Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.34 no.2 Pretoria 2004
The effect of additives on quality and nutrient degradability and digestibility of round bale silage
W. Nowak#; A. Potkański; S. Wylegała
Department of Animal Nutrition and Feed Management, Agricultural University Poznań, Wołyńska 33, 60-637 Poznań, Poland
ABSTRACT
The aim of the experiment was to determine the effect of applying an inoculant and formic acid at commercially recommended rates to big bale silage on the quality, protein and cell wall ruminal degradability and intestinal digestibility of undegraded protein. Wilted grass was ensiled with no additive, an inoculant (30 L/t) or formic acid (4 L/t). The composition of inoculated silage did not differ from that of the control silage. Formic acid supplementation decreased ammonia-N and lactic acid and increased acetic acid and water soluble carbohydrate content. The effective rumen degradability of silage N (determined in sacco) and the intestinal digestibility of undegraded protein (mobile bag) were not affected by the additive treatments. Cell wall degradation in the rumen was also not affected by inoculant or formic acid treatment.
Keywords: Silage, formic acid, inoculant, big bale silage, digestibility
Introduction
Big bale silage now comprises approximately 5% of the grass silage dry matter (DM) ensiled in Poland. The results of a series of experiments relating to baled silage were reported by O'Kiely et al. (1999). Some studies have shown the benefits of using both inoculant (Weddell, 1997) and chemical additives (Haigh et al., 1996) on big bale silage. The general effects of silage additives on cattle included increased silage intake (Petterson et al., 1997), increased milk yield (Gordon, 1989) and improved milk composition (Selmar-Olsen & Mo, 1997). Additives should be chosen carefully and appropriately, and applied properly if an economic response to their use is to be obtained (O'Kiely, 2001). Nitrogenous compounds in silage are rapidly degraded in the rumen, which may result in a poor utilisation of the ammonia-N for microbial N synthesis (Rooke et al., 1983). There is no clear evidence that silage additives improve the quality of silage protein by lowering ruminal protein degradability and/or increasing the intestinal digestibility of undegraded protein.
Forage intake and digestibility are limited by the presence of forage cell walls in plants, which are only partly digested by ruminal microorganisms (Nadeau et al., 1996). In some studies formic acid treatment slightly decreased the ADF concentration in the silage through a rapid decrease of pH (Marshall et al., 1993). However, in other studies formic acid treatment had no effect on cell wall degradation (Nadeau et al., 2000).
The present study compared the effects of inoculant and formic acid based additives on silage quality, protein quality and ruminal digestion kinetics of cell walls with those of untreated bale silage.
Materials and Methods
First cut herbage consisting mainly of Lolium perenne was cut late in May in Poland, and wilted to a mean DM content of 381 g/kg, and then baled with a fixed chamber baler. Forage was preserved either without an additive (control, CK) or with a lactic acid bacteria (LAB) inoculant (IN) or with a formic acid (FO) additive. The lactic acid bacteria inoculant, Pioneer Brand 1888, was obtained from Pioneer (Hi-Bred Services G.m.b.H) and the formic acid additive from NOFO, Norway. The additives were applied by spraying the herbage over the pick-up reel of the baler. The bales were wrapped on a turntable wrapper (four layers) and stored flat for 90 days.
The inoculant, consisting of four strains of Lactobacillus plantarum and two strains of Enterococcus faecium, was applied in liquid form at a rate of 30 L/t, which would provide 105 colony forming units (CFU)/g. Foraform 70%, containing 645 g formic acid/kg and 60 g NH3/kg, was applied at a rate of 4 L/t. A total of 24 bales of silage was made (3 treatments, 8 replications). One composite sample from each bale was taken and frozen for analyses. Frozen silage samples were chopped with a meat chopper, Spomasz MMU-10Z, to a length of 25 mm, than dried at 40 oCand sub-sampled prior to chemical analyses.
Silage DM content was determined by drying at 105 oC for 24 h, and not corrected for possible volatile fatty acids (VFA) and ethanol losses. Silage fermentation quality and water-soluble carbohydrate concentrations were determined as reported by Nowak et al. (2004). Original silage samples and in sacco residues were analysed for NDF and ADF levels according to the method of Van Soest et al. (1991). When ammonia-N was used as a quality criterion, a correction was used for the ammonia-N applied through Foraform in the FO treatment (Selmer-Olsen, 1995).
Two non-lactating cows fitted with ruminal and proximal duodenal cannulas were used to determine in situ degradation parameters. Quadruplicate nylon bags (two bags per cow) for each incubation time were filled with 5 g dry silage samples and incubated in the rumen for 0, 2, 4, 8, 12, 16, 24 and 48 h. Four additional bags per treatment (two per cow) were incubated for 12 h to be used subsequently in mobile bags to determine crude protein (CP) digestibility. The procedure was according to the method used by Nowak & Potkański (2002).
Four mobile bags per treatment (80 x 25 mm, pore size 9 μm) were filled with one gram dry samples of the 12 h ruminal incubation residue. The bags were incubated for 2 hours at 39 oC in a pepsin-HCl solution (100 mg pepsin-1:10000/L of 0.004 mol/L HCl solution, pH 2.4) as recommended by Madsen et al. (1995) and inserted into the intestine via the duodenal fistula approximately one hour after feeding (8 bags/cow/h). The bags were recovered in the faeces within 26 hours. Bags not recovered within 30 h were discarded. After recovery the bags were stored at -12 oC until analysed. Upon thawing, samples were washed, dried at 40 oC and weighed as described previously. The digestibility of undegradable protein was calculated as the amount of N lost from the bag during passage through the intestine expressed as a percentage of N in the bag before incubation.
Disappearance data were fitted to the non-linear regression equation P = a + b (1 - e-ct) where 'a' represents the soluble fraction and 'b' the potential degradable fraction which disappears at a constant rate 'c' per unit of time. The three constants were then used to calculate the effective ruminal degradability (ERD) as ERD = a + [(b x c) / (c + k)] assuming a fractional outflow rate (k) of 0.06/h (0rskov & McDonald, 1979).
The data were subjected to analysis of variance using Statgraphics 5.0 (2000). Differences between treatments were tested using Duncan test and significance was declared at P < 0.05.
Results and Discussion
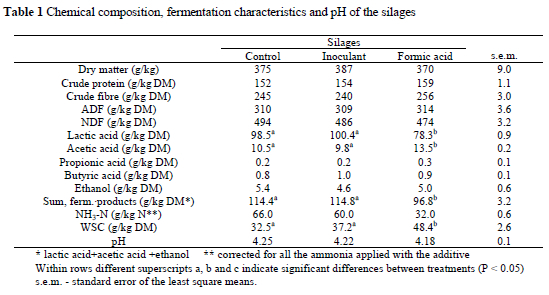
The DM content of the silages was within the range (320-400 g/kg DM) recommended by Pflaum (1997) for baled silages and close to the values reported by Lingvall (1997) and Tamm & Rausberg (1998). There were no significant differences between treatments regarding DM, CP, crude fibre, ADF and NDF levels (Table 1) and values were similar to those reported by Nadeau (1997) and Selmar-Olsen & Mo (1997). Bacterial inoculation can alter the composition of cell-wall carbohydrates during early fermentation when the pH is declining rapidly, but it has no effect on the final cell wall concentration (Jones et al., 1992; Nadeau, 1995). When silage was supplemented with pure formic acid, Haigh & Champple (1998) and Ramane et al. (1999) reported higher CP levels than what were found in the present study. Using the ammonia-N content and pH value as the basic criteria of fermentation, all silages were well preserved (Haigh & Chapple, 1998). However, acid treatment (FO) decreased the ammonia-N concentration (after correction) significantly. This would confirm the assumption that formic acid was probably a proteolysis inhibitor through the direct reduction in pH (Chamberlain & Quig, 1986). Similar results were obtained by Haigh & Parker (1985), Vagnoni et al. (1997), Haigh & Chapple (1998). However, Selmar-Olsen & Mo (1997) suggested that the subtracting of 100% of the ammonia added with the additive may sometimes lead to an overestimation of the results. In comparison to the untreated control (CO), the inoculant treatment (IN) did not affect the concentrations of ammonia-N, lactic acid, acetic acid, butyric acid, ethanol, WCS and the pH in big bale grass silage in the present study. Saarisalo et al. (2001) reported that inoculants were effective in decreasing protein degradation during ensiling. However, applying LAB inoculants to grass at ensiling did not result in a consistent change in silage fermentation characteristics, which may be partly due to the apparent domination of fermentation by the indigenous homofermentative LAB in the silage, thereby reducing the effect of inoculation (O'Kiely, 1996). Formic acid treatment (FO) decreased the sum of fermentation products mainly by decreasing the lactic and acetic acid concentrations and increasing the WSC content, a trend noted elsewhere (Gąsior & Brzóska, 2000). Formic acid inhibited lactic acid fermentation but not that of butyric acid. Contrary to our results, Haigh et al. (1996) found that formic acid big bale silage supplementation increased silage lactic acid and total acid contents and decreased the butyric acid content. In some studies, formic acid supplementation increased butyric acid (Keady & Murphy, 1996), while in others the addition of formic acid caused a decrease in butyric acid content (Haigh & Chapple, 1998; Pessi & Nousiainen, 1999). An even stronger effect of acid treatment was observed when restricting fermentation and preservation of WSC (McDonald et al., 1991). The high acetic acid content in grass silage supplemented with Foraform observed in this study is in agreement with that of other authors (Selmar-Olsen & Mo, 1997). However, formic acid would usually decrease the acetic acid content in material with a low N content and would increase it in material high in N and low in sugars (Davies et al., 1998). Formic acid treatment of big bales reduced proteolysis and the extent of fermentation resulting in silage with higher sugar concentrations. However, acid treatment did not alter moulding (Randby, 1996).
No differences occurred between silage treatments regarding any of the degradation parameters for any of the nutrients (Table 2). The effective protein degradability (EPD) was also not affected by silage additives, which is in agreement with a previous study (Gąsior & Brzóska, 2000) and most published values for silage. It appears that protein degradability in the rumen was not associated with the extent of proteolysis during ensiling. Formic acid and inoculant treatments did not protect silage protein from ruminal degradation. When silage was treated with formaldehyde, Beever et al. (1974) and Thomson et al. (1981) reported reduced protein degradability and an increase in the quantity of amino acids entering the small intestine.
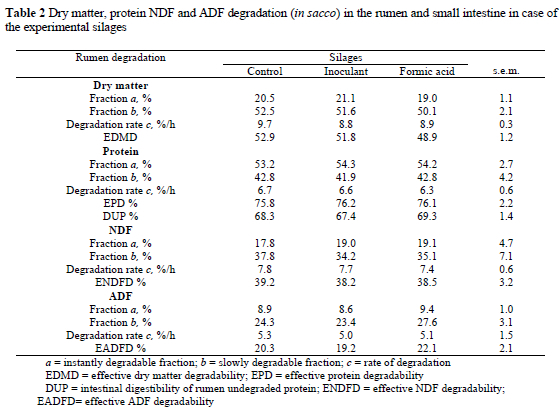
Effective protein degradability (EPD) of 76% for the formic acid treated silage (FO) was similar to results of Selmar-Olsen & Mo (1997) and Polan et al. (1998), but higher than the value of 65% reported by Thomas (1982). According to Keady & Steen (1994), formic acid additives decreased DM and protein degradation in the rumen. Nowak et al. (2004) found that formic acid slightly but not significantly decreased ruminal effective degradability of wilted grass silage. In a laboratory scale experiment Gąsior & Brzóska (1999) found that formic acid supplementation decreased protein degradability both in the silo during fermentation and in the rumen. However, in an experiment conducted by Gąsior & Brzóska (2000) on a commercial scale, they found no significant effect of formic acid and inoculant on EPD of grass silages. No significant effects of silage additives on rumen protein degradation were measured by the in sacco method (Rooke et al., 1983; Polan et al., 1998; Yang et al., 1998). Cushnahan & Mayna (1995) suggested that extensive silage fermentation may increase fraction a and reduce fractions b and c.
Intestinal digestibility of undegraded protein as measured by the mobile bag technique was not affected by formic acid or inoculant treatments (Table 2). This is in agreement with results reported by Rooke et al. (1983) for in vivo and in vitro studies. However, an improved in vivo total digestibility associated with an inoculant treatment had been shown by Keady & Steen (1994), but the precise mechanism of the increase was not evident. In another study, a significant increase in in vivo digestibility was associated both with inoculant and formic acid treatments (O'Kiely, 1996). A significant increase of intestinal digestibility of undegraded protein in formic acid treated silage was found in our earlier studies (Nowak et al., 2004). The higher intestinal digestibility in this trial may have resulted from lower protein ruminal degradability.
In the present study, treatments had no effect on ADF or NDF degradation in the rumen. Similarly, in a study of perennial ryegrass, formic acid treatment had no effect on ADF degradation in the rumen (Nagel & Broderick, 1992). A negative effect of formic acid on the digestibility of DM, NDF and cellulose was reported by Keady & Murphy (1996). Cushnahan & Mayne (1995) found no effect of restricting silage fermentation on digestibility. Gąsior & Brzóska (2000) found in an in vivo study that the apparent digestibility of crude fibre of inoculated silage increased by 5.5%, but the differences were not significant. They reported that inoculation significantly increased the digestibility of organic matter, ADF and NDF. An increase in the silage nutrient (OM, NDF and ADF) digestibility following inoculation probably reflects more extensive digestion in the abomasum and intestine, rather than in the rumen (Stokes, 1992). Yang et al. (1998) indicated that inoculation increased the nutrient digestibility of OM, energy, ADF and NDF significantly, suggesting that it may indicate a greater microbial activity in the rumen of animals offered inoculant-treated silage. Weimer (1996) suggested that the buffering effect of silage inoculant on rumen pH may be a possible explanation for the beneficial effects of inoculated silage on fibre digestibility. Weinberg et al. (2003) found that silage inoculants can survive in the rumen and modify fermentation. Yahaya et al. (2004) found that an epiphytic lactic acid bacteria treatment increased degradability of dry matter and NDF significantly. When combined enzymes and inoculants acted antagonistically to one another and did not improve organic matter digestibility (Stokes, 1992). In an experiment by Nadeau et al. (2000) cellulase and inoculant or formic acid had no significant effect on the in vitro DM digestibility.
Conclusion
The current study indicated that when formic acid was applied to big bale silage, higher levels of WCS and lower levels of fermentation products and ammonia-N were obtained. Lactic acid bacteria supplements in the inoculant did not significantly affect the quality of the silage. Both silage additives did not affect protein degradability, intestinal digestibility or cell wall degradability significantly above the untreated control silage.
References
- Beever, D.E., Cammell, S.B. & Wallace, A., 1974. The digestion of frozen and dried perennial ryegrass. Proc. Nutr. Soc. 33, 73A. [ Links ]
- Cushnahan, A. & Mayna, C.S., 1995. Effect of ensilage of grass on performance and nutrient utilization by dairy cattle. 1. Food intake and milk production. Anim. Sci. 60, 337-345. [ Links ]
- Davies, D.R., Merry, R.J., Willams, A.P., Bakewell, E.L., Leemans, D.K. & Tweed, J.K.S., 1998. Proteolysis during ensilage of forages varying in soluble sugar content. J. Dairy Sci. 81, 444-453. [ Links ]
- Gąsior, R. & Brzóska, F., 1999. Effect of formic acid and biological additives on the quality of silage made from grass and ground barley, on protein and fibre degradation during fermentation, and digestion in sacco. Ann. Anim. Sci. 26, 353-364. [ Links ]
- Gąsior, R. & Brzóska, F., 2000. The effect of wilting and additives on silage quality, protein degradation in the silo and in the rumen, and dairy cattle productivity. Ann. Anim. Sci. 27, 129-139. [ Links ]
- Gordon, F., 1989. An evaluation through lactating cattle of a bacterial inoculant as an additive for grass silage. Grass Forage Sci. 44, 169-179. [ Links ]
- Haigh, P.M. & Chapple, D.G., 1998. Effect of formic acid with formalin addition and wilting on silage fermentation and intake, and on liveweight change of young cattle. J. Agr. Eng. Res. 69, 179-183. [ Links ]
- Haigh, P.M. & Parker, J.W.G., 1985. Effect of silage additives and wilting on silage fermentation, digestibility and intake, and on liveweight change of young cattle. Grass Forage Sci. 40, 429-436. [ Links ]
- Haigh, P.M., Chapple, D.G. & Powell, T.L., 1996. Effect of silage additives on big-bale grass silage. Grass Forage Sci. 51, 318-323. [ Links ]
- Jones, B.A., Hatfield, R.D. & Muck, R.E., 1992. Effect of fermentation and bacterial inoculation on lucerne cell walls. J. Sci. Food Agric. 60, 147-153. [ Links ]
- Keady, T.W.J. & Murphy, J.J., 1996. Effect of inoculant treatment on ryegrass silage fermentation, digestilibility, rumen fermentation, intake and performance of lactating dairy cattle. Grass Forage Sci. 51, 232-241. [ Links ]
- Keady, T.W.J. & Steen, R.W.J., 1994. Effects of treating low dry-matter grass with a bacterial inoculant on the intake and performance of beef cattle and studies on its mode of action. Grass Forage Sci. 49, 438-446. [ Links ]
- Lingvall, P., 1997. Influence of inoculation on preventing clostride and fungi growth in silage. 8th Int. Symp. Forage Conser., Brno. pp. 138-139. [ Links ]
- Madsen, J., Hvelplund, T., Weisbjerg, M.R., Bertilsson, J., Olsson, I., Spörndly, R., Harstad, O.M., Volden, H., Tuori, M., Varvikko, T., Huhtanen, P. & Olafsson, B.L., 1995. The AAT/PBV protein evaluation system for ruminants. A revision. Norw. J. Agric. Sci., Suppl. No. 19. [ Links ]
- Marshall, S.A., Campbell, C.P. & Buchanan-Smith, J.G., 1993. Proteolysis and rumen degradability of alfalfa silages preserved with a microbial inoculant, spent sulfite liquor, formic acid or formaldehyde. Can. J. Anim. Sci. 73, 559-570. [ Links ]
- McDonald, P., Henderson, A.R. & Heron, S.J.E., 1991. The biochemistry of silage, 2nd ed., Chalcombe Publications, UK. pp. 340. [ Links ]
- Nadeau, E.M.G., 1995. Enzyme, inoculant, and formic acid effects on silage quality of orchard grass and alfalfa. PhD dissertation, Iowa State Univ., Ames, USA. [ Links ]
- Nadeau, E.M.G., 1997. Cellulase and bacterial inoculant effects on cocksfoot and Lucerne ensiled at high dry matter levels. J. Sci. Food Agric. 73, 369-376. [ Links ]
- Nadeau, E.M.G., Buxton, D.R., Lindgren, E. & Lingvall, P., 1996. Kinetics of cell-wall digestion of orchard grass and alfalfa silages treated with cellulase and formic acid. J. Dairy Sci. 79, 2207-2216. [ Links ]
- Nadeau, E.M.G., Buxton, D.R., Russell, J.R., Allison, M.J. & Young, J.W., 2000. Enzyme, bacterial inoculant, and formic acid effects on silage composition of orchard grass and alfalfa. J. Dairy Sci. 83, 1487-1502. [ Links ]
- Nagel, S.A. & Broderick, G.A., 1992. Effect of formic acid or formaldehyde treatment of alfalfa silage on nutrient utilization by dairy cows. J. Dairy Sci. 75, 140-154. [ Links ]
- Nowak, W. & Potkański, A., 2002. Effect of calcium soaps on rumen fermentation, protein degradability of barley, rapeseed meal and soybean meal. J. App. Anim. Res. 22, 65-74. [ Links ]
- Nowak, W., Potkański, A. & Wylegała, S., 2004. The effect of additives on quality, protein degradability, intestinal digestibility and feed intake of wilted grass silages. J. Anim. Feed Sci. 13, 101-100. [ Links ]
- O'Kiely, P., 1996. Performance of beef cattle offered grass silages made using bacterial inoculants, formic acid or sulphuric acid. Irish J. Agr. Food Res. 35, 1-15. [ Links ]
- O'Kiely, P., 2001. Producing grass silage profitably in northern Europe. NJF-seminar no.326, Lillehammer, pp. 1-18. [ Links ]
- O Kiely, P., Forristal, D. & Lenehan, J.J., 1999. Baled silage. Beef production series no. 11, Teagasc. p. 44. [ Links ]
- Ørskov, E.R., McDonald, I., 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 92, 499-502. [ Links ]
- Pessi, T. & Nousiainen, J., 1999. The effect of fermentation quality on the aerobic stability of direct cut or slightly prewilted grass silage. XII Int. Silage Conf., Uppsala. pp. 280-281. [ Links ]
- Petterson, D.C., Mayne, C.S., Gordon, F.J. & Kilpatrick, D.J., 1997. An evaluation of an inoculant/enzyme preparation as an additive for grass silage for dairy cattle. Grass Forage Sci. 52, 325-335. [ Links ]
- Pflaum, J., 1997. Role of new technology in improving silage quality. 8th Int. Symp. Forage Conservation, Brno. pp. 28-33. [ Links ]
- Polan, C.E., Stieve, D.E. & Garrett, J.L., 1998. Protein preservation and ruminal degradation of ensiled forage treated with heat, formic acid, ammonia, or microbial inoculant. J. Dairy Sci. 81, 765-776. [ Links ]
- Ramane, I., Kravale, D., Miculis, J. & Miettinen, H., 1999. Influence of different grass forage making technologies on feed composition, quality and milk production in dairy cows. XII Int. Silage Conf., Uppsala. pp. 207-208. [ Links ]
- Randby, A.T., 1996. Fermentation quality and moulding of round-bale silage. Proc.16th EGF Meeting Sept.15-19, Grassland Science in Europe. pp. 569-573. [ Links ]
- Rooke, J.A., Greife, H.A. & Armstrong, D.G., 1983. The digestion by cattle of grass silages made with no additive or with the application of formic acid or formic acid and formaldehyde. Grass Forage Sci. 38, 301-310. [ Links ]
- Saarisalo, E., Jaakkola, S., Skytta, E. & Jalava, T., 2001. Effects of biological additives and their combinations on fermentation quality and aerobic stability of wilted grass silage. NJF-seminar no.326, Lillehammer. pp. 37-41. [ Links ]
- Selmar-Olsen, I., 1995. Effect of ammonia applied with a formic acid-based silage additive on feed intake. In: Grassland into the 21st Century. Ed. Pollot, G.E., Proc. 50th Annual Meeting of the British Grassl. Soc., Harrogate, pp. 279-280. [ Links ]
- Selmar-Olsen, I. & Mo, M., 1997. The effects of three different silage additives on the extent of silage fermentation and the performance of dairy cows. Acta Agric. Scand. Sect. A-Anim. Sci. 47, 148-158. [ Links ]
- Stokes, M.R., 1992. Effects of an enzyme mixture, an inoculant, and their interaction on silage fermentation and dairy production. J. Dairy Sci. 75, 764-773. [ Links ]
- Statgraphics, 2000. Statgraphics plus for Windows 5.0. Manugistics, Inc. Rockville, Md., USA. [ Links ]
- Tamm, U. & Rausberg, P., 1998. Findings in feeding large quantities of grass silage to dairy cows. International Conference on Animal Nutrition, Tartu, pp. 56-60. [ Links ]
- Thomas, P.C., 1982. Utilization of conserved herbages. Occasional publication, Br. Soc. Anim. Prod., No. 6. pp. 67-78. [ Links ]
- Thomson, D.J., Beever, D.E., Lonsdale, C.R., Haines, M.J., Cammell, S.B. & Austin, A.R., 1981. The digestion by cattle of grass silage made with formic acid and formic acid-formaldehyde. Br. J. Nutr. 46, 193-208. [ Links ]
- Vagnoni, D.B., Broderick, G.A. & Muck, R.E., 1997. Preservation of protein in wilted lucerne using formic, sulphuric or trichloroacetic acid. Grass Forage Sci. 52, 5-11. [ Links ]
- Van Soest, P.J., Robertson, J.B. & Lewis, B.A., 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583-3597. [ Links ]
- Weddell, J., 1997. Quality in big bale grass silage. 8th International Symposium Forage Conservation, Brno, pp.142-143. [ Links ]
- Weimer, P.J., 1996. Why don't ruminal bacteria digest cellulose faster? J. Dairy Sci. 79, 1496-1503. [ Links ]
- Weinberg, Z.G., Muck, R.E. & Weimer, P.J., 2003. The survival of silage inoculant lactic bacteria in rumen fluid. J. Appl. Microbiol. 94, 1066-1071. [ Links ]
- Yahaya, M.S., Goto, M., Yimiti, W., Karita, S., Smerjai, B. & Kawamoto, Y., 2004. Additives effect of fermented juice of epiphytic lactic acid bacteria and acetic acid on silo fermentation and ruminal degradability of tropical elephant grass. J. Anim. Vet. Adv. 3, 116-122. [ Links ]
- Yang, T., Patterson, D.C., Gordon, F.J. & Kilpatrick, D.J., 1998. Effects of bacterial inoculation of unwilted and wilted grass silages 1. Rumen microbial activity, silage nutrient degradability and digestibility. J. Agric. Sci., Camb. 131, 103-112. [ Links ]
# Corresponding author. E-mail: nowakwl@jay.au.poznan.pl














