Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.33 no.4 Pretoria 2003
Chlorocholine chloride residue distribution in eggs, breast and femur meat of laying hens determined by labelled-15N estimates
NurhayatiI, II; J.D. KabasaI, III, #; G. ThinggaardI; U. ter MeulenI
IInstitute of Animal Physiology and Animal Nutrition, Göttingen University, Kellnerweg 6, D-37077, Germany
IIDepartment of Animal Nutrition and Feed Science, Jambi University, Kampus Pinang Masak Mendalo Darat, Jambi 36361 Indonesia
IIIFaculty of Veterinary Medicine, Makerere University, P.O. Box 7062, Kampala, Uganda
ABSTRACT
The distribution of chlorocholine chloride (CCC) residue or its metabolites in the meat and eggs of laying hens was studied using the 15N delta value (515N) and 15N atom % derived from 15N-CCC containing diets. In a completely randomised design, 20 laying hens were divided into four groups allocated four different diets namely; 0 mg 15N-CCC /kg feed a control diet (group A); 5 mg 15N-CCC /kg feed (group B), 50 mg 15N-CCC /kg (group C) and 100 mg 15N-CCC /kg (group D) for 11 days. During the seven days that followed, 15N-CCC diets were withdrawn and all hens were restored to feeding on the control diet. The 515N excess and 15N atom % excess in meat and eggs of hens fed diets containing 15N-CCC, were higher than in the control diet after 11 days of treatment and seven days after withdrawal of 15N-CCC, except for the egg yolk values of hens fed 5 mg 15N-CCC /kg feed. The 515N excess and 15N atom % excess of meat, egg yolk and egg albumen were dependent on dietary 15N-CCC concentrations and differed significantly between tissues for each of the three 15N-CCC concentrations examined. Femur meat 515N excess and 15N atom % excess were similar to that of breast meat but differed significantly from that of other tissues. The results show that tissue type is a factor in CCC residue/metabolite accumulation in chicken products suggesting differences in exposure or risk of CCC on consumers.
Keywords: Chlorocholine chloride residues, poultry products, labeled-15N
Introduction
Chlorocholine chloride (CCC), commonly known as Chlormequat, is a plant growth retardant widely used in commercial agriculture to improve the shape, size and yield of cereal crops. The CCC residues and its metabolites accumulate in cereal plants and can be detected in different plant products (Blinn, 1967; Bier & Dedek, 1970; 1972; Bohring, 1972; 1982). Although a small quantity of residue of CCC is allowed in foodstuffs, there are concerns that the residue may be harmful to both animals and humans. Consumption of excess CCC tended to increase the incidence of cancers such as leukaemia in rats (National Cancer Institute, 1979). Reports that CCC is completely metabolised into other compounds without leaving any residues in the tissues are not conclusive (Dekhuijzen & Bodlaender, 1973; Dekhuijzen & Vonk, 1974). To protect animals and humans from the intake of CCC residues in food, the European Union has set maximum CCC residue limits of 2 mg/kg for cereals, except for oats (5 mg/kg) and pears (10 mg/kg). Using radioactive-labelled CCC at different concentrations in feed, several workers demonstrated the presence of CCC residues in rats (Blinn, 1967; Romanowski, 1972; Gonzales, 1997), cows (Lampeter & Bier, 1970), oviducts of laying hens (Landazuri, 1992) and ovaries of pigs (Azem, 1996). When different concentrations (5, 50 and 250 mg) of 15N-labelled CCC/kg feed were fed to laying hens, significantly higher 15N concentrations were recorded in the egg yolk and albumen from hens fed the diets containing 250-mg/kg 15N-CCC (Songsang, 2000). The extent of CCC residue distribution in tissues may, however, differ due to differences in environmental factors, genotype, physiological and nutritional state of the individual (Ackermann et al., 1975; Sachse, 1977; Torner et al., 1999). High CCC concentrations in tissues increase the exposure risk of individuals consuming food products from such tissues. This study investigated the distribution of CCC residue and its metabolites in the eggs and meat of laying hens fed diets containing varying concentrations of CCC. For the purpose of this investigation the 15N-labelled CCC (15N-CCC) estimating method was used to trace the fate of the compound.
Materials and Methods
Twenty 280-day old Brown-breed laying hens, weighing between 1727 and 2269 g, were housed in individual layer cages and fed for seven days on a control diet consisting of 15N-CCC free wheat, maize, fish meal, a vitamin-mineral premix and feed lime. The diet contained 163.7 g crude protein/kg and 11.48 MJ ME/kg. Thereafter, the hens were divided randomly into four experimental groups. Each group was allocated one of the four experimental diets: a control diet containing 0 mg 15N-CCC/kg (group A) and diets containing 5 mg 15N-CCC/kg (group B), 50 mg 15N-CCC/kg (group C) and 100 mg 15N-CCC/kg (group D).
The 15N-CCC (atom 15N content, 99%) (MSD Isotopes, MERCK-FROSST Canada Inc., Montreal, Canada) was mixed in feed to concentrations of 5, 50 and 100 mg/kg in the final diet. The experimental diets were fed for 11 days, after which the diets containing 15N-CCC were withdrawn and the hens were fed the 15N-CCC free diet for seven days. Feed and water were offered ad libitum and cage temperatures were maintained at 19 - 21 °C during the experiment.
Eggs were collected daily, and the yolk and albumen portions of the eggs were separated immediately after collection using an egg separator (Labortek, Germany). The eggs from the different treatments were processed in separate rooms with separate equipment to avoid cross contamination with 15N. Meat samples were collected from the breast and femur muscles by biopsies on day 11 of the experimental period and seven days after 15N-CCC withdrawal. All samples were weighed, analysed for dry matter content and stored at -21 °C until further analyses.
The 11 days of experimental feeding were justified because yolk deposition takes place in 10 to 11 days (Etches, 1996; Hartmann, 2001). The seven days withdrawal period adopted was based on the observations of Coffman et al. (1999) that at least seven - eight days prior to slaughter are required to keep food derived from hens supplemented with antibiotics and/or drugs safe for human consumption.
The measurement of 15N was done according to the method described by Reineking et al. (1993) in the Forschungszentrum Waldökosysteme, Kompetenszentrum Stabile Isotope (KOSI) Göttingen University, Germany. The procedure involved using an elemental analyser (EA, Carlo-Erba 1500 nitrogen analyser) coupled into the Finnigan MAT Continuous Flow interface (ConFlo IITM), which connects to Finnigan MAT Isotope Ratio Mass Spectrometry (IRMS, Finnigan MAT 251). Acetanilide (C8H9NO) was the standard material for checking the analytical variability. It had a composition of 71.1% (C), 10.4% (N), 6.7% (H) and 11.8% (O) and contained 0.366943 of 15N atom % and 1.76 o/oo of δ15Ν. The relative δ15Ν was calculated using the formula of Mariotti (1983):
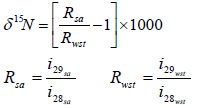
The 15N atom % was calculated based on the international standard of 15N in air (Mariotti, 1983) as follows:
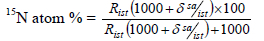
where:
R = absolute isotope ratios, measured for the sample and standard. The value of absolute isotope ratios of international standard in Goettingen was 0.003676496.
wst = work standard,
sa = sample,
i = current ion which i29 is ion of 15N and 14N, i28 is ion of 14N2
ist = international standard
Atom % excess (APE) and delta value excess (δ15ΝΕ) were calculated by difference between the 15N values of the treatment and that of the control. Similarly, the difference between δ value of the working standard and international standard of -13.468 was obtained.
Results and Discussion
The results of excess δ15 and excess atom % 15N in egg yolk, egg albumen, breast and femur meat of hens fed diets with varying levels of 15N-CCC for eleven days are shown in Table 1 and Figure 1. The 11 days of feeding diets containing 15N-CCC resulted in increased 515NE and APE in the meats and eggs of hens (P < 0.05). The significant increases were more in hens fed higher concentrations of 15N-CCC. The 515NE and APE values were highest in the egg yolks and decreased in the order: egg yolk > egg albumen > breast meat > femur meat.
The contribution of 15N in the meat N was lower than in the eggs at all doses examined. The results are similar to that of Wilbur (1975) who found that after 24 hours of oral application of 3 mg/kg CCC per laying hen per day, the CCC residue was higher in the yolk than in the albumen. However, residue levels in the muscle were not detectable (<0.01 mg/kg).
The pattern of the nitrogen distribution might be due to differences in tissue protein metabolism and flowing rate of nutrients during the production phase that is more in the egg than in muscle tissue. This is similar to reports by Paulson et al. (1983) that recorded the lowest pesticide residues concentration in the skeletal muscle of hens. Residue accumulation in the body is affected by several factors including post metabolism persistence of the applied compound and its rate of degradation (Paulson et al., 1983).
The results of excess δ15Ν value and that of excess atom % 15N (i.e. differences between the treatment and control values) in the eggs and meat of hens seven days after withdrawal of 15N-CCC diets are shown in Table 2 and Figure 2, respectively. Seven days after 15N-CCC diet withdrawal, δ15^ and APE concentrations in the meat of hens fed CCC treated feeds were still higher (P < 0.05) than in the control diets. For the eggs (yolk and albumen), the concentrations did not differ (P < 0.05) at any treatment level.
However, all values were lower than those observed after 11 days of feeding on treated diets. The δ15NE and APE in meat differed significantly at P < 0.05 among treatments, whereas those of egg yolk and albumen were similar during the same time period of eleven days.
Differences in tissue metabolism could explain these observations. Calsamiglia et al. (1996) observed differences in 15N levels and attributed their findings to variation in metabolism. The low mobility of 15N-CCC could also be a factor (Bohring, 1972; 1982) as it promotes tissue retention of the compound without any metabolism to the other compounds (Bier & Dedek, 1970; 1972). Other authors (Katz, et al., 1973; Beale et al., 1990; Shaikh & Chu, 2000; Furusawa et al., 2002) reported varying levels of drug residues in tissues, organs and eggs of poultry at different days after drug withdrawal. The residue levels were attributed to the body fat content that affects drug release back into the blood.
This study investigated the potential distribution of CCC residues in tissues of laying hens. Labelled 15N was used because it is a heavy isotope with a natural abundance of < 1% that is relatively constant in air. This abundance was approximately 0.366% at the time of the study. Since the CCC is a polar compound with low volatility, and the 15N was not degraded in the animal body under the experimental conditions, using 15N as a label of CCC was beneficial. The application of 15N in experimental diets enabled the quantification of the potential distribution of the dietary CCC and its metabolites in the chickens.
Conclusion
The results suggest that the CCC residue and/or its metabolites are distributed in chicken meat and eggs in varying concentrations. The 15N content was highest in egg yolk, followed by egg albumen and breast and lowest in femur meat. The nature of CCC metabolites in the chicken needs clarification.
Acknowledgements
We are grateful to the DUE-UNJA project (Development for Undergraduate Education Project at the University of Jambi), Departments of Tropical Animal Nutrition, Goettingen University, Germany; the Faculty of Animal Science, Jambi University, Indonesia and Physiological Sciences, Faculty of Veterinary Medicine, Makerere University, Uganda for invaluable support.
References
Ackermann, H., Kretzschmann, F., Beitz, H. and Banasiak, U., 1975. Untersuchungen zum Vorkommen von Chlormequat (CCC) in Weizenstroh und seine Exkretion mit der Milch nach Verfütterung chlormequathaltiger Strohpellets. Arch. Exp. Veterinär-med. 29(1), 157-161. [ Links ]
Azem, E., 1996. Ausscheidung und intermediäre Verteilung von 15N-markiertem Chlorcholinchlorid bei Schweinen nach einmaliger oraler Applikation. Doctoral Dissertation. Faculty of Agricultural Science, Georg-August University of Goettingen, Germany. pp. 119. [ Links ]
Beale, A.M., Fasulo, D.A. & Craigmill, A.L., 1990. Effects of oral and parenteral selenium supplements on residues in meat, milk and eggs. Rev. Environ. Contam. Toxicol. 115, 125-150. [ Links ]
Bier, H. & Dedek, W., 1970. Zur Frage des Abbaues von 15N- und 14C-Chlorcholinchlorid (CCC) in höheren Pflanzen. Biochem. Physiol. Pflanzen (BPP). 161, 403-410. [ Links ]
Bier, H. & Dedek, W., 1972. Transport, verteilung und rückstande von 14C- und 15N-CCC (2-chloroäthyl-trimethylammoniumchlorid) in weizen. Biochem. Physiol. Pflanzen (BPP). 168, 169-174. [ Links ]
Blinn, R.C., 1967. Biochemical behaviour in 2-chloroethyl trimethylammonium chloride in wheat and in rats. J. Agric. Food Chem. 15, 984-988. [ Links ]
Bohring, J., 1972. Abbau und Auswachsung von Chlorcholinchlorid bei Weizen. Z. Pflanzenernähr., Düngg. u. Bodenkde. 131(2), 179-190. [ Links ]
Bohring, J., 1982. Die Persistenz von Chlorcholinchlorid in Weizenpflanzen während der generativen Wachstumsphase und in lagernden Weizenkörnern. Z. Pflanzenernähr., Düngg. u. Bodenkde. 145, 278-287. [ Links ]
Calsamiglia, S., Stern, M.D. & Firkins, J.L., 1996. Comparison of nitrogen-15 and purine as microbial markers in continuous culture. J. Anim. Sci. 74, 1375-1381. [ Links ]
Coffman, J.R., Beran, G.W., Colten, H.R., Greig, C., Halloran, J., Hayes, D., Kaneene, J.B., McNutt, K., Meeker, D., Nickerson, S.C., Seay, T. & Stewart, R.G., 1999. The use of drugs in food animals: Benefits and risks. National Academy Press. Washington, D.C. pp. 115-119. [ Links ]
Dekhuijzen, H.M. & Bodlaender, K.B.A., 1973. Distribution and persistance of chlormequat in potatoes plants. Pestic. Sci. 4, 619-627. [ Links ]
Dekhuijzen, H.M. & Vonk, C.R., 1974. The distribution of chlormequat in wheat. Pest. Biochem. Physiol. 4, 346-355. [ Links ]
Etches, R.J., 1996. Reproduction in poultry. CAB International, Wallingford, UK. pp. 318. [ Links ]
Furusawa, N. & Kishida, K., 2002. Transfer and distribution profiles of dietary sulphonamides in the tissues of the laying hen. Food Addit. Contam. 19(4), 368-372. [ Links ]
Gonzales, V.M.S., 1997. Ausscheidung und Ruckstande von 15N-markiertem Chlorcholinchlorid (CCC) bei Albinoratten nach einmaliger oraler Verabreichehrung. M.Sc. thesis, Univ. Goettingen, Germany. [ Links ]
Hartmann, C., 2001. Selection for yolk production in laying hens. Doctoral thesis. Swedish University of Agricultural Sciences, Uppsala, Sweden. [ Links ]
JMPR. 1994. Joint FAO/WHO Meeting on pesticide residues: chlormequat. pp. 253-321. [ Links ]
Katz, S.E., Fassbender, C.A. & Dowling, J.J., 1973. Oxytetracycline residues in tissue, organs, and eggs of poultry fed supplemented rations. J. Assoc. Anal. Chem. 56, 77-81. [ Links ]
Lampeter, W. & Bier, H., 1970. Ausscheidung von Chlorcholinchlorid über Milch und Harn nach oraler Applikation von 1 g 15N-markiertem Chlorcholinchlorid an eine laktierende Kuh. Arch. Exper. Vet. Med. 24, 1027-1031. [ Links ]
Landazuri, J.C., 1992. Untersuchungen zum Stoffwechsel von CCC in Legehennen. M.Sc. thesis, Univ. Goettingen, Germany. [ Links ]
Mariotti, A., 1983. Atmospheric nitrogen as a reliable standard for natural 15N abundance measurements. Nature 303, 685-687. [ Links ]
National Cancer Institute, 1979. Bioassay of (2-Chloroethyl)trimethylammonium chloride for possible Carcinogenicity (DHEW Publication No. (NIH) 79-1714). Carcinogenesis Testing Programme, Division of Cancer Cause and Prevention, National Institutes of Health, Bethesda, MD, USA. [ Links ]
Paulson, G., Struble, C. & Mitchell, A., 1983. Comparative metabolism of sulfamethazine (4-amino-N-(4,6-dimethyl-2-pyrimidin) benzenesulfonamide) in the rat, chicken, pig and sheep. In: Pesticide chemistry: Human welfare and the environment. 3. Eds. Miyamoto, J. & Kearney, P.C., Pergamon Press, Oxford. pp. 375-380. [ Links ]
Reineking, A., Langel, R. & Schikowski, J., 1993. 15N, 13C-on-line measurements with an elemental analyser (Carlo Erba, NA 1500), a modified trapping box and a gas isotope mass spectrometer (Finnigan, Mat 251). Isotopenpraxis Environ. Health Stud. 29, 169-174. [ Links ]
Romanowski, H., 1972. Analytische Untersuchungen an CCC und sein Verhalten in veschidenen Medien und einigen Bodenarten. In: Probleme der Chlorcholinanwendung (CCC) in der Landwirtschaft. Die Nahrung, Ed. Engst, R..16 (I). pp. 56. [ Links ]
Sachse, J., 1977. Über die Bestimmung von Chlorcholinchlorid (CCC) in Getreide. Z. Lebensm. Unters.-Forsch. 163, 274-277. [ Links ]
Shaikh, B. & Chu, P.S., 2000. Distribution of total 14C residue in egg yolk, albumen, and tissues following oral (14C)sulfamethazine administration to hens. J. Agric. Food Chem. 48, 6404-6408. [ Links ]
Songsang, A., 2000. The effects of chlorocholine chloride in diets of laying hens on selected egg quality parameters and its distribution in the egg. Doctoral dissertation. Faculty of Agricultural Science, Georg-August University of Goettingen, Germany. [ Links ]
Torner, H., Blottner, S., Kuhla, S., Langhamer, M., Alm, H. & Tuchscherer, A., 1999. Influence of chlorocholinechloride-treated wheat on selected in vitro fertility parameters in male mice. Reprod. Toxicol. 13, 399-404. [ Links ]
Wilbur, R.D., 1975. Cycocel plant growth regulant: A study of the disposition of 14C-CL 38, 555: (1,2 14C-2-chloroethyl)-trimethyl ammonium chloride in laying hens; ACC report, Reg. Doc. BASF 75/10140. [ Links ]
# Corresponding author. E-mail: kabasajd@vetmed.mak.ac.ug














