Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.114 n.3 Pretoria Mar. 2024
http://dx.doi.org/10.7196/SAMJ.2024.v114i3.1338
RESEARCH
Cryoballoon ablation for atrial fibrillation in South Africa: One-year outcome from the Cryo Global Registry
A StanleyI; KA van BragtII; V ObidigboIII; B VeziIV
IMD; Private Practice, Netcare Sunninghill Hospital, Sandton, South Africa
IIPhD; Cardiac Ablation Solutions, Medtronic, Inc., Minneapolis, USA
IIIMSc; Cardiac Ablation Solutions, Medtronic, Inc., Minneapolis, USA
IVMD; Private Practice, Busamed Gateway Hospital, Umhlanga, South Africa
ABSTRACT
BACKGROUND: Pulmonary vein isolation (PVI) with cryoballoon catheter ablation (CBA) is a well-established and widely adopted method for the treatment of patients with atrial fibrillation (AF) to prevent recurrences of AF. CBA adoption in South Africa (SA) and outcome data in SA patients are limited.
OBJECTIVES: To evaluate real-world usage, safety and effectiveness of CBA in SA.
METHODS: In this sub-analysis of the Cryo Global Registry, 81 participants with paroxysmal AF (PAF) and persistent AF (PsAF) were enrolled between 2017 and 2021 across two private SA hospitals. Baseline characteristics, procedural characteristics, 12-month safety, effectiveness (atrial arrhythmia recurrence), healthcare utilisation (repeat ablation and all-cause hospitalisation), quality of life (QoL; measured by EQ-5D-3L) and predefined symptoms were reported on.
RESULTS: Participants in the SA cohort were a mean (standard deviation) of 60 (12) years old, 19 (23.5%) were female, and 48 (59.3%) presented with PAF. The overall presence of baseline comorbidities in the SA cohort was relatively low compared with the entire Cryo Global Registry cohort. The acute PVI success rate was high (98.8%). Two serious procedure-related adverse events occurred in 2 (2.5%) participants in the SA cohort. Freedom from arrhythmia recurrence was 97.4% (95% confidence interval (CI) 83.2 - 99.6%) in PAF and 78.4% (95% CI 58.1 - 89.7%) in persistent AF (p=0.035). Kaplan-Meier estimates for freedom from repeat ablations and all-cause hospitalisations were 97.0% (95% CI 88.4 - 99.2%) and 98.5% (95% CI 90.0 - 99.8%), respectively. Participants reported significant improvement in EQ-5D-3L index score and symptoms from baseline (0.90 (0.11)) - 12 months (0.97 (0.07), p<0.001.
CONCLUSION: CBA standard-of-care procedures in SA resulted in a high clinical freedom from arrhythmia recurrence, with a low risk of safety events within 12 months post ablation. In addition, participants experienced an improvement in QoL and high freedom from healthcare utilisation at 12 months. The obtained results will be important for guiding clinical decisions around CBA in SA.
Atrial fibrillation (AF) is imposing a rapidly increasing burden on healthcare systems worldwide. The reported prevalence of AF in South Africa (SA) is 3% in general practice,[1] and the rate of newly diagnosed AF was 4.6 - 5.9% in patients within specialised cardiac care.[2,3] Globally, catheter ablation (CA) is the recommended therapy for patients with symptomatic AF.[4] However, a national survey from 2014 (Jardine et al. [5]) showed that only 4.2% of AF patients in SA underwent CA for the treatment of AF. In fact, the number of CA procedures overall has steadily increased in the past decade.[6] SA is the only country in the sub-Saharan African region to perform complex cardiac ablations requiring three-dimensional (3D) mapping and trans-septal puncture.[7] Mkoko et al.[6] reported that AF ablation accounted for most of the CA cases, and radiofrequency ablation (RFA) was more commonly used than cryoballoon ablation (CBA; 94.7% v. 5.3%), respectively. CBA is a 'single-shot' anatomical ablation approach to pulmonary vein isolation (PVI), not requiring 3D mapping. Compared with RFA, CBA is associated with an increased long-term cost-effectiveness.[8,9] However, outcome data of CBA in the SA population are limited. This sub-analysis of the Cryo Global Registry assessed the procedural characteristics, safety and efficacy of CBA in patients with AF according to real-world practice in SA.
Methods
Study design
The Cryo Global Registry (NCT02752737) is a global multicentre, prospective, post-market study collecting data on AF ablation procedures conducted with the Arctic Front Family of cryoablation catheters (Medtronic, Inc., USA). This sub-analysis enrolled participants in two SA centres. Data collection adhered to the principles outlined in the Declaration of Helsinki, the National Department of Health's guidelines and the Health Professions Council of South Africa (HPCSA)'s General Ethical Guidelines for Health Researchers. Participants provided written informed consent prior to enrollment in the study. The Cryo Global Registry study protocol was reviewed and approved by an ethics committee (Pharma-Ethics, ref. no. 161115379) affiliated with both study sites in SA. A global steering committee of physicians advised on data quality, analysis and publication milestones. All medical procedures were performed according to the local standard of care. The aim of this sub-analysis of the Cryo Global Registry was to assess CBA usage, efficacy and safety in SA.
Study cohort
All AF participants aged >18 years undergoing a planned CBA procedure were eligible for inclusion into the Cryo Global Registry. There were no pre-existing characteristics or medical conditions that were considered exclusion criteria for participation. In this SA sub-analysis, data from participants with paroxysmal AF (PAF; episodes <7 days) and persistent AF (PsAF; episodes >7 days and <12 months)[4] were enrolled between May 2017 and August 2021; participants with longstanding persistent AF were excluded from this analysis for statistical considerations (N=3).
Cryoballoon ablation
CBA procedures in the Cryo Global Registry were performed according to local standard of care using a 23 or 28 mm CBA catheter (Arctic Front Advance or Arctic Front Advance Pro, Medtronic Inc., USA), as described previously.[10] In brief, dedicated, a 15-F steerable sheath (FlexCath or FlexCath Advance Steerable Sheath, Medtronic Inc., USA) was used to introduce the CBA into the left atrium via trans-septal puncture. The CBA catheter and sheath were then delivered to each pulmonary vein (PV) using a J-tip guidewire or a dedicated inner-lumen octopolar/decapolar circular mapping catheter (Achieve or Achieve Advance, Medtronic Inc., USA). Operators were required per protocol to confirm PVI by entrance and/or exit block using either a multi-electrode circular diagnostic catheter or the dedicated inner-lumen octopolar/decapolar circular mapping catheter. Phrenic nerve monitoring, oesophageal temperature monitoring and procedural imaging were operator determined. However, it was recommended (per protocol) that pacing and one other adjunctive method for phrenic nerve monitoring were used during all right-sided PV freeze applications. All freezes were halted upon detection of a decrease in diaphragmatic response. Post-ablation antiarrhythmic drug (AAD) initiation or continuation and participants' discharge occurred according to the centre's standard of care method.
Follow-up and endpoints
Participants were followed for 12 months according to standard of care at the participating centres, with a protocol-required visit at 12 months' follow-up. In this sub-analysis, all adverse events were reported and classified by the treating physician on seriousness (e.g. leading to death or a serious deterioration of health) and relatedness to the device or procedure. The primary efficacy endpoint was occurrence of a >30 second recurrence of AF, atrial flutter (AFL), or atrial tachycardia (AT) post a 90-day blanking period through 12 months. Atrial arrhythmia (AA) monitoring was performed according to each centre's standard of care and included (but was not limited to) the following methods: Holter monitor, electrocardiogram recording, trans-telephonic monitor, implantable cardiac monitor, pacemaker and/or implantable cardioverter defibrillator. Quality of life (QoL) over the study period was assessed by the EQ-5D-3L questionnaire[11] and participants were asked to report predefined AF symptoms at baseline and 12-month follow-up. Freedom from first (cardiovascular) hospitalisation and first repeat ablation through 12 months were assessed.
Statistical analysis
Continuous variables are summarised as mean and standard deviation and categorical variables are summarised as counts and percentages. Kaplan-Meier methods were used to estimate 12-month freedom from atrial arrhythmia recurrence, repeat ablation and hospitalisation. Standard error was approximated with Greenwood's formula. Changes in QoL from baseline to 12 months were assessed with a two-sided t-test, while changes in symptoms and usage of AAD from baseline to 12 months were assessed with a McNemar's test. Values of p<0.05 were considered statistically significant. Statistical analyses were performed with SAS software version 9.4 (SAS Institute, USA).
Results
Baseline characteristics
This sub-analysis of the Cryo Global Registry included 81 participants treated with CBA in SA. In total, 66of81 participants (81.5%) completed a 12-month follow-up visit. Twelve participants were lost to follow-up, 1 participant requested withdrawal from the study, 1 participant relocated to a different country and 1 (1.2%) died due to natural causes (unrelated to CBA) at 315 days after the procedure. Baseline characteristics are presented in Table 1. Participants were a mean (standard deviation (SD)) of 60 (12) years of age, 76.5% male and 59.3% had PAF. Mean (SD) time from AF diagnosis to enrollment was 34.5 (61.0) months, and the mean (SD) left atrial diameter was 42 (8) mm. Participants failed a mean (SD) of 0.6 (0.7) class I or III AAD prior to CBA, with 23.5% of participants receiving CBA as a first-line rhythm control therapy. A history of AFL was reported in 16.0% of participants.
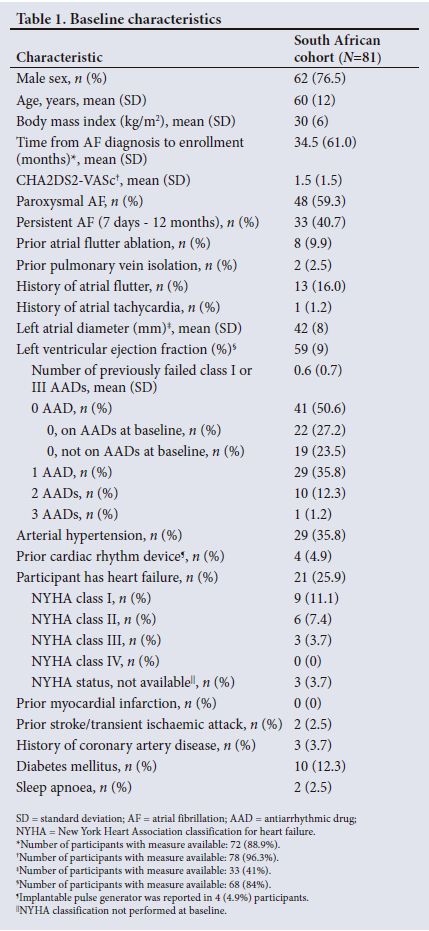
Procedural characteristics
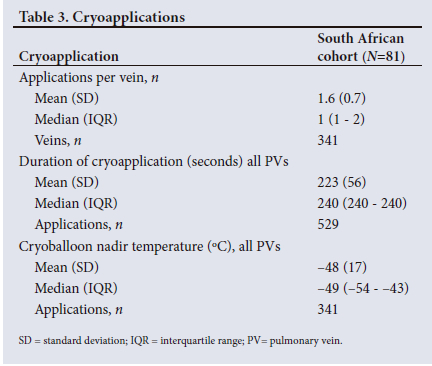
Mean (SD) procedure, left atrial dwell, and fluoroscopy times were 82 (27) minutes, 54 (20) minutes and 15 (7) minutes, respectively (Table 2). Most (98.8%) procedures were performed under general anaesthesia. Pre-procedural imaging with computed tomography (CT) or magnetic resonance imaging (MRI) and the use of intracardiac echocardiography (ICE) were unusual (9.9% and 0.0%, respectively). The phrenic nerve was monitored in 100% of cases. Acute PVI success was 98.8%. Participants received additional cavotricuspid isthmus (CTI) ablation in 22.2% and additional non-PVI and non-CTI ablation in 7.4% of cases. A total of 529 cryoapplications were applied to 341 veins, and the median (interquartile range (IQR)) application duration was 240 seconds. Additional cryoapplication information can be found in Table 3.
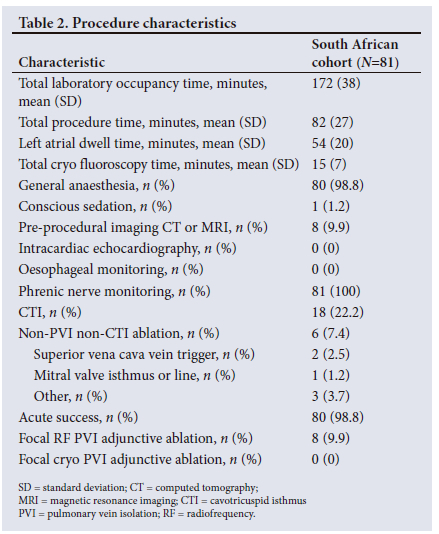
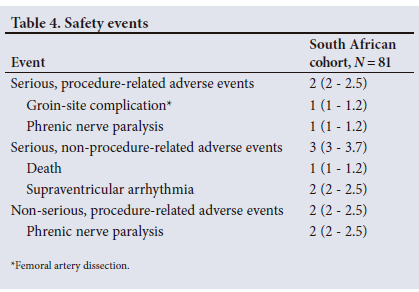
Safety and efficacy
All safety events in the SA cohort are reported in Table 4. Serious, procedure-related adverse events were reported in two participants (2.5%). One was an arteriovenous pseudoaneurysm. Surgical repair was needed to close the right femoral artery tear. The other was a phrenic nerve paralysis (PNP) classified by the physician as serious (owing to prolonged hospitalisation) and procedure related. The PNP resolved within 5 months of the procedure without further sequelae. No atrio-oesophageal fistula, cardiac perforations, or deaths related to the procedure were reported. In addition, three serious adverse events were reported that were not classified as related to the procedure (one death (morbidly obese patient failing attempts to lose weight) and two supraventricular arrhythmias that were both resolved during follow-up). Two more adverse events were reported that were classified as non-serious and procedure related. Both events were PNPs (one PNP was resolved at discharge, and the other resolved without further intervention during follow-up).
Clinical freedom from AA recurrence in the SA cohort is shown in Fig. 1A. The PAF cohort had a significantly higher freedom from 30 second AA recurrences at 12 months (97.4%) compared with the PsAF cohort (78.4%, p<0.05). In total, 41 of 81 (50.6%, Fig. 1B) participants received rhythm monitoring at least once during 12-month follow-up. Standard of care monitoring was mainly performed using 12-lead electrocardiography (ECG). The frequency of arrhythmia monitoring through 12 months was not statistically different between PAF and PsAF cohorts (p=0.99).
In 52% of participants, class I or III AADs were prescribed at discharge for the management of post-procedure arrhythmias (Fig. 2A). The AAD prescription was 30% at 12 months. Participants in the PsAF cohort were prescribed AADs post ablation more often than in the PAF cohort. The Kaplan-Meier estimate for freedom from repeat ablations was 97.2% (95% CI 81.9 - 99.6%) in PAF and 96.4% (95% CI 77.2 - 99.5%) in PsAF participants (p=0.78; Fig. 2B) at 12 months' follow-up. The Kaplan Meier estimate for freedom from all-cause hospitalisation was 97.5% (95% CI 83.5 - 99.6%) in PAF and 100% in PsAF participants (p=0.40; Fig. 2C) at 12 months' follow-up. Over the course of follow-up, one participant was hospitalised for supraventricular arrhythmia.
Participant-reported outcomes
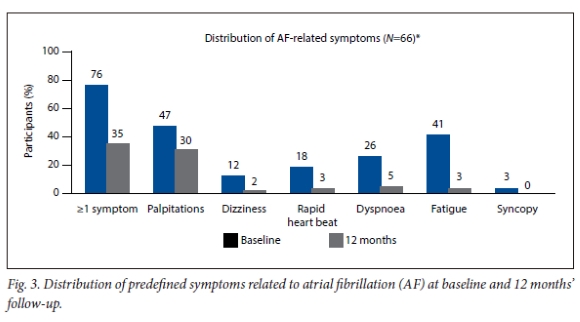
Reported EQ-5D-3L Index scores showed a significant and clinically relevant improvement in mean (SD) QoL from baseline to 12 months (0.90 (0.11) to 0.97 (0.07), p<0.001). Seventy-six percent of participants experienced at least one symptom related to AF in the 3 months before study enrollment (Fig. 3). The most common symptoms were palpitations (47.0%) and fatigue (40.9%). The proportion of participants with >1 symptom decreased at 12 months to 34.9%. The most common remaining symptom at 12 months was palpitations in 30.3% of participants post ablation, and the percentage of participants reporting fatigue decreased to 3.0%.
Discussion
Within the Cryo Global Registry platform, 81 participants were enrolled in two private hospitals in SA, of which 66 participants completed a 12-month follow-up. Participants were a mean (SD) of 60 (12) years old, 19 (23.5%) were female, and 48 (59.3%) presented with PAF at the time of enrollment. Procedures were lean and efficient, with an acute PVI success rate of 98.8%. Clinical freedom from a >30 second AA recurrence at 12 months was high in the PAF and PsAF cohort (97.4% and 78.4%, respectively), with a low occurrence of serious procedure-related events (n=2, 2.5%). In addition, participants had high freedom from repeat ablations and hospitalisations (97.0% and 98.8%, respectively) and showed a significant improvement in QoL and symptom burden at the 12-month follow-up.
Most participants in this SA sub-analysis of the Cryo Global Registry presented with PAF at study enrollment. In the general SA lower (32.1 - 50%), and more patients suffer from PsAF (21.2 - 29%) or permanent AF (12 - 46.7%).[5,13] The discrepancy with the general AF population may reflect that (i) all participants in the current registry were enrolled and treated in two private hospitals in SA, and (ii) higher success rates are observed in patients with PAF v. PsAF. Participants in the current sub-analysis also had a relatively low percentage of comorbidities when compared with other SA reports or the global Cryo Registry cohort.[2,5,10]
Efficacy in this SA cohort was high when compared with earlier published global results in the Cryo Global Registry (86.4% in PAF and 70.9% in PsAF at 12 months) and larger randomised controlled trials.[10,13-15] It is also likely that the rate of arrhythmia recurrences is underestimated in this analysis owing to limited cardiac monitoring during follow-up in SA. In this sub-analysis, cardiac monitoring was mainly driven by the presence of symptoms. As shown by Aguilar et al.,[16] monitoring frequency and monitoring modality both correlate with arrhythmia-free survival. In addition, 30% of participants in this SA sub-analysis were being prescribed AADs at 12 months, which may influence the number of recurrences. AAD prescription rates in the current registry are comparable with what is described earlier for SA patients undergoing AF ablation (63% at discharge and 26% at 9-month follow-up) and represent standard of care in SA.[17]
Regardless, CBA resulted in a significant improvement in QoL and AF-related symptoms at 12 months post ablation, and repeat ablations and rehospitalisations were uncommon in the SA cohort. Specifically, the low hospitalisation rate (n=1) is an important finding, considering that high rates of hospitalisation due to cardiovascular reasons were reported earlier for SA patients with AF (50.5% of rhythm control patients and 25.4% of rate control patients had at least one hospitalisation in 12 months).[5] Large randomised trials[18,19] have also demonstrated a favourable impact of CBA on healthcare utilisation and QoL. However, reported freedom from repeat ablations (84.3 - 88.2%) and all-cause hospitalisations (67.4%) were lower[18,19] compared with the results presented here. Further studies would be needed to better understand the reason for the low hospitalisation and repeat ablation rates. It has been shown that CBA results in clinically and statistically significant improvements in QoL. The QoL results using the EQ-5D-3L questionnaire were previously reported between 0.85 and 0.88 at baseline and between 0.88 and 0.93 at 12 months after CBA. Participants in this SA sub-analysis of the Cryo Global Registry scored higher at baseline and improved more from baseline to 12 months. In total, these data demonstrate the safety, efficacy and efficiency of CBA usage in this cohort of SA patients.
Limitations
In the Cryo Global Registry, participants were treated according to the local standard of care. In this SA sub-analysis, cardiac rhythm monitoring was not performed during follow-up in approximately half of the participants. The mean frequency of arrhythmia monitoring in the current cohort was 0.9 (1.2) times in 12 months, and mainly performed by 12-lead ECG. Also, it should be noted that in 15/81 (18.5%) of participants, the 12-month visit was missing.
Conclusion
Standard of care CBA in SA resulted in a high clinical freedom from arrhythmia recurrence with a low risk of safety events within 12 months post ablation. The perceived high costs of a catheter ablation for AF are countered by a significant improvement in symptom burden, QoL and a high freedom from healthcare utilisation at 12 months. These results support a wider adoption of CBA for the treatment of AF in SA.
Declaration. None.
Acknowledgements. The authors sincerely thank the Cryo Global Registry centres and staff for their commitment and contributions to the study. The authors also thank Ryan Radtke, Hae Lim, and Rachelle Kaplon from Medtronic for their support of the trial study and generation of this manuscript.
Author contributions. AS and BV were responsible for the acquisition of data. AS and KB have participated in the design of the mansucript, interpreting the results and writing the manuscript. VO was responsible for the analysis and validation of the data. All authors have critically reviewed and approved the manuscript.
Funding. This study is sponsored by Medtronic, Inc.
Conflicts of interest. KB and VO are employees of Medtronic. The remaining authors have nothing pertaining to this manuscript to disclose.
References
1. Connor M, Rheeder P, Bryer A, et al. The South African stroke risk in general practice study. S Afr Med J 2005;95(5):334-339. [ Links ]
2. Sliwa K, Carrington MJ, Klug E, et al. Predisposing factors and incidence of newly diagnosed atrial fibrillation in an urban African community: Insights from the Heart of Soweto Study. Heart 2010;96(23):1878-1882. https://doi.org/10.1136/hrt.2010.206938 [ Links ]
3. Mansoor E. De novo atrial fibrillation post cardiac surgery: The Durban experience. Cardiovasc J Afr 2014;25(6):282-287. https://doi.org/10.5830/CVJA-2014-067 [ Links ]
4. Hindricks G, Potpara T, Dagres N, et al. ESC Scientific Document Group. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42(5):373-498. http://doi.org/10.1093/eurheartj/ehaa612. Erratum in: Eur Heart J 2021;42(5):507. Erratum in: Eur Heart J 2021 Feb 1;42(5):546-547. Erratum in: Eur Heart J 2021;42(40):4194. [ Links ]
5. Jardine RM, Fine J, Obel IW. A survey on the treatment of atrial fibrillation in South Africa. S Afr Med J 2014;104(9):623-627. https://doi.org/10.7196/samj.8111 [ Links ]
6. Mkoko P, Barole N, Solomon K, Chin A. Feasibility and safety of interventional electrophysiology and catheter ablation in the South African public sector: Challenges and opportunities for comprehensive cardiac electrophysiology in South Africa. J Arrhythm 2022;38(6):1042-1048. https://doi.org/10.1002/joa3.12783 [ Links ]
7. Yuyun MF, Bonny A, Ng GA, et al. A systematic review of the spectrum of cardiac arrhythmias in sub-Saharan Africa. Glob Heart 2020;15(1):37. https://doi/org/10.5334/gh.808 [ Links ]
8. Metzner A, Straube F, Tilz RR, et al.; FREEZE Cohort Study Investigators. Electrophysiology lab efficiency comparison between cryoballoon and point-by-point radiofrequency ablation: A German sub-analysis of the FREEZE Cohort study BMC Cardiovasc Disord 2023;23(1):8. https://doi.org/10.1186/s12872-022-03015-8. Erratum in: BMC Cardiovasc Disord 2023;23(1):110. [ Links ]
9. Chun KRJ, Brugada J, Elvan A, et al.; FIRE AND ICE Investigators. The impact of cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation on healthcare utilisation and costs: An economic analysis from the FIRE AND ICE Trial. J Am Heart Assoc 2017;6(8):e006043. https://doi.org/10.1161/JAHA.117.006043 Erratum in: J Am Heart Assoc 2017;6(9). [ Links ]
10. Chun KRJ, Okumura K, Scazzuso F, et al.; Cryo Global Registry Investigators. Safety and efficacy of cryoballoon ablation for treating paroxysmal and persistent AF in a real-world global setting: Results from the Cryo AF Global Registry. J Arrhythm 2021;37(2):356-367. https://doi.org/10.1002/joa3.12504 [ Links ]
11. EQ-5D-3L questionnaire. https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/ (accessed 8 March 2024). [ Links ]
12. Thomas V, Schulein S, Millar RN, Mayosi BM. Clinical characteristics and outcome of lone atrial fibrillation at a tertiary referral centre: The Groote Schuur Hospital experience. Cardiovasc J Afr 2018;29(5):268-272. https://doi.org/10.5830/CVJA-2018-005 [ Links ]
13. Kuck KH, Brugada J, Fürnkranz A, et al.; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374(23):2235-2245. https://doi.org/10.1056/NEJMoa1602014 [ Links ]
14. Luik A, Radzewitz A, Kieser M, et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: The prospective, randomised, controlled, noninferiority freeze AF study. Circulation 2015;132(14):1311-1319. https://doi.org/10.1161/CIRCULATIONAHA.115.016871 [ Links ]
15. Andrade JG, Champagne J, Dubuc M, et al.; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: A randomised clinical trial. Circulation 2019;140(22):1779-1788. https://doi.org/10.1161/CIRCULATIONAHA.119.042622 [ Links ]
16. Aguilar M, Macle L, Deyell MW, et al Influence of monitoring strategy on assessment of ablation success and postablation atrial fibrillation burden assessment: Implications for practice and clinical trial design. Circulation. 2022;145(1):21-30. http://www.doi.org/10.1161/CIRCULATIONAHA.121.056109 [ Links ]
17. Lorgat F, Pudney E, van Deventer H, Chitsaz S. Robotically controlled ablation for atrial fibrillation: The first real-world experience in Africa with the Hansen robotic system. Cardiovasc J Afr 2012;23(5):274-280. https://doi.org/10.5830/CVJA-2012-015 [ Links ]
18. Kuck KH, Fürnkranz A, Chun KR, et al.; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: Reintervention, rehospitalisation, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016;37(38):2858-2865. https://doi.org/10.1093/eurheartj/ehw285 [ Links ]
19. Andrade JG, Macle L, Verma A, et al.; CIRCA-DOSE Study Investigators. Quality of life and health care utilisation in the CIRCA-DOSE study. JACC Clin Electrophysiol 2020;6(8):935-944. http://www.doi/10.1016/j.jacep.2020.04.017 [ Links ]
 Correspondence:
Correspondence:
A Stanley
anthys99@gmail.com
Accepted 21 November 2023














