Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 n.12 Pretoria Dec. 2023
http://dx.doi.org/10.7196/samj.2023.v113i12.1164
IN PRACTICE
Implementing E-MOTIVE for detection and treatment of postpartum haemorrhage in South Africa
M Singata-MadlikiI; S FawcusII; N F MoranIII; E ArendsIV; E MullerV; S MandondoVI; G J HofmeyrVII, VIII
IRN, PhD; Effective Care Research Unit, University of the Witwatersrand, Walter Sisulu University, East London, South Africa
IIMA, FRCOG; Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of Cape Town, South Africa
IIIBM BCh, FCOG; KwaZulu-Natal Department of Health; Department of Obstetrics and Gynaecology, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IVBCur; Retired: Western Cape Department of Health, South Africa
VBOccTher, MOccTher; Effective Care Research Unit, University of the Witwatersrand, Walter Sisulu University, East London, South Africa
VIMB BCh, FCOG; Eastern Cape Department of Health, East London, South Africa
VIIMRCOG, DSc; Effective Care Research Unit, University of the Witwatersrand, Walter Sisulu University, East London, South Africa
VIIIMRCOG, DSc; Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Botswana, Gaborone, Botswana
ABSTRACT
Postpartum haemorrhage (PPH) is the leading cause of preventable maternal mortality in South Africa (SA). In a significant breakthrough in the management of PPH, the E-MOTIVE trial found that a multifaceted health service intervention reduced severe PPH after vaginal delivery by 60% in 78 hospitals in Nigeria, Kenya, Tanzania and SA. The E-MOTIVE approach comprises objective blood loss measurement monitored every 15 minutes during the first hour after delivery to detect PPH early and trigger a bundle of first-line treatments, including massaging the uterus, oxytocin infusion, tranexamic acid infusion, intravenous crystalloid fluids, examination for the cause, emptying the bladder and, if necessary, escalation of care. E-MOTIVE was integrated into the existing Essential Steps in Managing Obstetric Emergencies (ESMOE) algorithm. Certain research-related elements of the trial setting cannot be replicated in routine practice. Therefore, we need to develop local strategies to ensure the essential clinical elements of the intervention are implemented. Potential strategies include incorporating the E-MOTIVE principles into national guidelines, ongoing training strategies and ensuring all facilities are equipped with necessary medication, equipment and delegations. This breakthrough intervention provides hope for women in SA, and requires a purposeful, co-ordinated implementation strategy on a national scale to reach all levels of the health service.
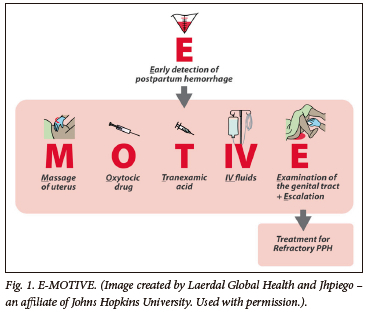
Haemorrhage, mainly postpartum haemorrhage (PPH), is the leading cause of avoidable maternal mortality in South Africa (SA), with 89.5% of haemorrhage deaths being potentially preventable.[1] Successive reports of the National Committee for Confidential Enquiries into Maternal Deaths (NCCEMD) have emphasised that management failures include late detection of PPH and substandard care ('too little, too late').[2] The announcement at the International Maternal and Newborn Health Conference in Cape Town in May 2023 of a major breakthrough in the management of PPH using the E-MOTIVE (Fig. 1) intervention[3] represents a beacon of hope. The great majority of births in SA are attended by midwives and non-specialist doctors in clinics and district hospitals. For childbearing South Africans to benefit from this new approach to PPH management, it will require a co-ordinated implementation strategy on a national scale, reaching all levels of the health service. This article summarises the details of the E-MOTIVE intervention and the results achieved in the trial, and discusses strategies specific to SA for wide-scale implementation of E-MOTIVE to all birthing facilities. While some of these strategies will be relevant to settings outside SA, there will be a need for locally feasible and relevant implementation strategies to be planned, taking into account the existing health systems in each setting.
Recent research: The E-MOTIVE trial results
The landmark E-MOTIVE trial[3] found that a multifaceted health service intervention reduced the primary outcome (severe PPH, laparotomy for bleeding or death from bleeding after vaginal birth) by a remarkable 60% (95% confidence interval 50% - 68% reduction). This evidence was generated by a large, parallel cluster randomised trial in 78 hospitals in Nigeria, Kenya, Tanzania and SA (14 hospitals in Western Cape, KwaZulu-Natal and Eastern Cape provinces). The study was not powered to compare maternal mortality, but the numbers were in keeping with a meaningful improvement (17 maternal deaths following 48 678 vaginal births in the intervention sites v. 28/50 044 in the control sites; maternal mortality per 100 000 vaginal births: 34.9 v. 56.0). There was also a 29% reduction in the use of blood transfusion for bleeding in the intervention sites. Dr Tedros Adhanom Ghebreyesus, current Director-General of the World Health Organization (WHO), responded to the results of the E-MOTIVE trial by tweeting that the trial 'shows highly promising results. This could represent a major breakthrough in reducing maternal deaths.'[4]
The E-MOTIVE intervention
The essential elements of the E-MOTIVE intervention (Fig. 1) at the SA sites included:
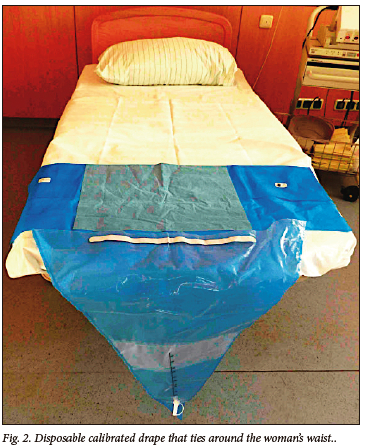
• objective assessment of blood loss after every vaginal birth, by use of a plastic calibrated blood collection drape placed under the woman's buttocks. Fig. 2 shows a picture of a calibrated drape. This drape was put in place by the midwife when preparing for vaginal delivery, or immediately after vaginal delivery. It was unrolled to allow blood collection after the delivery of the baby, but before delivery of the placenta.
• performing the required 15-minute post-delivery observations for 1 hour, as specified in the SA maternity case record, and adapting the observation chart to include blood volume measurements (see Fig. 3 for the modified observation chart used at all SA intervention sites during the trial).
• Early diagnosis of PPH when measured blood loss reached 500 mL, or earlier for obviously heavy bleeding or when abnormal vital signs suggested excessive blood loss
• A WHO-recommended 'bundle' of first-line treatments for PPH (MOTIVE), to be immediately administered when triggered by the early diagnosis of PPH:
• Massaging the uterus
• Oxytocin (10 IU in 100 - 200 mL infusion over 5 - 10 minutes, followed by maintenance oxytocin infusion (20 IU in 1 L over 4 - 8 hours)
• Tranexamic acid (TXA) (1 000 mg in 100 - 200 mL infusion over 10 minutes)
• IV - intravenous crystalloid fluids (this includes the fluids used for the infusions of oxytocin and tranexamic acid)
• Examination for the cause, Emptying the bladder and, if necessary, Escalation of care.
All these interventions were authorised to be performed by midwives, with medical support when indicated, and all components were to be initiated as soon as possible after the diagnosis of PPH was made, preferably within 15 minutes of the diagnosis. If there was a team of health workers attending to the woman with PPH, the various components of the bundle could be administered simultaneously, with the exception of the oxytocin and TXA infusions, which if given through the same IV line had to be administered sequentially, as there was concern about the possibility of an interaction between the two drugs if they were mixed in the same infusion bag.
Implementation of the E-MOTIVE intervention was facilitated by:
• provision of a 'PPH box' or carry-case equipped with essential commodities for treatment of PPH (except oxytocin, which had to be stored in the fridge)
• wall posters demonstrating the E-MOTIVE intervention
• 'Essential Steps in Managing Obstetric Emergencies' (ESMOE) PPH posters, modified to incorporate E-MOTIVE. The modified ESMOE algorithm is shown in Fig. 4. The important additions were accurate determination of blood loss, and a bundled rather than sequential treatment response. Key differences in the new approach were the oxytocin regimen given as an immediate 10 IU oxytocin infusion in 100 - 200 mL. The rationale for this was that the existing ESMOE protocol (20 IU in 1 L over 4 - 8 hours) administered oxytocin too slowly to be immediately effective. An infusion was used rather than a rapid bolus to reduce the risk of hypotension. This was followed by a maintenance oxytocin infusion (20 IU in 1 L). Immediate use of TXA was included in this first response bundle, based on evidence in the WOMAN trial[5] that early use of TXA for PPH reduces death, and WHO recommendation.[6] Where escalation of treatment for refractory PPH or where additional procedures were needed, usual ESMOE protocols were followed.
• hospital E-MOTIVE protocol signed by the senior doctor, authorising midwives to administer all E-MOTIVE bundle components
• designated midwife and doctor 'E-MOTIVE champions' at each site to promote the use of the E-MOTIVE intervention among all maternity staff
• train-the-trainer workshops for these champions to capacitate them to train the midwives and maternity doctors at their hospitals in how to conduct the E-MOTIVE intervention
• onsite small group E-MOTIVE training (90 - 120-minute sessions) for doctors and midwives at each intervention site, run by the champions, supported by research staff including research and implementation midwives. This training included simulation exercises for all participants, where the detection and management of PPH using E-MOTIVE was practised
• centrally co-ordinated monthly audit and feedback to intervention sites regarding the number of PPHs at that site and MOTIVE bundle use.
Discussion
The feedback about the E-MOTIVE intervention from midwives and maternity doctors at all the SA intervention sites was overwhelmingly positive. There was a perception that E-MOTIVE improved the quality of care and safety for women post delivery in the labour ward, which was borne out by the objective trial results. The staff at all the hospitals were keen to continue with the E-MOTIVE intervention even after the end of the trial.
The key changes in how women were managed post delivery with the introduction of E-MOTIVE can be summarised as follows:
(i) The blood loss was collected in such a way that it was clearly visible, and the attending midwife could easily note the cumulative volume of blood loss. This could be recorded on the adapted post-delivery observation chart every 15 minutes.
(ii) At a glance, the midwife could detect whether the PPH threshold of 500 mL had been reached. This means that few PPHs were missed, in contrast to the previous situation where PPHs were frequently missed unless severe.
(iii) The bundle principle meant that there was a simple 'one size fits all' first-response management once the PPH had been diagnosed, making PPH management simple to initiate without having to make decisions about which initial treatment to give depending on the cause of the PPH.
(iv) The diagnosis of PPH could be made and the initial bundle administered by the attending midwife without first getting confirmation of the diagnosis and authorisation for the treatment by a doctor. This saved time and empowered the midwives to manage the PPH promptly before it became severe.
How can the dramatic effects demonstrated in the E-MOTIVE trial be translated to improved outcomes from PPH in SA? Certain research-related elements of the trial setting cannot be replicated in routine practice, such as intensive dedicated face-to-face training, support from research midwives and centrally co-ordinated audit and feedback. Nonetheless, we need to develop local strategies to ensure that the essential clinical elements of the intervention (quantitative monitoring of blood loss after vaginal birth and early implementation of the MOTIVE bundle of first-line care) are implemented with the greatest possible level of fidelity and evaluate the impact through routine essential data collection or implementation research.
As a starting point for national dialogue on this effort, we suggest the following potential strategy:
• incorporation of the E-MOTIVE principles into the National Guidelines for Maternity Care in SA, which are currently undergoing revision
• incorporation of video-based E-MOTIVE simulation training into routine ESMOE training nationally, and locally as 'drills', so that all staff involved in postdelivery care can be trained to accurately measure blood loss and trigger the MOTIVE bundle for PPH (measured blood loss >500 mL and/or clinical features of PPH)
• distribution of wall posters outlining the E-MOTIVE approach integrated into ESMOE algorithms to all health facilities
• protocols at each delivery site authorising midwives to perform all components of the MOTIVE bundle, including timeous escalation for medical support
• approval of IV TXA as essential medication at primary care clinics which are designated delivery sites (e.g. midwife-led obstetric units)
• procurement of 'PPH boxes' or carry-cases for all labour wards that do not yet have them
• procurement of devices for objective measurement of blood loss after vaginal birth. If disposable calibrated drapes are unaffordable or unsustainable, consideration of use of the locally-developed reusable MaternaWell tray as an alternative or adjunctive tool for post-delivery blood loss measurement[7,8]
• amendment of the national maternity case record to include the adapted E-MOTIVE post-delivery observations page, which includes a place to document the cumulative blood loss at each time point
• ensuring staff are allocated to perform the 15-minute post-delivery observations of vital signs and measured blood loss (this can be done by enrolled nurses)
• developing a strategy to report on PPH, severe PPH and PPH complications as a measurable monthly indicator, which could be displayed for local monitoring and feedback by a simple wall chart.
Several strategies might be considered for monitoring of the impact of a national intervention. These might include monitoring of routinely collected data, or specific implementation research at sentinel sites. Ultimately, the evidence for effectiveness of such an intervention will be reflected in improvements in mortality from haemorrhage reported by the NCCEMD.
The E-MOTIVE trial was limited to an intervention following vaginal birth. For a more meaningful impact on maternal mortality nationally, we need to also address deaths from haemorrhage following caesarean birth, which have increased considerably in recent years.[1] Pending direct evidence from trials that may take place in the future, we need clinical consensus on the extent to which the evidence from the E-MOTIVE trial might be extrapolated to post-caesarean bleeding. The following may serve as a starting point for national dialogue.
Blood loss during or after caesarean birth is similar to that following vaginal birth, being related usually to placental site bleeding (atonic or non-atonic) or genital tract trauma, with the impact frequently potentiated by delayed diagnosis and management. However, accurate blood loss measurement is more challenging, and the steps and skills needed to arrest the bleeding and resuscitate the patient will often be different. Important strategies to reduce severe haemorrhage at caesarean delivery include surgical and anaesthetic skills training, anaesthetist/surgeon/nursing teamwork to monitor and control blood loss, as well as avoidance of unnecessary surgery. We can apply what we have learned from the E-MOTIVE trial to devise strategies for quantitative blood loss monitoring after caesarean birth and early, bundled management.
Declaration. None.
Acknowledgements. We thank the sponsors and investigators of the E-MOTIVE study and all the staff and patients who contributed to the study.
Author contributions. GJH conceived the paper and wrote the first draft.
All authors participated in the E-MOTIVE study, contributed to the content of the article and approved the final version.
Funding. No funding was received, but the E-MOTIVE trial was funded by the Bill & Melinda Gates Foundation; NCT04341662.
Conflicts of interest. GJH has an interest in the Maternawell Tray for blood loss monitoring after birth.
References
1. National Department of Health, South Africa. Saving Mothers; 2017 - 2019. The eighth report of the National Committee for Confidential Enquiry into Maternal Deaths in South Africa. Pretoria: NDoH, 2020. [ Links ]
2. Fawcus S. Maternal deaths from obstetric haemorrhage 2017 - 2019; are we overlooking deaths from postpartum haemorrhage after vaginal delivery? Obstetr Gynaecol For 2020;30(4):8-11. [ Links ]
3. Gallos I, Devall A, Martin J, et al. Randomised trial of early detection and treatment of postpartum hemorrhage. N Engl J Med 2023:389;11-21. https://doi.org/10.1056/nejmoa2303966 [ Links ]
4. Dr Tedros. Good news: a new trial to manage postpartum bleeding shows highly promising results. This could represent a major breakthrough in reducing maternal deaths. #HealthForAll. https://twitter.com/DrTedros/status/1655920280869412864?t=_6Odf8AjaAcYKrc7QFxyKQ&s=08 (accessed 10 November 2023). [ Links ]
5. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389(10084):2105-2116. https://doi.org/10.1016%2FS0140-6736(17)30638-4 [ Links ]
6. Althabe F, Therrien MNS, Pingray V, et al. Postpartum hemorrhage care bundles to improve adherence to guidelines: A WHO technical consultation. Int J Gynaecol Obstet 2020;148(3):290-299. https://doi.org/10.1002/ijgo.13028 [ Links ]
7. Singata-Madliki M, Hofmeyr GJ. A novel, re-usable "Safe birth Tray' for postpartum blood loss monitoring: A preliminary acceptability assessment. Int J Gynecol Obstetr 2021;155(3):553-555. https://doi.org/10.1002/ijgo.13817 [ Links ]
8. Esau J, De Waard L, Els C. The utility and acceptability of a Brass V Drape versus a blood loss monitoring device for the collection of postpartum blood loss in low-risk term vaginal deliveries; a prospective parallel randomised trial. 41st Conference on Priorities in Perinatal Care in Southern Africa 7 - 10 March 2023, Indaba Conference Centre, Gauteng, South Africa. Abstract, page 23. [ Links ]
 Correspondence:
Correspondence:
E Muller
elaviljoen@yahoo.com
Accepted 19 October 2023














