Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.114 no.2 Pretoria feb. 2024
http://dx.doi.org/10.7196/SAMJ.2024.v114i1.1159
RESEARCH
Household transmission of SARS-CoV-2 in a rural area in South Africa
G MaimelaI; CE MartinII; M ChersichIII; B BelloIV; J MautiV; T BarnighausenVI; S KohlerVII; A Almuedo-RieraVIII; S LuchtersIX; S SawryX
IMB ChB, MBA; Wits RHI, University of the Witwatersrand, Johannesburg, South Africa
IIMB ChB, MSc; Wits RHI, University of the Witwatersrand, Johannesburg, South Africa
IIIMB BCh, PhD; Wits RHI, University of the Witwatersrand, Johannesburg, South Africa
IVMSc; Centre for Statistical Analysis and Research, Johannesburg, South Africa
VDrSc, MSc; Heidelberg Institute of Global Health, Faculty of Medicine and University Hospital, Heidelberg University, Germany
VIMD, ScD; Heidelberg Institute of Global Health, Faculty of Medicine and University Hospital, Heidelberg University, Germany
VIIMD, PhD; Heidelberg Institute of Global Health, Faculty of Medicine and University Hospital, Heidelberg University, Germany
VIIIMD, MSc; ISGlobal, Hospital Clinic, University of Barcelona, Spain
IXMB ChB, PhD; Centre for Sexual Health and HIV/AIDS Research, Harare, Zimbabwe; Liverpool School of Tropical Medicine, Liverpool, UK
XMSc; Wits RHI, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Patterns of SARS-CoV-2 spread have varied by geolocation, with differences in seroprevalence between urban and rural areas, and between waves. Household spread of SARS-CoV-2 is a known source of new COVID-19 infections, with rural areas in sub-Saharan Africa being more prone than urban areas to COVID-19 transmission because of limited access to water in some areas, delayed health-seeking behaviour and poor access to care
OBJECTIVES: To explore SARS-CoV-2 infection incidence and transmission in rural households in South Africa (SA
METHODS: We conducted a prospective household cluster investigation between 13 April and 21 July 2021 in the Matjhabeng subdistrict, a rural area in Free State Province, SA. Adults with SARS-CoV-2 confirmed by polymerase chain reaction (PCR) tests (index cases, ICs) and their household contacts (HCs) were enrolled. Household visits conducted at enrolment and on days 7, 14 and 28 included interviewer-administered questionnaires and respiratory and blood sample collection for SARS-CoV-2 PCR and SARS-CoV-2 immunoglobulin G serological testing, respectively. Co-primary cases were HCs with a positive SARS-CoV-2 PCR test at enrolment. The incidence rate (IR), using the Poisson distribution, was HCs with a new positive PCR and/or serological test per 1 000 person-days. Associations between outcomes and HC characteristics were adjusted for intra-cluster correlation using robust standard errors. The secondary infection rate (SIR) was the proportion of new COVID-19 infections among susceptible HCs
RESULTS: Among 23 ICs and 83 HCs enrolled, 10 SARS-CoV-2 incident cases were identified, giving an IR of 5.8 per 1 000 person-days (95% confidence interval (CI) 3.14 - 11.95). Households with a co-primary case had higher IRs than households without a co-primary case (crude IR 14.16 v. 1.75, respectively; p=0.054). HIV infection, obesity and the presence of chronic conditions did not materially alter the crude IR. The SIR was 15.9% (95% CI 7.90 - 29.32). Households with a lower household density (fewer household members per bedroom) had a higher IR (IR 9.58; 95% CI 4.67 - 21.71) than households with a higher density (IR 3.06; 95% CI 1.00 - 12.35
CONCLUSION: We found a high SARS-CoV-2 infection rate among HCs in a rural setting, with 48% of households having a co-primary case at the time of enrolment. Households with co-primary cases were associated with a higher seroprevalence and incidence of SARS-CoV-2. Sociodemographic and health characteristics were not associated with SARS-CoV-2 transmission in this study, and we did not identify any transmission risks inherent to a rural setting
The World Health Organization estimated that 14.9 million excess deaths were directly or indirectly attributable to the COVID-19 pandemic in 2020 and 2021.'[1] In settings where transmission was characterised by cases that were clustered geographically and by widespread community transmission, household cluster investigations showed household SARS-CoV-2 spread to be a major source of new COVID-19 cases.[2,3] Household transmission has in fact been shown to be a greater contributor to the spread of SARS-CoV-2 than community spread in settings where movement was restricted and social distancing imposed at community level to curb the spread of infection.[4,5] Factors enabling transmission of SARS-CoV-2 in households include closed spaces, overcrowding, close contact for prolonged periods, and reduced use of protective equipment.[6]
Patterns of SARS-CoV-2 virus spread have varied by geolocation, with differences in seroprevalence between urban and rural areas, and between waves.[7,8] Several features of rural areas in sub-Saharan Africa may increase the risk of SARS-CoV-2 transmission and COVID-19 disease. For example, many rural communities do not have sufficient access to soap and water for handwashing, an established public health intervention for the prevention and control of many infectious diseases.[9] Rural communities may also be more vulnerable to poor COVID-19 disease outcomes and mortality as a result of delayed health-seeking behaviour, poor access to care, or additional strain on already limited health systems and resources.[7,10-12]
Understanding SARS-CoV-2 transmission patterns and rural dimensions of COVID-19 disease may help in implementing control and mitigation initiatives on the African subcontinent. Lessons learned from transmission studies in different contexts may be relevant to infections other than SARS-CoV-2, and aid our understanding of and response to future pandemics. The objectives of this study were therefore to define epidemiological parameters of SARS-CoV-2 infection, including the transmissibility and clinical disease spectrum, and assess demographic, behavioural and household-level risk factors for infection and transmission in rural households in South Africa (SA).
Methods
Study population
The study investigated adults aged >18 years with SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR) tests and their household members.
Study design
We conducted a prospective household cluster investigation of SARS-CoV-2 incidence between 13 April and 21 July 2021.
Study setting
The study was conducted in the Matjhabeng subdistrict, a rural area in Free State Province, SA, with a population of ~407 020, a population density of 79 persons/km2 and an average household size of 3.1.[13] Enrolment and follow-up of participants took place prior to and during the early part of the third wave of COVID-19 (dominated by the Delta variant of SARS-CoV-2), which occurred between June and September 2021. At the start of the study, most restrictions on movement of people had been lifted, but from 1 June people were encouraged to remain at home except for travel to work or school or for medical care. Attendance at sporting events and post-funeral gatherings remained prohibited throughout the study period, and social distancing and mask wearing in public spaces and at the workplace were mandatory. Vaccination against SARS-CoV-2 was implemented for healthcare workers from February 2021. During the study period, vaccination was rolled out for those aged >60 years from 17 May and for those aged >50 years from 1 July.
Data collection
For index case (IC) identification, a list of new cases of SARS-CoV-2 infection (age >18 years), confirmed by reverse transcriptase PCR (RT-PCR) tests conducted by the National Health Laboratory Service in the district, was received daily (or as the list was updated) from the local Department of Health. Pre-screening was conducted by the study nurse and fieldworker. Potential IC participants were then contacted telephonically and screened for eligibility. If eligible, they were invited to participate in the study. Once interest to participate in the study was confirmed for the IC, and permission had been sought to contact household members, a household visit was scheduled for the initial study visit, at which written informed consent was obtained from ICs and household members, including children aged <12 years with parental consent.
Household visits were conducted at enrolment and on days 7, 14 and 28 after enrolment. A study questionnaire was completed by each participant aged >12 years at each visit. Children aged <12 years did not complete the questionnaire. The questionnaires collected information on sociodemographics, household characteristics and water sources, experience of symptoms, self-reported smoking habits and alcohol use, and past medical history including HIV and antiretroviral treatment (ART) status. The study nurse collected blood samples (5 mL whole blood in a gel separator (serum separator) tube for >12-year-olds and a dried blood spot on Whatman 903 protein saver cards (Cytiva, USA) for <12-year-olds) for SARS-CoV-2 immunoglobulin G serological testing, and respiratory samples (nasal swabs from those aged <12 years and nasopharyngeal swabs from those aged >12 years, both in 15 mL Falcon tubes (Corning Inc., USA) containing viral transport media) for SARS-CoV-2 PCR testing. Household members completed paper-based symptom diaries daily from days 1 to 28, in which they recorded any symptoms they experienced each day. In the case of children aged <12 years, diaries were completed by a parent. These were reviewed at each study follow-up visit and collected on day 28. Participants testing positive for SARS-CoV-2 were notified of their results and advised on isolation and other prevention measures, and to seek medical care if required; they were not retested for SARS-CoV-2 infection at subsequent visits. Respiratory and blood samples were transported on the same day to a central Bio Analytical Research Corporation South Africa (BARC SA) laboratory for analysis. Serum samples were tested using the Abbott IgG chemiluminescent microparticle assay (Abbott Diagnostics, USA) to detect SARS-CoV-2 antibodies for seroprevalence. Nasal and nasopharyngeal specimens were tested for the presence of SARS-CoV-2 nucleic acids by RT-PCR, using the Abbott RealTime SARS-CoV-2 assay (Abbott Diagnostics, USA).
Case definitions
An IC was the first member in a household who tested positive for SARS-CoV-2 by RT-PCR, aged >18 years and living in Matjhabeng in a household with at least one other member. An incident case was any household contact (HC) testing RT-PCR negative for SARS-CoV-2 at the first visit, and later having evidence of infection through either a positive SARS-CoV-2 RT-PCR test or serological conversion. A co-primary case was any HC who tested positive for SARS-CoV-2 on RT-PCR at study enrolment. Asymptomatic cases were any participants with SARS-CoV-2 infection confirmed by RT-PCR or serological testing who did not report any symptoms. A household was defined as any group of two or more people living in the same residence. A household member or contact (HC) was any person residing in the same household as the IC in the past 4 days and planning on residing in that household for the next 28 days. Households were enrolled within 7 days of the test specimen being taken from the IC.
Outcome measures
Household density was calculated as the number of persons in the household divided by the number of bedrooms. A household transmission risk score was calculated by summing 12 transmission risk behaviour variables (sharing a room with the IC; spending the last 7 consecutive nights at home; and taking care of, hugging, kissing or shaking hands with, sharing a meal with, eating with hands from the same plate as, sharing a drinking cup/glass or cutlery with, sleeping in the same room as, or sharing a toilet with the IC during the time s/he was ill), with each risk behaviour assigned a score of 1. The composite variable, with a total possible score of 12, was dichotomised as no risk (composite score of 0) and any risk (composite score of 1 - 12).
Statistical analysis
The incidence rate (IR) was calculated as the number of incident cases among HCs per 1 000 person-days at risk, using a Poisson distribution. Participants were censored when they became positive or exited the study, or at the end of the study follow-up period. The secondary infection rate (SIR) was calculated as the proportion of HCs with confirmed SARS-CoV-2 infection by day 28 among the total number of SARS-CoV-2-susceptible HCs enrolled and with known SARS-CoV-2 infection status at day 28. The incubation period was calculated as the median number of days from exposure to the primary case to the first sign or symptom of disease, among symptomatic cases. Poisson distribution univariable and multivariable models (log link function) were used to explore the association of potential risk factors for SARS-CoV-2 incidence, seroprevalence and symptomatic fraction. All analyses took into account the household cluster design and adjusted for intraclass correlation using robust standard errors. All analyses were conducted using Stata 15 (StataCorp, USA).
Patient and public involvement
Community leaders in the study subdistrict and managers of local health facilities were informed about the study and its objectives prior to and during the study through various meeting platforms. The study team also collaborated closely with the Department of Health at subdistrict level, specifically the local structures involved in the management of the COVID-19 response in the subdistrict.
Data sharing statement
The data that support the findings of this study are available from the corresponding author, GM, upon reasonable request.
Ethical considerations
The study was approved by the human research ethics committees at the University of the Witwatersrand (ref. no. 200914) and the University of Heidelberg (ref. no. S-837/2020). All study participants provided written informed consent prior to participation. Parental consent was provided for minors, and those aged >7 years also gave their assent for participation.
Results
Sample characteristics
Between 13 April and 21 July 2021, 26 ICs and 95 HCs were enrolled in the study. Participants from three households (3 ICs and 12 HCs) withdrew from the study and were not included in analyses, two after the first visit and one after the day 7 visit. Of the 23 households, 14 (61%) had <5 members living in the household at the time of the study, with most households (n=15; 65%) having one or two bedrooms; 11 households (48%) had a household density of >2 persons per bedroom. Piped water was available to all households.
The median (interquartile range (IQR)) age of ICs (40 (33 - 52) years) was higher than that of HCs (17 (9 - 33) years) (p<0.001). Approximately half (n=12; 52%) of the ICs were male and 60% (n=50) of HCs were female. Unemployment among HCs was high (n=28; 76%); just over 70% of those who were unemployed were women, and most (47%) were young adults aged 18 - 29 years (data not shown). Smoking and alcohol consumption were low among HCs aged >13 years, at 11% and 15%, respectively (Table 1). Overall, 8 participants (11%) reported being HIV infected, 7 (88%) of whom were on ART. The proportion of ICs who reported having chronic conditions (n=5; 22%) was double that of HCs (n=9; 11%). Of the 5 ICs with chronic conditions, 1 had diabetes and all had hypertension. Of the 9 HCs, 2 had diabetes and 7 had hypertension; none reported having both conditions. The median body mass index for ICs was higher than that for HCs (30.8 v. 28.0, respectively; p<0.001), with 78% of ICs and 65% of HCs being either overweight or obese at enrolment (Table 1). No participants reported having cancer.
SARS-CoV-2 infection among HCs at enrolment and during the follow-up period
Of the 83 HCs, 20 (24%) from 11 households had a positive SARS-CoV-2 RT-PCR at enrolment; these were classified as co-primary cases. Nine of the 20 co-primary cases were seropositive at enrolment. An additional 16 HCs were seropositive at enrolment. During follow-up, of the 63 HCs who were SARS-CoV-2 RT-PCR negative at enrolment, 5 became RT-PCR positive - 4 at day 7 and 1 at day 28 (Fig. 1).
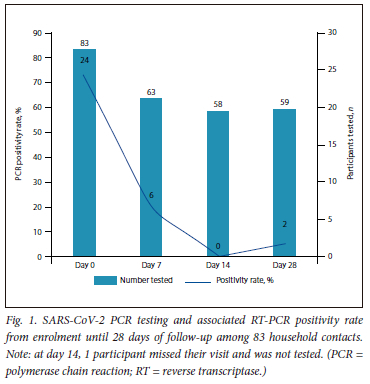
IR of SARS-CoV-2 among HCs
The 63 HCs who were RT-PCR negative at enrolment contributed a total follow-up duration of 1 710 person-days. Cumulatively, 10 HCs had incident SARS-CoV-2 infection; 4 were RT-PCR positive at day 7, 1 was RT-PCR positive at day 28, and a further 5 had SARS-CoV-2 antibodies detected at day 28 (Table 2). The IR was estimated as 5.8 per 1 000 person-days (95% confidence interval (CI) 14 - 11.95).
Factors associated with incidence of SAR-CoV-2 infection
The incidence of SAR-CoV-2 in households with a lower household density (number of household members per bedroom) was significantly higher (IR 9.58; 95% CI 4.67 - 21.71) than in those with a higher household density (IR 3.06; 95% CI 1.00 - 12.35) (Table 3). Households with symptoms at any time during follow-up had a higher incidence (IR 12.07; 95% CI 5.23 - 28.77) than those with without any symptoms (IR 2.65; 95% CI 0.48 - 34.97). Having a co-primary case in the household resulted in a significantly higher crude IR compared with having no co-primary case (14.16 v. 1.75; p=0.054). Stratification of incidence by HIV infection, obesity and the presence of chronic conditions did not materially alter the crude IR.
SIR, incubation period and serial interval
At the end of the study follow-up period, 10 incident cases were identified among 63 non-co-primary HCs, translating to an SIR of 15.9% (95% CI 7.90 - 29.32). These incident cases originated from 9 (39%) of the households. Household-specific SIR was determined for 22 households; all HCs in one household were co-primary cases. Overall, the mean SIR at household level was 22%; 13 households (59%) had an SIR of 0.0%, 1 (5%) an SIR of 25%, 2 (9%) an SIR of 33%, 4 (18%) an SIR of 50%, and 2 (9%) an SIR of 100%. The median (IQR) incubation period among 33 symptomatic HCs was 8 (1 - 16) days, and the median serial interval was 24 (15 - 28) days.
Symptomatic cases
Overall, 33 HCs (40%) reported the presence of any of the nine symptoms at any visit (Table 4). A slightly higher proportion of females (42%) than males (36%) reported any symptoms during the study period (p=0.380). The proportion of HCs reporting symptoms decreased with increasing age (p=0.017). A significantly higher proportion of non-smokers than smokers reported symptoms (40% v. 0%, respectively; p<0.001), and more obese HCs reported symptoms (40%) than non-obese HCs (32%) (p=0.496). As would be expected, more incident cases reported being symptomatic (70%) than non-incident contacts (28%), and 55% of co-primary contacts reported symptoms during the follow-up period (p=0.079) (Table 4). Of note, 45% of co-primary cases and 30% of incident cases were asymptomatic throughout the follow-up period. Among the 10 HCs with incident SARS-CoV-2 infection, 33% (n=1/3) of asymptomatic and 57% (n=4/7) of symptomatic contacts were diagnosed using SARS-CoV-2 RT-PCR; the rest were diagnosed through serology.
SARS-CoV-2 seroprevalence among HCs
Seropositivity increased as the study progressed, from 30% on day 0 to 46% on day 28. Overall, 43 of 83 HCs (52%) were seropositive at some point during the study. Associations between participant characteristics and seropositivity at weeks 1 and 4 and any time during the study were explored (Supplementary Table 1, available online at https://www.samedical.org/file/2147). As seen with the IR, persons from households with a co-primary case at enrolment were approximately three times as likely to be seropositive, at all three time points, compared with those from households with no co-primary cases (day 0 prevalence ratio (PR) 2.60; 95% CI 1.07 - 6.19, day 28 PR 3.67; 95% CI 1.51 - 8.90, any time PR 4.27; 95% CI 1.83 - 9.98).
Discussion
In this study describing the characteristics of household transmission of SARS-CoV-2 among 106 participants from 23 households (23 Cs and 83 HCs) in a rural district in SA, we found an IR of 5.8 per 1 000 person-days and an SIR of 15.9% among 63 participants who were SARS-CoV-2 RT-PCR negative at enrolment. Factors associated with incidence were having a co-primary case in the household, households with symptoms at any time during follow-up, and households with a lower density. The seroprevalence rate among the 83 HCs was 30% at enrolment and 46% by day 28. Overall, at the time of diagnosis, 30% of incident SARS-CoV-2 infection cases and 45% of co-primary cases in our study were asymptomatic.
The SIR of 15.9% in our study is comparable to the secondary attack ate of 16.4% reported from a meta-analysis of similar household transmission studies conducted through to October 020,[6] the 20.4% reported among rural households in SA at a similar time,[7] and the 18.9% reported in an updated meta-analysis of household studies published between October 2020 and June 2021.[14] The seroprevalence in the present study is similar to two large SA cross-sectional studies conducted in rural settings after the second wave, reporting seroprevalences of 26% in a community-based study of 1 211 participants[8] and 46% among 4 858 blood donors.[15]
However, compared with these two studies, we found no association between seroprevalence and any sociodemographic or health characteristics.[8,15] This may be because our sample was limited to HCs in a particular type of setting, making direct comparisons between studies difficult. Interestingly, HCs with a co-primary case were significantly more likely to be seropositive at any visit. Given the high number of asymptomatic infections (60%) seen in this and another SA study,[7] this seropositivity could be a result of a recent related infection in the household, with the current IC possibly not being the true IC. There was also some indication that the incidence of SARS-CoV-2 was associated with a co-primary case at enrolment, which may be due to a higher viral burden because of the number of active cases in the household, although we did not measure SARS-CoV-2 viral load.
We found a wide variation in the SIR (from 0% to 100%) among households. Clustering of cases within households has been reported in other household transmission studies.[6] The overall number of SARS-CoV-2 PCR-positive incident cases was small (n=5/63) and suggests that PCR testing of HCs should not be a priority strategy in case identification, but this should be determined through a formal evaluation of the cost-effectiveness of the strategy.
The rate of asymptomatic cases in the present study was much lower than was found in an SA seroprevalence study where rates of asymptomatic cases were reported at 85%,[7] probably because our study was a targeted, household study following known SARS-CoV-2 ICs where contacts may have had a lower threshold for reporting symptoms. We also found that 30% of non-incident contacts reported symptoms, further highlighting that symptom screening may not be useful in case identification.[7]
Transmission dynamics of other respiratory illnesses would suggest overcrowding to be associated with a higher IR of infection. This was not a finding in this study or in another SA-based study.[7] Despite exploring the size of the houses and the numbers of rooms and bedrooms, we could not find a clear explanation for this finding.
Findings from the present study suggest that symptom screening and SARS-CoV-2 RT-PCR testing of HCs through community outreach may not be a cost-effective strategy for case identification in future COVID-19 waves, but this needs to be determined in a formal study.
Study strengths and limitations
Strengths of this study include its prospective nature and the intensity of follow-up over the 28-day period, with molecular and serological testing, as well as the fact that the study included participants of all ages. Additionally, conducting the study in a rural area in SA provides a unique perspective on household SARS-CoV-2 transmission in this population.
The study had several limitations. Although incident cases were assumed to have been acquired from the IC, we did not conduct genotypic testing, so we were unable to confirm that this was the case. We were also unable to confirm whether the IC was the true primary case in households with co-primary cases. Given the high number of co-primary cases that were not included in the IR analysis, our results are probably an underestimate of the true IR. The 14-day gap between the 3rd and 4th study visit may have resulted in incident cases occurring during this time being missed, although serological testing was conducted to mitigate this. We did not quantify the SARS-CoV-2 viral load among incident cases, and were therefore not able to determine the role of viral load in household transmission.
Conclusion
We found a high SARS-CoV-2 infection rate in HCs in a rural setting, with 48% of households having a co-primary case at the time of enrolment. Households with co-primary cases were associated with a higher seroprevalence and incidence of SARS-CoV-2. Sociodemographic and health characteristics were not associated with SARS-CoV-2 transmission in this study, and we did not identify any transmission risks inherent to a rural setting.
Declaration. None.
Acknowledgements. The authors thank all the individuals who kindly agreed to participate in the study, including the field staff Modiehi Mopeli and Emily Lekitlane and the Wits RHI staff Nkululeko Mngomezulu, Arabang Letebele, Ntombikayise Tiwani, Pitso Rasiile and Enoch Manyame, as well as the BARC laboratory staff who worked tirelessly to implement the study. We would also like to thank the COREP Consortium Partners, including Eunice Irungu and Anthony Ngugi from Aga Khan University, Kenya.
Author contributions. Conception and design of study: GM, CEM, SS, JM, TB, MC and SL. Acquisition of data: GM, CEM and SS. Analysis and/or interpretation of data: BB, SS, MC, CEM and GM. Drafting the manuscript: GM, CEM, SS and MC. Revising the manuscript critically for important intellectual content: GM, SS, CEM, BB, JM, TB, MC, AA-R, SK and SL. Approval of the version of the manuscript to be published: all authors.
Funding. This study was supported by the European and Developing Countries Clinical Trials Partnership (EDCTP), grant no. RIA2020EF-3026.
Conflicts of interest. None.
References
1. World Health Organization. 14.9 million excess deaths associated with the COVID-19 pandemic in 2020 and 2021. Updated 5 May 2022. https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021 (accessed31 May 2023). [ Links ]
2. WHO-China Joint Mission. Report of the WHO-China Joint Mission on coronavirus disease 2019 (COVID-19 ). 28 February 2020. https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19 (accessed 15 August 2020). [ Links ]
3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19 ) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648 [ Links ]
4. Curmei M, Ilyas A, Evans O, Steinhardt J. Constructing and adjusting estimates for household transmission of SARS-CoV-2 from prior studies, widespread-testing and contact-tracing data. Int J Epidemiol 2021;50(5):1444-1457. https://doi.org/10.1093/ije/dyab108 [ Links ]
5. Wang Z, Ma W, Zheng X, Wu G, Zhang R. Household transmission of SARS-CoV-2. J Infect 2020;81(1):179-182. https://doi.org/10.1016/j.jinf.2020.03.040 [ Links ]
6. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: A systematic review and meta-analysis. JAMA Netw Open 2020;3(12):e2031756. https://doi.org/10.1001/jamanetworkopen.2020.31756 [ Links ]
7. Cohen C, Kleynhans J, von Gottberg A, et al. SARS-CoV-2 incidence, transmission, and reinfection in a rural and an urban setting: Results of the PHIRST-C cohort study, South Africa, 2020 - 21. Lancet Infect Dis 2022;22(6):821-834. https://doi.org/10.1016/s1473-3099(22)00069-x [ Links ]
8. Kleynhans J, Tempia S, Wolter N, et al. SARS-CoV-2 seroprevalence in a rural and urban household cohort during first and second waves of infections, South Africa, July 2020 - March 2021. Emerg Infect Dis 202137(12):3020-3029. https://doi.org/10.3201/eid2712.211465 [ Links ]
9. Jiwani SS, Antiporta DA. Inequalities in access to water and soap matter for the COVID-19 response in sub-Saharan Africa. Int J Equity Health 2020;19(1):82. https://doi.org/10.1186/s12939-020-01199-z [ Links ]
10. Geldsetzer P, Reinmuth M, Ouma PO, et al. Mapping physical access to health care for older adults in sub-Saharan Africa and implications for the COVID-19 response: A cross-sectional analysis. Lancet Healthy Longev 2020;1(1):e32-e42. https://doi.org/10.1016/S2666-7568(20)30010-6 [ Links ]
11. Jassat W Mudara C, Ozougwu L, et al. Difference in mortality among individuals admitted to hospital with COVID-19 during the first and second waves in South Africa: A cohort study. Lancet Glob Health 2021;9(9):e1216-e1225. https://doi.org/10.1016/S2214-109X(21)00289-8 [ Links ]
12. Ogunkola IO, Adebisi YA, Imo UF, Odey GO, Esu E, Lucero-Prisno DE 3rd. Rural communities in Africa should not be forgotten in responses to COVID-19 . Int J Health Plann Manage 2020;35(6):1302-1305. https://doi.org/10.1002/hpm.3039 [ Links ]
13. Statistics South Africa. Matjhabeng Local Municipality 2011. https://www.statssa.gov.za/?page_id=993&id=matjhabeng-municipality (accessed 15 August 2020). [ Links ]
14. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Factors associated with household transmission of SARS-CoV-2: An updated systematic review and meta-analysis. JAMA Netw Open 2021;4(8):e2122240. https://doi.org/10.1001/jamanetworkopen.2021.22240 [ Links ]
15. Sykes W, Mhlanga L, Swanevelder R, et al. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and Free State provinces of South Africa in January 2021. Res Sq 2021. https://doi.org/10.21203/rs.3.rs-233375/v1 [ Links ]
 Correspondence:
Correspondence:
G Maimela
gmaimela@wrhi.ac.za
Accepted 30 October 2023














