Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.114 no.2 Pretoria Fev. 2024
http://dx.doi.org/10.7196/SAMJ.2024.v114i2.1538
RESEARCH
Postmortem minimally invasive tissue sampling to ascertain the cause of death in South African children: A case for implementing as standard of care
J du ToitI; K StorathII; I DunnIII; P MakekengIV; M MoosaV; K MothibiVI; N UmunezaVII; C A ReesVIII, IX; D M BlauX; S LalaXI; Y AdamXII; S VelaphiXIII; M HaleXIV; P SwartXV; J WadulaXVI; L MothibiXVII; A WiseXVIII; V BabaXIX; P JaglalXX; S MahtabXXI; SA MadhiXXII, XXIII; Z DangorXXIV
IMB BCh; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIBClinMed; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIBHSc Hons; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVBCMP; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VMBBS; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIMSc; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIIMSc; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIIIMD, MPH; Division of Pediatric Emergency Medicine, Emory University School of Medicine, Atlanta, USA
IXMD, MPH; Department of Emergency Medicine, Children's Healthcare of Atlanta, USA
XDVM, PhD; Global Health Center, US Centers for Disease Control and Prevention, Atlanta, USA
XIMB BCh, PhD; Office for Teaching and Learning; and Paediatric Education and Research Ladder, Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XIIMB BCh, FCOG SA; Department of Obstetrics and Gynaecology, University of the Witwatersrand, and Chris Hani Baragwanath Academic HospitalJohannesburg, South Africa
XIIIMB ChB, PhD; Department of Paediatrics and Child Health, School of Clinical Medicine, Faculty of Health Sciences, Chris Hani Baragwanath Academic Hospital and University of the Witwatersrand, Johannesburg, South Africa
XIVMB ChB, FC Path; Department of Anatomical Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XVMB BCh, MMed; Department of Anatomical Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XVIMD, FC Path Micro; Department of Clinical Microbiology and Infectious Diseases, Faculty of Health Sciences, University of Witwatersrand, National Health Laboratory Services, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
XVIIMB BCh, FC Path Micro; Department of Clinical Microbiology and Infectious Diseases, Faculty of Health Sciences, University of Witwatersrand, National Health Laboratory Services, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
XVIIIMB BCh, MMed; Rahima Moosa Mother and Child Hospital, Obstetrics and Gynaecology, University of the Witwatersrand, Johannesburg, South Africa
XIXM BChB, FCOG SA; Department of Obstetrics and Gynaecology, University of the Witwatersrand, and Chris Hani Baragwanath Academic Hospital Johannesburg, South Africa
XXMB BCh, MMed; Department of Clinical Microbiology and Infectious Diseases, Faculty of Health Sciences, University of Witwatersrand, National Health Laboratory Services, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
XXIMBBS, PhD; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XXIIMB BCh, PhD; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XXIIIMB BCh, PhD; Wits Infectious Diseases and Oncology Research Institute, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XXIVMB BCh, PhD; South African Medical Research Council Vaccines and Infectious Diseases Analytics Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Determining the death burden for prioritising public health interventions necessitates detailed data on the causal pathways to death. Postmortem minimally invasive tissue sampling (MITS), incorporating histology, molecular and microbial culture diagnostics, enhances cause-of-death attribution, particularly for infectious deaths. MITS proves a valid alternative to full diagnostic autopsies, especially in low- and middle-income countries. In Soweto, South Africa (SA), the Child Health and Mortality Prevention Surveillance (CHAMPS) programme has delineated over 1 000 child and stillbirth deaths since 2017. This SA CHAMPS site supports advocating for the use of postmortem MITS as routine practice, for more granular insights into under-5 mortality causes. This knowledge is crucial for SA's pursuit of Sustainable Development Goal 3.2, targeting reduced neonatal and under-5 mortality rates. This commentary explores the public health advantages and ethicolegal considerations surrounding implementing MITS as standard of care for stillbirths, neonatal and paediatric deaths in SA. Furthermore, based on the data from CHAMPS, we present three pragmatic algorithmic approaches to the wide array of testing options for cost-effectiveness and scalability of postmortem MITS in South African state facilities.
There has been a reduction in the global under-5 mortality rate from 93 deaths per 1 000 live births in 1990 to 38 in 2021.[1] Nevertheless, under-5 mortality rates remain high in low- and middle-income countries (LMICs). Ascertaining the cause of childhood death remains challenging in LMIC settings, and mainly premised on verbal autopsy and vital registration data.[2] The verbal autopsy tool was developed to establish cause of death where other methods are unavailable, despite the low sensitivity (<33.3%).[3,4] Determining the cause of death, even in health facilities in LMICs, is often hampered by the scarcity of diagnostic tools, multiple coexisting conditions, and paucity of postmortem sampling of the decedents.[2,5,6] Furthermore, there is a lack of data on the cause of stillbirths, of which 98% of the 2.6 million annual cases occur in LMICs.[7] More granular insight into the causes of deaths in children under 5 years of age from LMICs could inform interventions to achieve the Sustainable Development Goal 3.2 of reducing neonatal and under-5 mortality to as low as 12 and 25 per 1 000 live births, respectively, by 2030.
Complete diagnostic autopsy remains the gold standard for determining cause of death. However, social, cultural and religious factors, including fears of body mutilation or organ removal, and relatively short times to burial related to cultural and religious beliefs, preclude routine use of complete diagnostic autopsies in most LMICs.[8,9] Further constraints to undertaking complete diagnostic autopsy in LMICs include personnel and laboratory resource constraints, and funding of competing health priorities.[5] The use of postmortem minimally invasive tissue sampling (MITS) has emerged as a valuable tool in accurately determining cause of death, particularly from infectious causes. Furthermore, the use of MITS is acceptable among LMIC communities, and can be undertaken within a reasonable period following death.[10,11] A standardised operating protocol for the MITS procedure using biopsy needles was piloted in Mozambique and is widely used in other programmes.[12] Findings from Mozambique showed 83% correlation in the cause of death determination using MITS compared with complete diagnostic autopsy, including stillbirths, and in the absence of antemortem clinical data.[12-14] The Child Health and Mortality Prevention Surveillance (CHAMPS) programme is a multicentre surveillance across seven LMIC settings. CHAMPS aims to determine the causal pathway to death on the causes of stillbirths and under-5 childhood deaths. The investigations in CHAMPS include clinical data abstraction, verbal autopsies and systematic multi-organ MITS, which is submitted for histopathology, molecular and microbiologic diagnostics.[2]
In this commentary, leveraging on experience with MITS in Soweto, South Africa (SA), we discuss the social and cultural considerations of undertaking MITS and the materialising public health benefits. We provide an argument for the implementation of MITS as a standard-of-care practice in paediatric departments, with the aim of improving diagnostic accuracy of cause-of-death attribution to inform public health and clinical interventions to reduce childhood mortality. Furthermore, based on the data from CHAMPS, we present three pragmatic algorithmic approaches to the wide array of testing options for cost-effectiveness and scalability of postmortem MITS in SA state facilities.
Determining cause of death: Procedure and process
The MITS procedure has been previously described.[12,15-17] Briefly, following consent from the guardian/parent, the decedent is cleansed with alcohol and iodine solution within 24 hours of death in an unrefrigerated body or within 72 hours if the corpse was refrigerated. Investigation includes photographs of the decedent, measuring of height and weight and tissue sampling. The body organ samples include core biopsy samples from the brain, liver and lungs. Blood is taken by intracardiac puncture, cerebrospinal fluid is collected from below the occipital bone and swabs are taken from nasopharyngeal space and rectum. The entire sampling procedures can be completed within 30 minutes, albeit dependent on the expertise of the technician and the number of sites being sampled.
The cerebrospinal fluid is sent for microscopy, culture and susceptibility (MC&S) testing as well as for multiplexed nucleic acid amplification tests using polymerase chain reactions (PCR) on four custom-designed syndromic TaqMan array cards (TAC; ThermoFisher Scientific, USA).[16] The TAC cards used in CHAMPS have been designed to detect 116 different pathogens. Blood samples are sent for HIV PCR, malaria thick and thin smears, MC&S and TAC. The rectal swab is sent for GeneXpert MTB and TAC, and nasopharyngeal swab is sent for TAC.[16] Tissue sampling from lung, liver and brain is done with a core biopsy needle for histopathological assessment and special stains or immunohistochemistry, as guided by the TAC results (Fig. 1). Limitations to the current testing process include the inability to diagnose congenital heart lesions. Kidney sampling could be attempted should the clinician feel that histological confirmation of renal disease would be helpful.
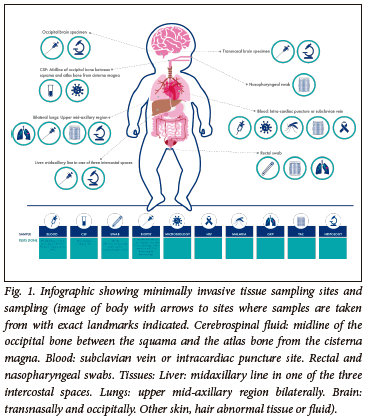
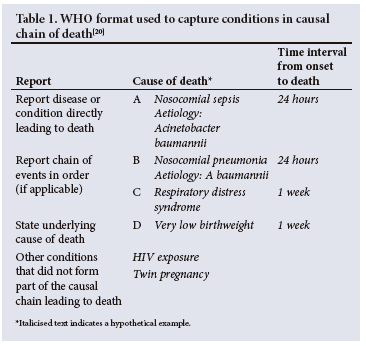
Demographics, clinical findings, antemortem test results, postmortem test results and verbal autopsy are used by the Determination of Cause of Death (DeCoDe) panel for the cause of death ascertainment. The DeCoDe panel consists of local paediatricians, neonatologists, obstetricians and gynaecologists, microbiologists, and anatomical pathologists.[18] Using the World Health Organization coding of death, the panel ascribe an underlying and an immediate cause of death, and any other causes that are directly or indirectly in the pathway to death.[19] For deaths attributed to a single condition, that condition is considered the underlying cause of death. For deaths in which more than one condition played a role, underlying, antecedent and immediate causes are assigned. The underlying cause of death is the condition considered to have precipitated a series of events that ultimately resulted in death. The immediate cause of death is the most proximal event which resulted in the death, and comorbid conditions are events between the underlying and immediate cause of death. A hypothetical example is illustrated in Table 1. The results from the DeCoDe panel are relayed to the family, who are offered grief counselling, and provided any recommendations from the panel to prevent future deaths.
Ethicolegal considerations for conducting MITS in South Africa
According to SA law, persons are entitled to their inherent dignity even when they have demised. The South African Constitution, Section 15(1) states that 'everyone has the right to freedom of conscience, religion, thought, belief, and opinion.[21] Respecting the family's choice of their deceased loved ones is to be upheld. Notably, the law permits autopsies outside 'other than natural deaths', with consent from the families. Similarly, upholding human rights to dignity and freedom when considering the MITS procedure is of critical importance.[21] MITS conducted in the CHAMPS study aligns with SA legislature, as it promotes the respect to human dignity of the deceased as well as the rights of their families.
Furthermore, the 'right to access healthcare services, including reproductive health care', and therefore the preventability of death, is recognised in section 27(1)(a) of SA law.[21] Informed by the results of MITS in CHAMPS, some of the preventable factors of a death include access to high-quality healthcare, appropriate health-seeking behaviours, adequate antenatal care and access to family planning services. This also draws attention to some of the potentially targetable public health measures to reduce childhood mortality.
With regard to autonomy, it is particularly important that the family is not coerced into having the MITS procedure on the deceased. Families are often receptive to the procedure if the principles of improving lives and outcomes of others in the future are made known. In a multi-country mixed-method study, 75% of 194 participants were willing to know the cause of death of a relative, with an additional 16% being willing to know the cause of death only under certain circumstances (e.g. sudden death or unclear clinical diagnoses).[9] A retrospective survey in Kenya reported that 81% of people who had previously declined a full autopsy would have consented to MITS.[22] Unpublished data in Soweto suggest that the mothers of decedents found that the information garnered by MITS in CHAMPS assisted them with closure and initiated their healing processes. Furthermore, mothers' perceptions were that the timeous undertaking of MITS did not interfere with religious and traditional practices. It is important that the procedure is thoroughly explained to the parents and that details about the sampling are included. This includes a description of the puncture sites and that small needle marks may be visible on the body after the procedure.
Ethically, it is permissible for bodies of the decedents to be used for medical research if it promotes beneficence and will improve the livelihood of those who are living, provided consent has been given. Feasibility of the MITS procedure ensures the bioethical principle of justice by providing people with a clearer understanding of the causes of death of their loved ones.[21]
The benefits of the results of MITS extend to women who deliver stillbirths, by understanding factors that may have contributed to death. Therefore, MITS fulfils the reproductive rights of women and girls in SA by equipping them with information related to their previous pregnancies, which can inform preventive measures for subsequent pregnancies.
Acceptability of standardisation of MITS among healthcare professionals
Prior studies suggest that 90% of healthcare practitioners found MITS to be either 'as acceptable' or 'more acceptable' than complete diagnostic autopsy, and that a less invasive procedure would make the process of discussing postmortem examination with parents and relatives easier.[23] A qualitative study among healthcare workers conducted in India reported that MITS was perceived to offer accurate diagnoses, and might identify less prevalent co-existing illnesses.[6] Healthcare workers also found MITS acceptable in cases where the diagnosis or cause of death was undetermined in the antemortem period.[6] Currently, in SA, there are no laws in place that prohibit standardisation of autopsy procedures. The standardisation of the MITS procedure in SA could help alleviate the burden of cost and limited personnel to conduct full autopsies within the SA healthcare system.
The experience gained since 2017 from the CHAMPS study in Soweto has established a valuable foundation that underscores the importance and feasibility of broadly implementing MITS as a standard practice. Nevertheless, there are certain challenges in LMICs that may impede routine deployment of MITS, including the clinical workload of healthcare professionals, the time required to perform MITS and the limited laboratory facilities to undertake testing.[6] These perceived hurdles to routine use of MITS to investigate the causes of under-5 childhood deaths need to be weighed against the benefit of undertaking the procedure. For example, CHAMPS noted that clinician antemortem diagnosis often differed from the DeCoDe panel, which had access to the MITS results.
Public health benefits of undertaking MITS in LMICs
SA has an extensive backlog of pending complete diagnostic autopsies owing to the high rate of 'other than natural deaths' in adults and children, coupled with the scarcity of qualified personnel to perform complete diagnostic autopsies.[24,25] It is estimated that there are ~70 000 autopsies needing to be performed for medicolegal purposes annually in SA.[25] The MITS procedure is less complex than a complete diagnostic autopsy and does not require the same level of skill as forensic pathologists. Furthermore, results from MITS can be provided to families in a shorter time, assisting with the grieving process and providing closure and clarity on the cause of the death.[8]
The dissemination of cumulative cause-of-death results provides an important opportunity for key targeted public health interventions. For example, in Kenya, the launch of the Kisumu County Nutrition Action Plan was informed, in part, by the large number of childhood deaths from underlying malnutrition identified through CHAMPS.[26] Furthermore, national folate food fortification has been instituted in Ethiopia following the large number of childhood deaths attributable to neural tube defects identified through CHAMPS. In SA, interventions targeted at common hospital-acquired pathogens such as Klebsiella pneumoniae and Acinetobacter baumannii are being implemented, again informed by the findings from the use of postmortem MITS.[18 Our experience in CHAMPS has also revealed an increasing number of young mothers not attending antenatal care, and, resultantly, conditions such as syphilis and pre-eclampsia are undiagnosed in the antenatal period. These missed opportunities to seek healthcare offer us the chance to educate local women about the importance of antenatal care and health-seeking behaviour.
Pragmatic approach to MITS in South Africa
The ideal situation would be for extensive testing of all samples; however, MITS as performed in CHAMPS is intensive and costly (~USD609 - 1028).[27] Given that most public sector hospitals in SA are resource constrained, a cost-effective approach is to limit testing to key informed areas and less for routine surveillance purposes. Based on the data from the CHAMPS study in Soweto, we propose using age-specific algorithms that provide a pragmatic cost-effective approach, albeit with some limitations associated with such rationalisation (Figs 2A-C). Consequently, the clinician or clinical department will ultimately determine the extent of testing depending on the availability of resources. Notably, causes of death may change with time and algorithms may not be generalisable to all regions within SA. Importantly, consent, a verbal autopsy and review of the clinical data will need to be done timeously in non-refrigerated bodies, a limitation for busy low-staffed clinical units.
Stillbirths algorithm
The paucity of data in LMICs on causes of stillbirths has made it difficult to establish effective interventions.[28] The maternal medical history and placental investigation play a pivotal role in providing more granular evidence of the causes of stillbirths.[29] The placenta is not, however, always available, and a pathologist is required to undertake appropriate placental histology investigation. Extracting information from the maternal file for obstetric complications, and use of a verbal autopsy, are also useful to inform the causes of the stillbirth, particularly when the placenta is not available. Our experience with MITS in Soweto demonstrates limited value of brain, liver and lung samples when investigating stillbirths because tissue samples are often autolysed.[29]
Neonatal algorithm The 'neonatal algorithm' provides a diagnostic guide for deaths in the neonatal period where the babies were hospitalised from birth (i.e. not discharged home), and is largely determined by whether the neonate was born prematurely or full term, and the duration of hospital stay. Neonates who are diagnosed with severe congenital abnormalities that preclude them from timeous discharge from hospital will either die from the severity of the congenital abnormality or from a hospital-acquired infection. Sampling of these cases would not provide additional diagnostic information. In term neonates, antemortem medical records and sampling the placenta are useful if the history is consistent with a congenital infection or having experienced intrauterine hypoxia.
For prematurely born neonates (or low birthweight as a proxy for preterm birth), MITS sampling of extremely low birthweight neonates (<700 g) would not be necessary as death is most likely because of severe prematurity in the first 48 hours; however, sampling the placenta and review of maternal obstetric history may provide insight to the cause of the premature delivery. For preterm births >700 g without an obvious cause, taking a blood sample for culture or a lung biopsy may identify congenital infections if death occurs in the first 48 hours.
Neonates who remain in hospital for >48 hours often succumb to nosocomial infections, as a previous pilot indicated that 74.4% of infection-related neonatal deaths were hospital acquired.[30] These infections have been identified primarily as A. baumannii and K. pneumoniae in our setting, and may differ elsewhere. [30] Further testing may provide more granularity regarding identification of the organisms involved, but this needs to be balanced with the cost. In contrast, decedents who gradually deteriorated
from the time of birth would require a lung biopsy and blood sample for MC&S to establish whether there was a source of infection that caused the death.
Paediatric algorithm
Deaths following discharge from hospital can be stratified into three different groups: (i) previously well child with no comorbidities; (ii) untreated/virologically unsuppressed HIV or severe acute malnutrition; and (iii) death on arrival to hospital. Unfortunately, MITS cannot accurately diagnose poisoning, and this is a limitation when it comes to more directed sampling.
Decedents with an underlying traumatic incident would not usually require testing, as the underlying cause of death is usually attributed to the traumatic event (e.g. motor vehicle accident). Should the child survive the traumatic event and require a prolonged hospitalisation, their immediate cause of death would then likely be a nosocomial infection, and directed sampling would indicate the causative organism.
In children presenting to a health facility as 'death on arrival' (DOA), the verbal autopsy would be useful to determine further testing. For DOAs without well-documented preceding events, a complete diagnostic autopsy would be recommended. In children with a history of an infectious cause of death, we recommend sampling of blood, cerebrospinal fluid (CSF) and a lung aspirate/biopsy for culture and/or molecular testing.
In a pilot study in Soweto, it was shown that 36% of paediatric cases were HIV exposed at birth, including 13% who were infected with HIV. Furthermore, 62% of the children were malnourished, confirming the magnitude of the immunocompromised paediatric population in Soweto.[31] Immunocompromised children admitted for >48 hours are prone to hospital-acquired infections, and if resources allow, further testing will provide more granular information of the involved organisms. Community-acquired infections are often the cause of death in these children admitted for <48 hours, and blood, CSF and lung biopsy or culture have been demonstrated to be reliable in our setting for determining the infectious agent. Infection, mainly from pneumonia or sepsis, was found to be the immediate cause of death in 94% of cases where HIV was the underlying cause of death[31]
Children who are not immunocompromised commonly present to hospital with signs of sepsis, diarrhoeal disease, or signs of an acute respiratory tract infection.[31] Our experience has suggested that the best way to investigate these three categories of disease is with blood, CSF and lung culture. Molecular testing, if available, can provide a broader organism identification.
Conclusion
The CHAMPS study has provided an opportunity to better understand the burden of disease in LMICs. Using data-informed pragmatic algorithms, a limited MITS procedure can be cost-effective and scalable in LMICs. The public health benefits and acceptability among the community and healthcare practitioners make this a feasible standard of care practice to improve the assessment of death in children. Efforts to achieve Sustainable Development Goal 3.2 should be targeted at the evolving findings of causes of death in children, neonates and stillbirths. The findings from MITS are providing more granular information on current causes of death, and this could guide further interventions to achieve the Sustainable Development Goals. The long-term impact would result in deaths averted by changes in health-seeking behaviour, antenatal care, and preventable childhood diseases.
Declaration. None.
Acknowledgements. None.
Author contributions. JdT led the first draft with KS, ID, PM, MM, KM, NU writing certain sections. All authors provided critical input and reviewed the drafts.
Funding. The CHAMPS network is funded by the Bill & Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest. DMB is employed by the US Centers for Disease Control and Prevention (CDC). The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the CDC.
References
1. CHAMPS. The CHAMPS story. https://champshealth.org/about/. (accessed 2 February 2023). [ Links ]
2. Salzberg NT, Sivalogan K, Bassat, Q. Mortality surveillance methods to identify and characterise deaths in child health and mortality prevention surveillance network sites. Clin Infect Dis 2019;69(Suppl 4):S262-S273. https://doi.org/10.1093/cid/ciz599 [ Links ]
3. Rakislova N, Jordao D, Ismail MR. Accuracy of verbal autopsy, clinical data and minimally invasive autopsy in the evaluation ofmalaria-specific mortality: An observational study. BMJ Glob Health 2021;6(6):e005218. https://doi.org/10.1136/bmjgh-2021-005218 [ Links ]
4. Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br Med Bull 2009,92(1):7-32. https://doi.org/10.1093/bmb/ldp028 [ Links ]
5. Fligner CL, Murray J, Roberts DJ. Synergism of verbal autopsy and diagnostic pathology autopsy for improved accuracy ofmortality data. Popul Health Metr 2011,9(1):25. https://doi.org/10.1186/1478-7954-9-25 [ Links ]
6. Das M, Arora N, Rasaily R. Perceptions of the healthcare providers regarding acceptability and conduct ofminimal invasive tissue sampling (MITS) to identify the cause of death in under-five deaths and stillbirths in North India: A qualitative study. BMC Health Serv Res 2020,20(1):833. 10.1186/s12913-020-05693-6 [ Links ]
7. Lawn JE, Blencowe H, Waiswa P. Stillbirths: Rates, risk factors, and acceleration towards 2030. Lancet 2016,387(10018):587-603. https://doi.org/10.1016/S0140-6736(15)00837-5 [ Links ]
8. Munguambe K, Maixenchs M, Anselmo R. Consent to minimally invasive tissue sampling procedures in children in Mozambique: A mixed-methods study. PLoS ONE 2021,16(11):e0259621. https://doi.org/10.1371/journal.pone.0259621 [ Links ]
9. Maixenchs M, Anselmo R, Zielinksi-Gutierrez E. Willingness to know the cause of death and hypothetical acceptability of the minimally invasive autopsy in six diverse African and Asian settings: A mixed methods socio-behavioural study. PLoS Med 2016,13(11):e1002172. https://doi.org/10.1371/journal.pmed.1002172 [ Links ]
10. Paganelli CR, Goco NJ, McClure EM. The evolution of minimally invasive tissue sampling in postmortem examination: A narrative review. Glob Health Action 2020,13(1):1792682. https://doi.org/10.1080/16549716.2020.1792682 [ Links ]
11. Menendez C, Castillo P, Martinez MJ. Validity of a minimally invasive autopsy for cause of death determination in stillborn babies and neonates in Mozambique: An observational study. PLoS Med 2017,14(6):e1002318. https://doi.org/10.1371/journal.pmed.1002318 [ Links ]
12. Rakislova N, Fernandes F, Lovane L. Standardisation of minimally invasive tissue sampling specimen collection and pathology training for the child health and mortality prevention surveillance network. Clin Infect Dis 2019,69(Suppl 4):S302-S310. https://doi.org/10.1093/cid/ciz565 [ Links ]
13. Hailu R, Desta T, Berkuretsion Y. Minimally invasive tissue sampling in preterm deaths: A validation study. Glob Pediatric Health 2020,7:2333794X20953263.10.1177/2333794X20953263 [ Links ]
14. Tanko NM, Bakytkaly I, Issanov A. Validating a minimally invasive tissue sampling (MITS) method in determining cause of death in stillbirths and neonates. Children 2021,8(12):1095. https://doi.org/10.3390/children8121095 [ Links ]
15. Taylor AW Blau DM, Bassat Q. Initial findings from a novel population-based child mortality surveillance approach: A descriptive study. Lancet Glob Health 2020,8(7):e909-e919. https://doi.org/10.1016/S2214-109X(20)30205-9 [ Links ]
16. Diaz MH, Waller JL Theodore MJ. Development and implementation of multiplex TaqMan array cards for specimen testing at Child Health and Mortality Prevention Surveillance site laboratories. Clin Infect Dis 2019,69(Suppl 4):S311-S321. https://doi.org/10.1093/cid/ciz571 [ Links ]
17. Martines RB, Ritter JM, Gary J. Pathology and telepathology methods in the Child Health and Mortality Prevention Surveillance network. Clin Infect Dis 2019,69(Suppl 4):S322-S332. https://doi.org/10.1093/cid/ciz579 [ Links ]
18. Breiman RF, Blau DM, Mutevedzi P Postmortem investigations and identification of multiple causes of child deaths: An analysis of findings from the Child Health and Mortality Prevention Surveillance (CHAMPS) network PLoS Med 2021,18(9):e1003814. https://doi.org/10.1371/journal.pmed.1003814 [ Links ]
19. Mahtab S Madhi SA, Baillie VL. Causes of death identified in neonates enrolled through Child Health and Mortality Prevention Surveillance (CHAMPS), December 2016 - December 2021. PLOS Glob Public Health 2023, 3(3): e0001612. https://doi.org/10.1371/journal.pgph.0001612 [ Links ]
20. World Health Organization. Cause of death certification flyer: A tool for certifying physicians. Geneva: WHO, 2015. [ Links ]
21. South Africa. Constitution of the Republic of South Africa. 1996. [ Links ]
22. Bunei M, Muturi P Otiato F. Factors influencing acceptance of post-mortem examination of children at a tertiary care hospital in Nairobi, Kenya. Ann Glob Health 2019,85(1):95. https://doi.org/10.5334/aogh.2504 [ Links ]
23. Ben-Sasi K, Chitty LS, Franck LS. Acceptability of a minimally invasive perinatal/paediatric autopsy: Healthcare professionals' views and implications for practice. Prenat Diagn 2013,33(4):307-312. https://doi.org/10.1002/pd.4077 [ Links ]
24. Statistics South Africa. Mortality and causes of death in South Africa: Findings from death notification 2018. Pretoria: Stats SA, 2018. [ Links ]
25. Toit-Prinsloo LD. Performance of autopsies in South Africa: Selected legal and ethical perspectives Continuing Med Educ 2012,30(2):53-55. http://wwwcmej.orgza/index.php/cmej/artícle/view/2326/2188 (accessed 26 June 2023). [ Links ]
26. Anyango L. Nutrition investment evidence. Kisumu 2023. https://www.kisumu.go.ke/nutrition-investment-evidence/ (accessed 23 May 2023). [ Links ]
27. Morrison LTR, Brown EG, Paganelli CR. Cost evaluation of minimally invasive tissue sampling (MITS) implementation in low- and middle-income countries. Clin Infect Dis 2021,73(Suppl 5):S401-S407. https://doi.org/10.1093/cid/ciab828 [ Links ]
28. Aminu M, Bar-Zeev S White S. Understanding cause of stillbirth: A prospective observational multi-country study from sub-Saharan Africa. BMC Pregn Childbirth 2019,19(1):470. https://doi.org/10.1186/s12884-019-2626-7 [ Links ]
29. Madhi SA, Pathirana J, Baillie V. An observational pilot study evaluating the utility of minimally invasive tissue sampling to determine the cause of stillbirths in South African women. Clin Infect Dis 2019,69(Suppl 4):S342-S350. https://doi.org/10.1093/cid/ciz573 [ Links ]
30. Madhi SA, Pathirana J, Baillie V Unraveling specific causes of neonatal mortality using minimally invasive tissue sampling: an observational study. Clin Infect Dis 2019,69(Suppl 4):S351-S360. 10.1093/cid/ciz574 [ Links ]
31. Chawana R, Baillie V Izu A. Potential of minimally invasive tissue sampling for attributing specific causes of childhood deaths in South Africa: A pilot, epidemiological study. Clin Infect Dis 2019,69(Suppl 4):S361-S373. 10.1093/cid/ciz550 [ Links ]
 Correspondence:
Correspondence:
J du Toit
jeanie.dutoit@wits-vida.org
Accepted 22 November 2023














