protection. On the other hand, male ostriches do not seem to be very effective because ostrich females can parasitise nests of other males (Kimwele & Graves, 2003), suggesting that females also play a role in paternity. Numbers of sperm supplied to females and time of last copulation would be crucial for a territory-holding male if he is to gain a 'respective' share of his paternity. Male ostriches have large testes for their body size (testes mass index greater than 0.4; Malecki - unpublished data) suggesting they could produce sperm in relatively large numbers. Current estimates of ostrich ejaculate volume and sperm concentration do not support this view (Bertshinger et al., 1992; Hemberger et al., 2001; Ya-jie et al., 2001; Rosenboim et al., 2003). Artificial semen collection methods may, however, not be satisfactory and ejaculates might not reflect real output (Gvaryahu et al., 1984), or ostrich males may use a high testis output to supply relatively small numbers of sperm at frequent intervals. Since sperm storage is very efficient in this species, it could be that, prior to commencement of a clutch, the male ostrich supplies his females with relatively low but frequent numbers of sperm. During egg laying, his efforts may then be mainly directed towards deterring predators and competing males.
Ostriches appear to be highly prolific birds and the industry should take advantage of that potential. High numbers of sperm observed in this and in the previous study (Malecki & Martin, 2003b) are indicative of an ability of males to supply large numbers of sperm, certainly sufficient for the eggs produced by more than one female. Colonies instead of pairs and trios should guarantee high fertilisation rates. Even though high numbers of sperm are frequently present in eggs, low numbers are also associated with fertilisation, so that overall flock fertility can remain high provided copulation frequency does not become lower than the minimum required to maintain female fertility. As females store sperm for a prolonged time, and lay eggs on alternate days, they could guarantee a relatively large number of fertilised eggs following natural or artificial insemination. Variation between individuals in sperm numbers supplied by males and sperm use by females is likely to result from between-male variation in copulation frequency and ejaculate size, and from between-female variation in sperm transfer efficiency into and from the sperm storage tubules (Bakst et al., 1994; Wishart & Staines, 1995; Wishart, 1997; Malecki & Martin, 2003b). These sources of variation could be used to identify superior individuals for those fertility traits for usage in breeding programs. In addition, even though separation of sexes generally reduced the rate of lay, this effect was not observed in all females, while others did not seem to be affected, regardless how far away the male was taken. The cause of this variation is likely to be complex. Nevertheless, a better understanding of the factors affecting the ovulatory cycle, combined with selection of females that are less affected by separation, could result in flocks of females that can lay in the absence of males.
Conclusion
Research on establishing a feasible artificial insemination protocol for ostriches has thus far focused on sperm collection and evaluation techniques (Hemberger et al., 2001; Rosenboim et al., 2003). The present study suggests that the long sperm storage duration in ostrich females, while being a useful breeding strategy in the wild, could also provide a basis for the establishment of a viable artificial insemination system for ostrich enterprises. Such a development would facilitate ostrich data recording and genetic evaluation schemes, by reducing the level of confounding between male and female effects commonly observed in pair-bred flocks. The latter attribute was pointed out as a challenge to the establishment of a viable ostrich genetic evaluation scheme (Cloete et al., 1998). An additional benefit would be increased selection intensity on the male side in breeding operations (Cloete et al., 2002).
Acknowledgment
We thank S.J. van Schalkwyk, Z. Brand and B. Pfister from the Klein Karoo Agricultural Research Centre, and C. Reed and P. Cowl from the University of Western Australia for their valuable assistance. This work was supported by the Rural Industries Research and Development Corporation in Australia, the Western Australian Branch of the Australian Ostrich Association and the Elsenburg Agricultural Development Institute in South Africa.
References
Abacus Concepts, 1989. SuperANOVA, Abacus Concepts, Inc., Berkeley, CA, USA. [ Links ]
Badley, A.R., 1997: Fertility, hatchability and incubation of ostrich (Struthio camelus) eggs. Poult. Avian Biol. Rev. 8, 53-76. [ Links ]
Bakst, M.R., Wishart, G. & Brillard, J-P., 1994. Oviducal sperm selection, transport, and storage in poultry. Poult. Sci. Rev. 5, 117-143. [ Links ]
Bezuidenhout, A.J., Soley, J.T., Groenewald, H.B. & Burger, W.P., 1995. Sperm-storage tubules in the vagina of the ostrich (Strutio camelus). Onderstepoort J. Vet. Res. 62, 193-199. [ Links ]
Bertram, B.C.R., 1980. Vigilance and group size in ostriches. Anim. Behav. 28, 278-286. [ Links ]
Bertschinger, H.J., Burger, W.P., Soley, J.T. & de Lange, J.H., 1992. Semen collection and evaluation of the male ostrich. Proc. Biennial Congr. S. Afr. Vet. Assoc., 7-10 September 1992, Grahamstown, South Africa. pp. 154-158. [ Links ]
Birkhead, T.R., 1988. Behavioural aspects of sperm competition in birds. Adv. Study Behav. 18, 35-72. [ Links ]
Birkhead, T.R. & Møller, A.P., 1992. Numbers and size of sperm storage tubules and the duration of sperm storage in birds: a comparative study. Biol. J. Linn. Soc. 45, 363-372. [ Links ]
Birkhead, T.R. & Fletcher, F., 1994. Sperm storage and the release of sperm from the sperm storage tubules in Japanese quail Coturnix japonica. Ibis 136, 101-105. [ Links ]
Birkhead, T.R., Atkin, L. & Møller, A.P., 1987. Copulation behaviour of birds. Behaviour 101, 101-133. [ Links ]
Birkhead, T.R., Hunter, F.M. & Pellatt, J.E., 1989. Sperm competition in the zebra finch, Taeniopygia guttata. Anim. Behav. 38, 935-950. [ Links ]
Bunter, K.L., Cloete, S.W.P., Van Schalkwyk, S.J. & Graser, H-U., 2001. Factors affecting reproduction in farmed ostriches. Proc. Assoc. Advmnt Anim. Breed. Gen. 14, 43-46. [ Links ]
Bunter, K.L. & Graser, H-U., 2000. Genetic evaluation for Australian Ostriches. Publication No 00/153, Rural Industries Research and Development Corporation. [ Links ]
Burger, J. & Gochfeld, M., 1988. Effect of group size and sex on vigilance in ostriches (Struthio camelus): anti-predator strategy or mate competition. Ostrich 59, 14-20. [ Links ]
Cloete, S.W.P., van Schalkwyk, S.J. & Brand, Z., 1998. Ostrich breeding - progress towards a scientifically based strategy. Proc. 2nd Int. Ratite Congr., Oudtshoorn, South Africa, 21-25 September 1998. pp. 55-62. [ Links ]
Cloete, S.W.P., Van Schalkwyk, S.J. & Bunter, K.L., 2002. Progress in ostrich breeding research. Ed. Horbanczuk, J.O., Proc. 2nd World Ostrich Congr., September 2002, Warsaw, Poland. pp 23-37. [ Links ]
Davies, S.J.J.F., 1976. The natural history of the emu in comparison with that of other ratites. Eds. Frith, H.J. & Calaby, J.H. The 16th Int. Ornithological Congr., Australian Academy of Science, Canberra. pp. 109-120. [ Links ]
Davies, S.J.J.F., 2002. Ostrich and elephant birds. In: Ratites and Tinamous, Oxford University Press, New York. pp. 257-271. [ Links ]
Handford, P. & Mares, M.A., 1985. The mating systems of ratites and tinamous: an evolutionary perspective. Biol. J. L. Soc. 25, 77-104. [ Links ]
Hemberger, M.Y., Hospes, R. & Bostedt, H., 2001. Semen collection, examination and spermiogram in ostriches. Reprod. Dom. Anim. 36, 241-243. [ Links ]
Holm, L., Ruziwa, S.D., Dantzer, V., Ridderstrâle, Y., 2000. Carbonic anhydrase in the utero-vaginal junction of immature and mature ostriches. Br. Poult. Sci. 41, 244-249. [ Links ]
Jarvis, M.J.F., Jarvis, C. & Keffen, R.H., 1985. Breeding seasons and laying patterns of the southern African Ostrich Strutio camelus. Ibis 127, 442-449. [ Links ]
Kimwele, C.N. & Graves, J.A., 2003. A molecular genetic analysis of the communal nesting of the ostrich (Struthio camelus). Mol. Ecol. 12, 229-236. [ Links ]
Lake, P.E., 1975: Gamete production and the fertile period with particular reference to domesticated birds. Symp. Zool. Soc. London 35, 225-244. [ Links ]
Malecki, I.A. & Martin, G.B., 2002a. Fertile period and clutch size in the emu (Dromaius novaehollandiae). Emu 102, 165-170. [ Links ]
Malecki, I.A. & Martin, G.B., 2002b. Fertility of male and female emus (Dromaius novaehollandiae) as determined by spermatozoa trapped in eggs. Reprod. Fert. Dev. 14, 495-502. [ Links ]
Malecki, I.A. & Martin, G.B., 2003a. Distribution of spermatozoa in the outer perivitelline layer from above the germinal disc of emu and ostrich eggs. Reprod. Fert. Dev. 15, 263-268. [ Links ]
Malecki, I.A. & Martin, G.B., 2003b. Sperm supply and egg fertilization in the ostrich (Struthio camelus). Reprod. Dom. Anim. 38, 429-435. [ Links ]
Møller, A.P., Birkhead, T.R., 1991. Frequent copulations and mate guarding as alternative paternity guards in birds: a comparative study. Behaviour 118, 170-186. [ Links ]
Møller, A.P., 1992. Frequency of female copulations with multiple males and sexual selection. Am. Nat. 139, 1089-1101. [ Links ]
Rosenboim, I., Navot, A., Snapir, N., Rosenshtrauch, A., El Halawani, M.E., Gvaryahu, G. & Degen, A., 2003. Method for collecting semen from the ostrich (Struthio camelus) and some of its quantitative and qualitative characteristics. Br. Poult. Sci. 44, 607-611. [ Links ]
Sauer, E.G.F. & Sauer, E.M., 1966. The behavior and ecology of the South African Ostrich, Struthio camelus australis. Living Bird 5, 45-75. [ Links ]
Sax, A., Hoi, H. & Birkhead, T.R., 1998. Copulation rate and sperm use by female bearded tits, Panurus biarmicus. Anim. Behav. 56, 1199-1204. [ Links ]
Swart, D., Siebrits, F.K. & Hayes, J.P., 1993. Utilisation of metabolizable energy by ostrich (Struthio camelus) chicks at two different concentrations of dietary energy and crude fibre originating from lucerne. S. Afr. J. Anim. Sci. 23, 136-141. [ Links ]
Wishart, G.J. & Staines, H.J., 1995. Assessing the breeding efficiency of broiler breeder flocks by measuring sperm transfer into laid eggs. Br. Poult. Sci. 36, 317-323. [ Links ]
Wishart, G.J., 1987. Regulation of the length of the fertile period in the domestic fowl by numbers of oviducal spermatozoa, as reflected by those trapped in laid eggs. J. Reprod. Fert. 80, 493-498. [ Links ]
Wishart, G.J., 1988. Numbers of oviducal spermatozoa and the length of the fertile period in different avian species. 11th Int. Congr. Animal Reproduction and Artificial Insemination, University College Dublin, Ireland, June 26-30 1988. V. 3, 3 pp. [ Links ]
Wishart, G.J., 1997. Quantitative aspects of sperm : egg interaction in chickens and turkeys. Anim. Reprod. Sci. 48, 81-92. [ Links ]
Ya-jie, J.I., Yan-bo, Y. & Wu-zi, D., 2001. Studies on ostrich semen character and semen storage at low temperature. J. Econ. Anim. 5, 49-54. [ Links ]
# Corresponding author. E-mail: imalecki@agric.uwa.edu.au














