Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.12 Pretoria dic. 2023
http://dx.doi.org/10.7196/samj.2023.v113i11.1127
IN PRACTICE
Informal gold miners with mercury toxicity: Novel asymmetrical neurological presentations
J GeorgeI; E SadiqII; I MoolaII; S MaharajIII; A MochanIV
IMB BCh; Division of Neurology, Department of Neurosciences, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB BCh, FC Neurol (SA); Division of Neurology, Department of Neurosciences, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMB BCh; Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVFCP (SA) Neurology, MD; Division of Neurology, Department of Neurosciences, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Mercury is a highly toxic heavy metal that may cause neurological, respiratory, gastrointestinal and dermatological illnesses. Previously described neurological manifestations of mercury toxicity are symmetrical, and include a pancerebellar syndrome, generalised seizures and encephalopathy. Mercury is used in the gold mining process, and in artisanal or illicit gold mining, often without necessary protection. Here we describe the cases of two artisanal gold miners from western Johannesburg, South Africa, who presented with atypical neurological manifestations of mercury toxicity. Patient 1 presented with focal seizures, an asymmetrical cerebellar syndrome and an acute encephalopathy. Patient 2 had unilateral cerebellar ataxia. Both patients had toxic mercury levels, with no other cause identified for their symptoms. Patient 1 responded well to chelation therapy, but patient 2 refused admission and further medical treatment. The neurological manifestations of mercury toxicity are typically symmetrical, whereas our two patients presented with markedly asymmetrical features. It is important to maintain a high index of suspicion for mercury poisoning, even in patients with atypical and unilateral or asymmetrical presentations. A prompt diagnosis and the commencement of early chelation therapy have the potential to produce good outcomes.
Mercury is a highly toxic compound. It has been used for many years in medicine, agriculture and industry, making it a ubiquitous environmental pollutant.[1] The clinical presentation of mercury toxicity depends on the type and quantity of the mercury exposure, and may include neurological, respiratory, renal and gastrointestinal syndromes. The neurological manifestations of mercury toxicity include anxiety, encephalopathy, seizures and cerebellar ataxia. As with most toxin-related neurological syndromes, the clinical deficits are almost exclusively symmetrical in their presentation.[2]
Mercury poisoning gained public interest with the ingestion of contaminated seafood during the Minamata Bay disaster in Japan, and with cases in Iraq from exposure of grain to mercury-containing fungicide.[3,4] Here we report two cases of mercury toxicity in artisanal gold miners in South Africa (SA). These illegal, unregulated mining operations lack occupational safety precautions and can lead to exposure to dangerously high levels of mercury.[5]
Of particular interest here were the strikingly asymmetrical neurological deficits in our patients, which to our knowledge have not been described previously.
Ethical considerations
Patient informed consent was obtained for a written case report. Ethical approval was obtained from the Human Research Ethics Committee (Medical) of the University of the Witwatersrand, Johannesburg, South Africa (ref. no. M221096).
Case presentations
Case 1
A 32-year-old male was referred to Helen Joseph Hospital, Johannesburg, SA, with a 2-month history of progressively worsening tremors of his right upper and lower limbs. One week prior to the presentation he had become increasingly confused, experienced auditory and visual hallucinations and displayed aggressive behaviour. He had no past medical history. His occupational history revealed that he had been an informal gold miner for the past 6 months. His work entailed burning a mixture of gold ore and elemental mercury in order to extract the gold. He did not use any personal protective equipment (PPE) for this purpose. There was no history of exposure to any other toxins or drugs.
On arrival at our institution, he was markedly encephalopathic, not talking, not obeying instructions, making non-purposeful movements and extremely anxious and apprehensive of anyone trying to approach him. He was noted to have focal seizures of his right upper limb, and was markedly ataxic axially and in all four limbs. Notably, this ataxia was strikingly more prominent on the right side of his body compared with the left. Routine blood investigations and cerebrospinal fluid (CSF) analysis were within normal limits (Table 1). Urgently obtained blood mercury levels were grossly elevated at 146 μg/L (normal range 10 -15 μg/L). A computed tomography (CT) scan of the brain was normal; magnetic resonance imaging (MRI) showed a non-enhancing left frontal cortical hyperintensity on T2 FLAIR, which was isointense on T1-weighted sequences (Fig. 1).
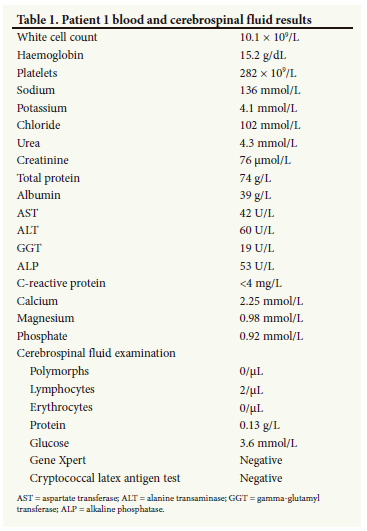
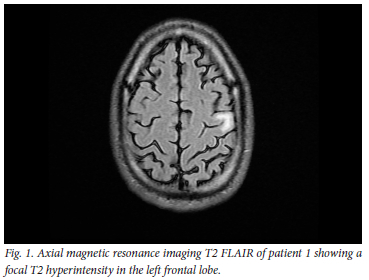
A diagnosis of mercury poisoning secondary to occupational exposure was made, with resultant encephalopathy, right focal motor seizures and a markedly asymmetrical cerebellar syndrome.
Chelation therapy with N-acetyl penicillamine was initiated within hours of admission at a dose of 300 mg per os 6-hourly. Sodium valproate was commenced for focal seizures.
For the first 3 days, the patient remained encephalopathic and the focal seizures extended to the right side of his face. On day 5 of treatment, the patient became less agitated, and was able to obey simple instructions. He was unable to verbalise, responding with groans only. He remained markedly ataxic, with the right side still worse than the left. The focal seizures persisted with involvement of his face, but occurred less frequently. Chelation therapy was continued. By day 10 of treatment, the patient was able to speak, but with a severe cerebellar dysarthria. He was orientated to person, but not to place or time. A repeat mercury level on day 10 was 72.8 μg/L. By day 20, he had markedly improved, was fully orientated and was able to follow complex commands. His dysarthric speech was now intelligible and his asymmetrical ataxia, although still present, had improved to the extent of being able to feed himself and mobilise independently. There were no further focal seizures. He had no recall of the events directly preceding his admission, but he did remember working in the gold mine and using mercury.
On day 25 of admission, his liver function test revealed mild transaminitis (alanine aminotransferase of 170 U/L and aspartate aminotransferase of 107 U/L), and penicillamine was discontinued. The deranged liver enzymes normalised, and he was discharged from hospital on day 27 for outpatient follow-up. At 1 month after discharge, he had mild residual ataxia. On his 2-month review, the ataxia had completely resolved, and he had returned to baseline. He had vague recollections of his admission to hospital, but his memory before and after the hospital stay was normal.
Case 2
A 26-year-old male presented with a 1-week history of poor balance associated with slurred speech and inco-ordination of his left arm. He had been working on an informal gold mine for the past year. There was no history of exposure to drugs or toxins. His neurological examination revealed normal higher functions, truncal ataxia and a unilateral hemispheric cerebellar syndrome, with left-sided dysmetria, dysdiadochokinesia and intention tremor.
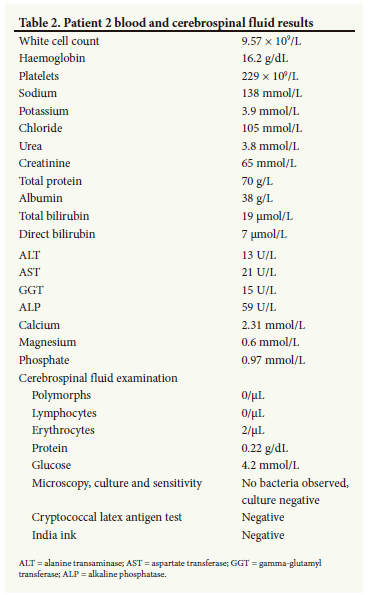
Basic blood investigations and CSF analysis (Table 2) were normal. A contrasted CT scan of the brain was normal. His urine mercury level was 141.13 μg/L (normal <35 μg/L), confirming the diagnosis of mercury toxicity. Despite extensive counselling, the patient refused to stay in the hospital for treatment. He was discharged into the care of his family and subsequently lost to follow-up.
Discussion
Mercury exists in organic, inorganic and elemental forms, each leading to different intoxication syndromes.[1] Organic mercury exposure usually occurs from ingestion of contaminated food, while inorganic mercury exposure is seen with laxative abuse, skin lightening creams and antiseptics.[1] Both are primarily absorbed through the gastrointestinal tract, and secondarily through intact skin. They mainly cause renal and gastrointestinal toxicity, while organic mercury toxicity may additionally cause neurological symptoms, which are characteristically delayed.[2,4,6]
Elemental mercury is used in manufacturing and industrial processes (mining, smelting), as well as household, medical and electrical devices (e.g. thermometers, electrical switches and dental amalgam).[1,5] It is primarily absorbed by inhalation, is highly fat soluble, and readily crosses the blood-brain barrier.[7] Thus pulmonary (interstitial pneumonitis) and CNS disorders are the typical manifestations of elemental mercury toxicity. The classically involved CNS regions are the cerebral cortex (pyramidal cells and astrocytes) and the cerebellum (Purkinje cells, granule cells and deep cerebellar nuclei).[1,2,6] At low levels of toxicity, patients have nonspecific symptoms of headache, nausea and anorexia.[8] At higher levels, presentations comprise cerebellar ataxia, encephalopathy and a neuropsychiatric syndrome known as erethism, characterised by extreme fearfulness, anxiety, excessive sensitivity and irritability.[8,9]
Here we have described two cases of elemental mercury toxicity in artisanal gold miners presenting at a tertiary medical institution in SA's Gauteng Province. Artisanal and small-scale gold mining is considered the largest source of mercury pollution globally.[10] This mainly occurs in underdeveloped countries in Asia, South America and Africa. It is often the only source of income for the impoverished, with minimal or no professional expertise required.[11,12] A multinational review of artisanal gold miners from Ecuador, Indonesia, the Philippines, Tanzania and Zimbabwe by Steckling et al.[13] reported chronic mercury intoxication in 24 - 29% of their primary data set.
In Johannesburg, the gold mining industry started with the discovery of the gold reef in 1886.[14] Numerous abandoned mines around Johannesburg invite illicit gold mining operations.[15] Their illegal nature makes the implementation of occupational health and safety regulations very difficult.
Mercury is used to inexpensively and effectively extract gold from the amalgamated ore sludge. Upon heating the mixture, elemental mercury evaporates and is subsequently inhaled, particularly if adequate PPE is not utilised. This is the most likely mechanism of exposure in both of our patients.[5,6]
The clinical presentations of our patients are unique in that they presented with strikingly asymmetrical neurological signs. The first patient had focal motor seizures with an asymmetrical cerebellar syndrome, while the second patient had a purely unilateral cerebellar syndrome.
This asymmetry is most peculiar and difficult to explain. Toxin-induced neurological disorders are known for their symmetrical presentations.[2,16,17] A literature search could not yield any possible explanation or even any previous cases describing asymmetrical CNS features in patients with mercury toxicity. MRI scans of the brains in some of the Minamata Bay disaster victims showed bilateral involvement of cerebral and/or cerebellar cortices.[18] The MRI scan of our first patient showed a unilateral T2 FLAIR hyperintensity involving the left frontal cortex. This correlated well with the location of his focal seizures (right arm and face).
Seizures have previously been described in patients with mercury poisoning (including 2.86% of the Minamata Bay-exposed).[19]
However, to our knowledge there have been no previous reports of focal seizures in the literature, as were noted in our first patient. Our second patient presented with purely unilateral cerebellar signs, with no corresponding structural explanation on imaging, although we acknowledge that only a CT scan was obtained.
Alternative aetiologies for the asymmetrical presentations were reasonably excluded, and resolution of the asymmetrical deficits with chelation therapy in patient 1 implies that mercury toxicity was the most likely aetiology of these asymmetrical signs.
Dimercaprol, succimer and N-acetyl penicillamine are the mainstay chelating agents used in mercury poisoning.[7] The SA Standard Treatment Guidelines and Essential Medicines List includes both dimercaprol and N-acetyl penicillamine.[20] N-acetyl penicillamine was used in our first patient and was effective in chelation.
Regarding the duration of treatment, we had treated our first patient with N-acetyl penicillamine for 5 days as is standard.[20] However, as there was no improvement after 5 days, and in consultation with our national poison centre and expert opinion, we opted to continue treatment until the patient was asymptomatic or until the mercury blood level returned to within normal range.[9,21] In this patient, we elected to treat for 25 days as, despite significant improvement, the patient remained mildly symptomatic. We stopped treatment upon the diagnosis of a mild drug-induced liver injury, which resolved spontaneously. His cerebellar ataxia and encephalopathy had resolved markedly after 25 days of chelation, and continued to improve over the next 2 months, returning to baseline function.
Study limitations
Limitations of our case descriptions include the non-availability of MRI and lack of follow-up of our second patient, as well as the lack of a post-chelation repeat MRI for the first patient.
Teaching points
Neurological manifestations of mercury poisoning are universally symmetrical in nature.
Here we present two cases with markedly asymmetrical neurological manifestations.
History-taking and a high index of suspicion are very important. Prompt diagnosis and commencement of chelation treatment has good outcomes.
Conclusion
Artisanal gold mining is a thriving, unregulated industry in parts of SA and elsewhere in the developing world, resulting in an excessive risk of mercury toxicity to its workers and possibly the community at large. Neurological disorders associated with toxin exposure, including mercury, are inevitably symmetrical in presentation. Our two patients with markedly asymmetrical neurological manifestations of elemental mercury toxicity are a novel finding. Despite severe neurological impairment, prompt diagnosis and treatment resulted in a favourable outcome in one of the patients.
While there is no clear pathophysiological explanation for the atypical presentations, our findings highlight the need to keep a high index of suspicion for mercury toxicity, particularly in high-risk individuals, despite a markedly atypical asymmetrical clinical picture.
Declaration. None.
Acknowledgements. None.
Author contributions. JG, ES: conceptualisation, data acquisition and interpretation, manuscript drafting, review and editing. IM: conceptualisation, data acquisition, manuscript review. SM: data interpretation, manuscript drafting and review. AM: conceptualisation, manuscript review and editing. All authors approved the final version of the manuscript submitted for publication.
Funding. None.
Conflicts of interest. None.
References
1. Syversen T, Kaur P. The toxicology of mercury and its compounds. J Trace Elem Med Biol 2012;26(6):215-226. https://doi.org/10.1016/j.jtemb.2012.02.004 [ Links ]
2. Berlin M, Zalups RK, Fowler BA. Handbook on the Toxicology of Metals. 3rd ed. London: Elsevier, 2005. [ Links ]
3. Environmental Health Department, Ministry of the Environment, Japan. Minimata Disease: The History and Measures. Tokyo: Government of Japan, 2002. [ Links ]
4. Bakir F, Damluji SF, Amin Zaki I. Methylmercury poisoning in Iraq: An interuniversity report. Science 1973;181(4096):230-241. [ Links ]
5. Ronald E. Mercury hazards from gold mining to humans, plants, and animals. Rev Environ Contam Toxicol 2004;181:139-198. https://doi.org/10.1007/0-387-21733-9_4 [ Links ]
6. Robin A, Bernhoft. Mercury toxicity and treatment: A review of the literature. J Environ Public Health 2012;2012:460508. https://doi.org/10.1155/2012/460508 [ Links ]
7. Hursh JB, Clarkson TW, Miles EF, et al. Percutaneous absorption of mercury vapor by man. Arch Environ Health 1989;44:120-127. https://doi.org/10.1080/00039896.1989.993438 [ Links ]
8. Jackson AC. Chronic neurological disease due to methylmercury poisoning. Can J Neurol Sci 2018;45(6):620-623. https://doi.org/10.1017/cjn.2018.323 [ Links ]
9. Gillian B, Kusin S, Carl-Gustaf E. Mercury toxicity. Uptodate: 13 Jun 2022. [ Links ]
10. Mhlongo SE, Amponsah-Dacosta F, Muzerengi C, et al. The impact of artisanal mining on rehabilitation efforts of abandoned mine shafts in Sutherland goldfield, South Africa. Jamba J Disaster Risk Stud 2019;11(2):688. https://www.jamba.org.za/index.php/JAMBA/article/view/688 (accessed 14 February 2022). [ Links ]
11. Ali M, Hery S, Putri SA. Mercury toxicity potential from artisanal and small-scale gold mines in Lebong Regency, Bengkulu Province. E3S Web Conference 2018;73:06002. https://doi.org/10.1051/e3sconf/20187306002 [ Links ]
12. Veiga MM, Hinton JJ. Abandoned artisanal gold mines in the Brazilian Amazon: A legacy of mercury pollution. Nat Resour Forum 2002;26(1):15-26. [ Links ]
13. Steckling N, Tobollik M, Plass D, et al. Burden of disease of mercury used in artisanal small-scale gold mining. Ann Glob Health 2017;83(2):234-247. https://doi.org/10.1016/j.aogh.2016.12.005 [ Links ]
14. City of Johannesburg. Mining. Johannesburg: City of Johanesburg, 2018. https://www.joburg.org.za/work_/keysectors/Pages/Mining.aspx#:~:text=The%20major%20gold%20and%20diamond,South%20Africa's%20total%20mineral%20production (accessed 13 May 2023). [ Links ]
15. Mining.com. Gang battles over abandoned gold mines in South Africa. Mining.com, 2015. https://www.mining.com/gangs-battle-over-abandoned-gold-mines-in-south-africa/ (accessed 13 May 2023). [ Links ]
16. Kim Y, Kim JW. Toxic encephalopathy. Saf Health Work 2012;3(4):243-256. https://doi.org/10.5491/SHAW.2012.3.4.243 [ Links ]
17. Ropper AH, Adams RD, Victor M, et al Adams and Victor's Principles of Neurology. 8th ed. New York: McGraw-Hill, 2005. [ Links ]
18. Korogi Y, Takahashi M, Okajima T, et al MR findings of Minamata disease - organic mercury poisoning. J Magn Reson Imaging 1998;8(2):308-316. https://doi.org/10.1002/jmri.1880080210 [ Links ]
19. Yukun Y. Methylmercury: A potential environmental risk factor contributing to epileptogenesis. Neurotoxicology 2012;33(1):119-126. [ Links ]
20. National Department of Health, South Africa. Essential Drugs Programme. Hospital Level (Adults) Standard Treatment Guidelines and Essential Medicines List. 5th ed. Pretoria: NDoH, 2019. [ Links ]
21. Poison informations helpline. https://www.westerncape.gov.za/service/poisons-information-helpline-western-cape (accessed 13 May 2023). [ Links ]
 Correspondence:
Correspondence:
E Sadiq
eitzaz.sadiq@wits.ac.za
Accepted 18 September 2023














