Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.114 no.1 Pretoria Jan. 2024
http://dx.doi.org/10.7196/SAMJ.2024.v114i1.1477
IN PRACTICE
Recurrent venous thrombosis - an unusual first presentation of autoimmune polyendocrinopathy syndrome type 3B
N LeteteI; D VazII, III; P H MalishiIV; J J PotgieterV, VI; P RheederVII
IMB ChB, DipHIVMan; National Health Laboratory Services, Tshwane Academic Division, Pretoria, South Africa
IIMB BCh, MMed (Haem); National Health Laboratory Services, Tshwane Academic Division, Pretoria, South Africa
IIIMB BCh, MMed (Haem); Department of Haematology, Steve Biko Academic Hospital and Faculty of Health Sciences, University of Pretoria, South Africa
IVMB ChB, MMed (Int Med); Department of Internal Medicine, Steve Biko Academic Hospital and Faculty of Health Sciences, University of Pretoria, South Africa
VMB ChB, MMed (Haem); National Health Laboratory Services, Tshwane Academic Division, Pretoria, South Africa
VIMB ChB, MMed (Haem); Department of Haematology, Steve Biko Academic Hospital and Faculty of Health Sciences, University of Pretoria, South Africa
VIIMB ChB, PhD; Department of Internal Medicine, Steve Biko Academic Hospital and Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
A 45-year-old female presented with unprovoked recurrent venous thromboembolism (VTE), in unusual sites, and pancytopenia, posing a complex diagnostic challenge. Work-up for inherited thrombophilia, antiphospholipid syndrome (APLS) and paroxysmal nocturnal haemoglobinuria were unremarkable. Investigations revealed autoimmune thyroid disease, and a mixed iron/vitamin B12 deficiency due to pernicious anaemia and resultant atrophic gastritis. Hyperhomocysteinaemia due to vitamin B12 deficiency was identified as a potential contributor to her recurrent VTE. This case highlights the unusual initial presentation of autoimmune polyendocrinopathy syndrome type 3B (APS-3B) with recurrent thromboembolism, and emphasises the importance of considering hyperhomocysteinaemia in unprovoked and atypical VTE cases.
Recurrent venous thromboembolism (VTE) is a complex clinical problem, and there is a lack of expert consensus regarding the management of this presentation. Autoimmune conditions have long been associated with an increased risk of thrombosis via multiple mechanisms such as chronic inflammation, immune complex deposition and immunoparesis.
In the late 20th century, a link emerged between vitamin B12 deficiency (commonly associated with pernicious anaemia) and thrombosis. This connection was attributed to the presence of hyperhomocysteinaemia (HHCys). It has remained a topic of debate to this day, and randomised controlled trials are yet to display a definitive causal relationship. We report the case of a patient diagnosed with autoimmune polyendocrinopathy syndrome 3B (APS-3B), after presenting with recurrent venous thrombosis, a rare initial manifestation of the disease.
This case study was approved by the Faculty of Health Sciences Research Ethics Committee of the University of Pretoria (ref. no. 605/2023).
Case presentation
A 45-year-old female was referred to the haematology clinic from the vascular surgery department for the work-up of recurrent VTEs. Her medical history was significant for three separate venous thromboembolic events, namely: portal vein thrombosis, complicated by portal hypertension and splenomegaly (on propranolol) superior mesenteric vein thrombosis, and most recently, iliofemoral deep vein thrombosis (DVT), for which she underwent surgical thrombolysis 1 month prior to her being seen at our clinic.
The patient had completed 3 months of warfarin therapy after her first thromboembolic event, 3 years before this presentation. She had no history of constitutional symptoms, melena stool, abnormal uterine bleeding or medication history. She had no significant smoking or alcohol history and endorsed no history of illicit drugs, traditional medication or over-the-counter medications. Her family history was not significant for autoimmune diseases or coagulopathies. The patient also denied any history of long-distance travel, recent periods of prolonged immobilisation or any major surgical interventions before each of her thrombotic events.
Her examination was significant for pallor, tachycardia, bounding pulses, active precordium and hepatosplenomegaly. She also had mild right lower limb swelling with minimal tenderness on palpation from the hip to the ankle, with pulses palpable bilaterally. There was no neurological fallout, tongue abnormalities or Woltman's sign present. Laboratory investigation uncovered pancytopenia, increased red cell distribution width, absolute lymphopaenia and a reduced absolute reticulocyte count (Table 1). Her renal and liver function tests were unremarkable.
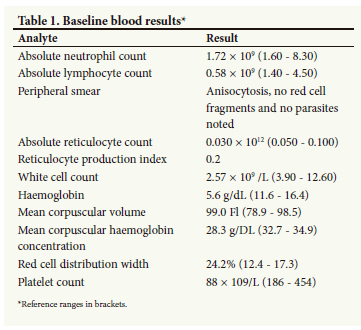
She previously had unremarkable work-ups for inherited thrombophilia: mutational analysis for factor V Leiden and prothrombin G20210A, and functional analysis for protein C, protein S and antithrombin deficiencies. Acquired thrombophilia in the form of antiphospholipid antibodies and lupus anticoagulant was consistently ruled out during non-anticoagulated periods and in the absence of acute thrombotic events.
Her thrombotic history and pancytopenia were suspicious for paroxysmal nocturnal haemoglobinuria (PNH) or occult malignancy.
A week later, the decision was made to admit her for the initiation and optimisation of anticoagulation treatment (warfarin), a bone marrow aspirate and trephine investigation and diagnostic upper endoscopy. These investigations yielded a non-functional World Health Organization (WHO) grade 1 duodenal neuroendocrine tumour, trilineage dysplasia secondary to nutritional deficiencies and unremarkable cytogenetic/molecular studies.
Given the mixed nutritional deficiency, pancytopenia and thrombotic history, the leading differential diagnoses were: PNH with bone marrow failure syndrome; gastrointestinal malignancy; or autoimmune disease process with malabsorption (pernicious anaemia/coeliac disease).
In view of her biochemical profile (Table 2), her clinical picture is best summarised in the following manner:
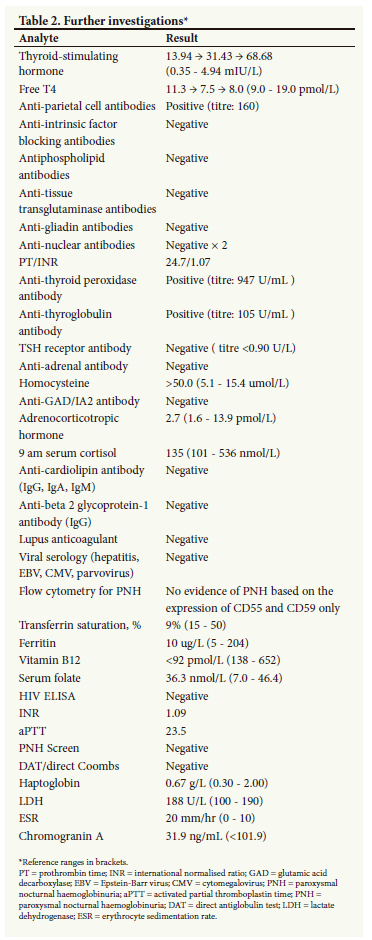
• chronic atrophic gastritis (noted on biopsy from 2019) and achlorhydria secondary to pernicious anaemia, complicated with iron deficiency anaemia
• pancytopenia: secondary to mixed vitamin B12 and iron deficiencies
• autoimmune thyroid disease in the form of Hashimoto's thyroiditis
• incidentaloma: in the form of an asymptomatic neuroendocrine tumour (non-functional)
• recurrent VTE likely driven by HHCys; however, the contribution of the neuroendocrine tumour cannot be discounted.
The patient was started on vitamin B12, iron and folate supplementation, with improvement of symptoms, and was discharged with a therapeutic INR (international normalised ratio) on life-long anticoagulation (warfarin). Given her clinical presentation, the results of her special investigations and her response to treatment, the diagnosis of APS-3B was favoured.
Owing to deteriorating thyroid function tests and positive autoimmune antibodies, she was referred to the endocrine clinic for further management of her autoimmune thyroid disease. The patient also returned to the gastroenterology clinic for further work-up of the incidental neuroendocrine tumour that was found on upper endoscopy. She continued to follow up at the haematology department, and her cytopenias improved with vitamin B12, iron and folate supplementation.
Discussion
In our patient, it is likely that the presence of previous VTEs and subsequent endothelial dysfunction played a role in the ensuing recurrent VTEs. We postulate that her vitamin B12 deficiency and hypothyroidism resulted in HHCys, which - in conjunction with her non-functional, localised neuroendocrine tumour - may have predisposed her to recurrent VTEs.
There has long been a debate regarding the causal relationship between elevated homocysteine levels and thrombosis. In 1969, McCully[1] first described a potential association between elevated homocysteine levels and arterial thrombosis.
Homocysteine is an amino acid that is involved in two distinct metabolic pathways - remethylation and trans-sulphuration (Fig. 1). These pathways are dependent on vitamin B12, B6 and folate. Elevations in homocysteine reflect disruptions in one of the components of these two processes.[2]
Elevated levels of homocysteine have been described with vitamin B12/B6/folate deficiency, chronic kidney disease, hypothyroidism and congenital defects in homocysteine metabolism (mutations in enzymes involved in homocysteine metabolism, e.g. homozygous defect of gene encoding for methylenetetrahydrofolate (MTHFR) (Table 3).[2]
Hyperhomocysteinaemia is postulated to increase the risk of atherosclerosis by promoting endothelial injury, inflammation and oxidative stress.[3] Other hypotheses include increased platelet aggregation, increased low-density lipoprotein, abnormal fibrinolysis (decreased activity of antithrombin and protein C) and increased smooth muscle proliferation.[3]
Although the literature clearly indicates that cardiovascular, cerebrovascular disease and thromboembolic diseases are associated with elevated levels of homocysteine, there is a lack of robust evidence displaying an improvement in outcomes with the lowering of homocysteine levels. Therefore the causal relationship remains unclear and an area for future research.
APSs, also known as polyglandular syndromes, are a group of rare, heterogeneous conditions that have the common features of organ-specific autoantibodies and T lymphocyte autoreactivity.[4]
APSs were first classified according to clinical criteria (Table 4) by Neufeld et al.[5] in 1980. APSs can also be categorised based on their associated genetic abnormalities. APS-1 is characterised as a monogenic defect of the autoimmune regulator gene on chromosome 21, and typically presents in childhood. APS-2 is a polygenic disorder, of early adult onset and female preponderance, whereas APS-3 is typically seen in middle-aged females and is the most common subgroup of APS.
In our review of the existing literature, we found case reports documenting the co-existence of autoimmune polyglandular syndrome 3, and one episode of deep vein thrombosis (DVT),[6] as well as cerebral venous sinus thrombosis.[7] There have, however, been no documented cases of recurrent thromboembolism as the first sign of APS-3B.
Atypical presentations of APS-3B include diverse clinical manifestations. Among these, there have been reported cases of impending pericardial tamponade attributed to hypothyroidism,[8] highlighting the complex interplay between endocrine dysregulation and cardiovascular complications in this syndrome. In addition, severe and longstanding vitamin B12 deficiency has been linked to subacute combined degeneration as the initial presentation,[9] revealing neurological involvement as another facet of APS-3B's heterogeneity.
These unusual presentations underscore the importance of considering APS-3B as a potential underlying cause in patients presenting with seemingly unrelated clinical features.
Moreover, the scarcity of local data on the incidence of hyperhomocysteinemia and its association with thrombosis is evident in the South African context. Mehta et al.[10] addressed this knowledge gap by reporting two compelling case studies from North West Province. One case involved a right middle cerebral artery infarct, whereas the other presented with a right femoral vein DVT. Both cases were attributed to elevated homocysteine levels resulting from vitamin B12 deficiency.
A recent case report from Johannesburg highlights the significance of HHCys as a potential cause of thrombosis.The report described a 40-year-old woman with a history of thrombotic stroke who presented with chronic thromboembolic pulmonary disease, with haematological features of severe vitamin B12 deficiency, emphasising the need to consider HHCys in cases of recurrent and early-onset VTE.
Additionally, several published cases in the literature have demonstrated that elevated homocysteine can lead to thrombosis in atypical sites. These cases include concurrent cerebral artery and vein thrombosis, acute myocardial infarction, upper extremity DVT, superior ophthalmic vein thrombosis, inferior vena cava thrombosis and submassive pulmonary embolism.[12-17]
A meta-analysis conducted by Ray[18] revealed a significant association with HHCys as a risk factor for VTE disease across a wide spectrum of patients, with first or recurrent VTEs. Notably, this risk appears to be most significant for patients with VTE disease <60 years old. As a result, it is suggested that measurement of homocysteine levels may be worthwhile in younger patients if it will alter their clinical management, by determining the risk for VTE recurrence and in the use of anticoagulants for long-term prophylaxis or during high-risk states (e.g. pregnancy or perioperatively).
Regarding thrombosis in unusual sites, such as the superior mesenteric vein and portal vein, the British Society of Haematology (BSH) offers valuable guidance on the recommended work-up. This work-up includes myeloproliferative neoplasms and paroxysmal nocturnal haemoglobinuria. The BSH guidelines, importantly, advise that testing for MTHFR mutations and homocysteine levels should not be included in the thrombophilia panel unless features of homocystinuria are present.[19] It is worth noting that in line with international guidelines, local thrombosis guidelines do not currently include HHCys assessment as a routine part of VTE evaluation.[20]
The management of recurrent thrombosis consists of lifelong anticoagulation in unprovoked cases, and long-term anticoagulation in those with persistent/long-term risk factors. While vitamin K antagonists (VKAs) remain the current standard of care, direct oral anticoagulants are a reasonable choice for extended anticoagulant therapy because they are convenient to patients and their physicians, are as effective as the VKAs and confer a lower risk of bleeding. However, their performance in the long term remains unknown.[21]
The potential role of B vitamins (folate, B6 and B12) has been postulated to reduce cardiovascular risk by lowering homocysteine levels, which correlate strongly with the risk of coronary disease and stroke. Daily supplementation with folate was found to reduce homocysteine (Hcys) levels by 25%, and adding vitamin B12 further lowers levels by 7%.[22,23] However, a meta-analysis of randomised controlled trials found no association between vitamin supplementation, whether used for primary or secondary prevention, and reduced major adverse cardiovascular events, total mortality, cardiac death, myocardial infarction or stroke.[24]
Conclusion
This is the first reported case of recurrent thrombosis in unusual sites as the initial presentation of APS-3B. It highlights the importance of considering HHCys as a potential cause in unprovoked and atypical VTE cases. While HHCys testing may not be widely recommended, this case suggests a need for further research into screening practices and the management of HHCys-related thrombosis. Furthermore, it highlights a scarcity of data regarding APS in sub-Saharan Africa, emphasising the necessity for future research to bridge this gap. This case also serves to illustrate the diverse clinical presentations of APS, underpinning the significance of comprehensive investigation and multidisciplinary management.
Teaching points:
• Highlight atypical manifestations of autoimmune syndromes, as evident in this case where recurrent venous thromboembolism was the initial presentation of APS type 3B.
• Consider hyperhomocysteinaemia as a potential contributor to thrombotic risk in the absence of traditional risk factors
• When managing patients with autoimmune disorders, emphasise the significance of investigating and excluding coexisting autoimmune conditions, as they may contribute to complex clinical presentations and guide treatment strategies.
Declaration. None.
Acknowledgements. None.
Author contributions. NL: responsible for conceptualising, researching, writing and editing. DV: conceptualising and editing. PM: editing. JP: editing, PR: editing.
Funding. None.
Conflicts of interest. None.
References
1. McCully KS. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am J Pathol 1969;56(1):111-128. https://doi.org/10.1177/56.L111 [ Links ]
2. Veeranki S, Gandhapudi SK, Tyagi SC. Interactions of hyperhomocysteinemia and T cell immunity in causation of hypertension. Can J Physiol Pharmacol 2017;95(3):239-246. https://doi.org/10.1139/cjpp-2016-0401 [ Links ]
3. Park WC, Chang JH. Clinical implications of methylenetetrahydrofolate reductase mutations and plasma homocysteine levels in patients with thromboembolic occlusion. Vasc Specialist Int 2014;30(4):113-119. https://doi.org/10.5758/vsi.2014.30.4.113 [ Links ]
4. Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med 2004;350(19):2068-2079. https://doi.org/10.1056/NEJMra030158 [ Links ]
5. Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann 1980;9(9):154-162. https://doi.org/10.3928/0090-4481-19800901-08 [ Links ]
6. Horsey M, Hogan P, Oliver T. Deep vein thrombosis, an unreported first manifestation of polyglandular autoimmune syndrome type III. Endocrinol Diabetes Metab Case Rep 2016;2016:16-0034. https://doi.org/10.1530/EDM-16-0034 [ Links ]
7. Terunuma D, Egashira S, Doijiri R, Kimura N, Hashimoto Y, Kikuchi T. Autoimmune polyglandular syndrome type 3 diagnosed with cerebral venous sinus thrombosis: A case report. Rinsho Shinkeigaku 2023;63(5):298-304. https://doi.org/10.5692/clinicalneurol.cn-001830 [ Links ]
8. Syed S, Fatima S. Impending pericardial tamponade as the initial presentation of autoimmune polyglandular syndrome 3B. Chest 2021;160(4):A272. https://doi.org/10.1016/j.chest.2021.07.610 [ Links ]
9. Apolinario M, Brussels A, Cook CB, Yang S. Autoimmune polyglandular syndrome type 3: A case report of an unusual presentation and literature review. Clin Case Repo 2022:e05391. https://doi.org/10.1002/ccr3.5391 [ Links ]
10. Mehta R, Daude A, Variava E. Vitamin B12 deficiency and hyperhomocysteinemia: A description of two cases with thrombosis. Wits J Clin Med 2022;4(2):103-106. https://doi.org/10.18772/26180197.2022.v4n2a6 [ Links ]
11. Ndaba LW, van Blydenstein SA, Hodkinson KE. Vitamin B12 deficiency presenting with pulmonary embolism: An unusual presentation. Afr J Thoracic Crit Care Med 2023;29(3):e285. https://doi.org/10.7196/AJTCCM.2023.v29i3.285 [ Links ]
12. Vinash BL, Kamath V, Ganguly S. An interesting case of hyperhomocysteinemia presenting as acute myocardial infarction and cerebral venous thrombosis. APIK J Int Med 2021;9(2):116-119. https://doi.org/10.4103/AJIM.AJIM_1_20 [ Links ]
13. Varlamos C, Pappas C, Kiouri E, Kosmas N, Benetou DR, Rallidis LS. Hyperhomocysteinemia as the only risk factor in a young man presenting with ST-elevation myocardial infarction. J Cardiol Cases 2021;23(3):112-114. https://doi.org/10.1016/j.jccase.2020.10.004 [ Links ]
14. Sud S, Kumar Y, Bhardwaj S, Dwivedi D. Homocysteinemia-induced upper-extremity deep-vein thrombosis: A sinister at high altitude. Indian J Vasc Endovasc Surg 2021;8(3):274-276. https://doi.org/10.4103/ijves.ijves_112_20 [ Links ]
15. Khaliq L, Kabir KF, Pyai K, Hadid T, Collins-Hamel B. A simple vitamin deficiency with life-threatening complications: A case of B12 deficiency and hyperhomocysteinemia-induced thrombosis. Cureus 2023;15(8):e42908. https://doi.org/10.7759/cureus.42908 [ Links ]
16. Kovalenko O, Kassem AN, Jenkins M. Hyperhomocysteinemia and pulmonary embolism in a young male. Cureus 2020;12(4):e7818. https://doi.org/10.7759/cureus.7818 [ Links ]
17. Acharya R, Shrestha A, Simkhada S, et al. Inferior vena cava thrombosis attributable to hyperhomocysteinemia: A case report from Nepal. S Afr Med J 2022;112(1):1-6. https://doi.org/10.1002/ccr3.6605 [ Links ]
18. Ray JG. Meta-analysis of hyperhomocysteinemia as a risk factor for venous thromboembolic disease. Arch Intern Med 1998;158(19):2101-2106. https://doi.org/10.1001/archinte.158.19.2101 [ Links ]
19. Rachchillage DJ, Mackillop L, Chandratheva A, Motawani J, MacCallum P, Laffan M. Thrombophilia testing: A British Society for Haematology guideline. Br J Haematol 2022;198(3):443-458. https://doi.org/10.1111/bjh.18239 [ Links ]
20. Jacobsen BF, Louw S, Büller H, on behalf of the Southern African Society of Thrombosis and Haemostasis. Venous thromboembolism: Prophylactic and therapeutic practice guideline. S Afr Med J 2013;103(4):260-267. https://doi.org/10.7196/SAMJ.6706 [ Links ]
21. Kyrle PA. How I treat recurrent deep-vein thrombosis. Blood 2016;127(6):696-702. https://doi.org/10.1182/blood-2015-09-671297 [ Links ]
22. Homocysteine Lowering Trialists' Collaboration. Lowering blood homocysteine with folic acid-based supplements: Meta-analysis of randomised trials. BMJ 1998;316:894-898. https://doi.org/10.1136/bmj.316.7135.894 [ Links ]
23. Homocysteine Lowering Trialists' Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomised trials. Am J Clin Nutr 2005; 82:806-812. https://doi.org/10.1093/ajcn/82.4.806 [ Links ]
24. Zhang C, Wang Z-Y, Qin Y-Y, Yu F-F, Zhou Y-H. Association between B vitamins supplementation and risk of cardiovascular outcomes: A cumulative meta-analysis of randomised controlled trials. PLoS ONE 2014;9(9):e107060. https://doi.org/10.1371/journal.pone.0107060 [ Links ]
 Correspondence:
Correspondence:
N Letete
nena.letete@gmail.com
Accepted 30 October 2023














