Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.11 Pretoria Nov. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i11.845
RESEARCH
Biliary atresia: The profile, management and outcome of patients treated at a tertiary hospital in central South Africa
E BritsI; S M le GrangeII
IMMed (Paed Surg), FC Paed Surg (SA); Department of Surgery, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
IIMMed (Surg), Cert Paed Surg (SA); Department of Surgery, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
ABSTRACT
BACKGROUND: Biliary atresia (BA) is an obstructive inflammatory disease of the bile ducts. Without intervention, the disease rapidly progresses to liver cirrhosis and fibrosis, with end-stage liver failure and death occurring within the first 3 years of life. It is the most common indication for liver transplantation (LT) in the paediatric population. The management of BA in South Africa (SA) faces multiple challenges, such as late referrals and socioeconomic burdens, with suboptimal outcomes
OBJECTIVES: To determine risk factors and shortcomings that are detrimental to the outcome of the paediatric patient population by reviewing the profile, management and outcome of patients with BA treated at Universitas Academic Hospital Complex (UAHC), Bloemfontein, SA
METHODS: This was a retrospective analytical record review of all patients diagnosed with BA and treated at UAHC from 1 January 2009 to 31 December 2019
RESULTS: In total, 67 patients were included; 74.6% were female, and 86.6% were black Africans. Most (62.7%) had isolated BA. A Kasai portoenterostomy (KPE) was performed in 32 patients (47.8%). Of 5 patients referred for LT evaluation, 2 received a transplant. Of 55 patients with known outcomes, 5.5% (n=3) survived and 94.5% (n=52) died after receiving a KPE or palliative treatment. Of the 3 patients who were alive at the end of the study period, 1 had a KPE and 2 had LTs
CONCLUSION: Late presentation, cholangitis and cessation of bile flow after an initial successful KPE, and socioeconomic challenges are issues of concern and had a detrimental influence on the outcome of BA in our study population. Implementing screening measures and education programmes at the primary healthcare level is essential to diagnose and refer BA patients timeously. Establishing support systems to assist socioeconomically disadvantaged patients will enable them to qualify for LT
Biliary atresia (BA) is an idiopathic progressive, fibro-obliterative cholangiopathy affecting both the intra- and extrahepatic bile ducts and occurring during the perinatal period. Without intervention, the disease rapidly progresses to hepatic fibrosis and cirrhosis, with end-stage liver failure and death within the first 3 years of life.[1] Although BA is a rare disease, it is the most common indication for liver transplantation (LT) in the paediatric population.[2]
The incidence of BA varies. It is common in the East, e.g. in Taiwan and Japan (1 in 5 000 - 10 000),[3,4] and less prevalent in the West, e.g. in Europe and North America (1 in 15 000 - 20 000).[5-7] In addition, the incidence is higher in females than in males, and in Asian and black than in white infants.[8-10] The reasons for these differences remain unclear, but are probably related to genetic, environmental and cultural factors.[11] However, the clinical presentation of BA is typical, with progressive obstructive jaundice, pale stools and dark urine. Other symptoms include vitamin K-dependent coagulopathy, ascites, portal hypertension and splenomegaly.[12]
Since the aetiology of BA is unknown, there are many theories as to its cause. Several mechanisms and factors are thought to lead to the disease, rather than a single cause.[13] These include cytomegalovirus (CMV) infection, immune dysregulation, autoimmune conditions, vascular lesions, gene mutations and toxin exposure.[14] Recent studies suggest that the initial insult is perinatal, because elevated conjugated bilirubin levels have been noted in the first 3 days of life in neonates who developed BA. This finding may provide a means of screening newborns to identify BA early.[15]
BA can be subclassified into four major variants, namely isolated BA (IBA, 80%), BA splenic malformation (BASM, 5 - 15%), cystic BA (CBA, 5 - 10%) and CMV-associated BA (5 - 10%).[16-21] IBA varies in presentation time, level of biliary tree obliteration and degree of inflammation, with no other congenital anomalies. In contrast, BASM is frequently associated with laterality malformations such as situs inversus, asplenia, polysplenia, malrotation, interrupted inferior vena cava and cardiac anomalies. CBA is detectable during gestation and is therefore the only type of BA identifiable by prenatal ultrasound. While the causes of these different variants are unclear, it has been shown that their clinical courses differ,[16-20] with BASM and CMV-associated BA having the worst prognosis.[19,20]
Assessing the extent of liver fibrosis in BA is vital for management decision-making and prognostic purposes.[22] A liver biopsy is the gold standard for evaluating the degree of liver injury.[23] Histopathological parameters, including the degree of liver fibrosis, can predict early failure of Kasai portoenterostomy (KPE).[24] It is also clear that the presence of bile duct proliferation, giant cells and fibrosis indicates a significant change in fibrosis progression and decreased native liver survival rates (NLSRs) in BA.[25]
The current standard of care for BA is surgical management, with KPE and LT the only therapeutic options. KPE is considered the first-line treatment, even though it is not curative despite clearance of jaundice. Most patients will ultimately require LT by early adulthood owing to progressive hepatic dysfunction.[26,27] Therefore, despite excellent graft (90%) and patient (97%) survival,[28] the question of whether to abandon KPE and proceed to primary LT has arisen. However, the inadequate number of organs available and the burden of immunosuppressive therapy during childhood make this option unfeasible. Maximising the efficacy of KPE with resultant improved NLSRs is therefore crucial.[29]
Current ways to improve the NLSR after KPE are to perform the surgery as early as possible and to use postoperative adjuvant therapy. Jaundice can be cleared in 50 - 60% of patients operated on before 70 days of age.[30] Moreover, if KPE is done before 45 days, the NLSR can be improved to 65.5% at 2 years and 40.5% at 15 years.[31] Additionally, adjuvant treatment is a major area of research in BA to help improve KPE outcomes by decreasing the detrimental effect of the inflammatory response on the bile ducts, improving bile flow, and preventing recurrent cholangitis.[32]
BA remains a very challenging disease to manage, with a considerable variation in outcome globally (0 - 97% survival) and unfavourable results, especially in resource-poor countries (low- to middle-income countries (LMICs) and low-income countries (LICs)).[28,33-36] The reasons for these poor outcomes are thought to be multifactorial, and may include socioeconomic factors resulting in delayed presentation, nutritional deficits, a high rate of CMV-associated BA, HIV exposure, non-Caucasian ethnicity, suboptimal management, and loss to follow-up (LTFU).[19,21,35-37]
The above challenges are exacerbated by a lack of centralised BA management. In South Africa (SA), for example, patients are treated at their nearest tertiary paediatric surgical referral centre with KPE if BA is diagnosed before the onset of liver cirrhosis. Patients with a failed KPE or established liver cirrhosis should be referred for evaluation by the LT teams at Red Cross War Memorial Children's Hospital (RXWMCH) in Cape Town[38] or Wits Donald Gordon Medical Centre (WDGMC) in Johannesburg.[35,39] In contrast, in countries such as the UK, centralising BA cases to designated units and concentrating expertise in surgical techniques and multidisciplinary management have led to survival benefits.[34] While WDGMC has the expertise to perform living related donor transplankts, it is unfortunately a privately funded facility that provides therapeutic options to children in the state sector on a limited basis.[39]
There are many obstacles for patients treated at Universitas Academic Hospital Complex (UAHC) who are suitable for evaluation for LT. These include the vast distances to the centres mentioned above (400 km and 1 000 km, respectively), while family socioeconomic challenges make work-up and follow-up visits difficult, contributing to disqualification from LT. State patients from Lesotho are not accepted for LT in SA. Additional challenges in the management of BA at UAHC include late referral of jaundiced patients, with established histological liver injury at the time of referral, many CMV immunoglobulin M (CMV IgM)-positive BA cases, HIV exposure, patients who pass pigmented stools after KPE but whose jaundice does not clear, post-KPE cholangitis, ongoing liver damage, and poor compliance with follow-up visits. While BA has been studied in other centres in SA,[35,36,39-41] no such studies have been conducted at UAHC.
The present study aimed to determine the risk factors and shortcomings (e.g. late presentation, CMV-associated BA, HIV exposure, degree of perioperative liver injury, postoperative cholangitis, LTFU, not qualifying for LT, etc.) that are detrimental to outcomes in this patient population by reviewing the profile, management and outcome of patients treated at UAHC. We hope to identify possible areas for improvement and to show gaps in our knowledge of our patient population. Areas for improvement may include establishing education and screening programmes to diagnose BA earlier, determining the effects of HIV exposure on BA outcome, and using non-invasive methods to determine the degree of liver injury at presentation.
Methods
Study context
UAHC is in Bloemfontein in Free State Province, SA. It provides paediatric surgical care in the public health sector to central SA (Free State, Northern Cape and parts of Eastern Cape provinces) and Lesotho. This large geographical area involves cross-provincial and cross-border referrals that may contribute to delays. Patients from Lesotho contribute ~25% of index paediatric surgery cases at UAHC. Furthermore, Lesotho and SA are LICs to LMICs with many socioeconomic burdens that may influence BA prognosis and ability to qualify for LT.
Study sample and measurement
This study was a retrospective analytical record review of all 67 patients diagnosed with BA and treated at UAHC from 1 January 2009 to 31 December 2019. These patients were identified by doing an International Statistical Classification of Diseases, 10th revision (ICD-10) code (Q44.2) search on the electronic note-keeping system (MediTech version 3.25a, Medical Information Technology, Inc., SA) at UAHC.
The following information was captured on a data collection sheet from the electronic patient files: (i) demographics (gender, age at KPE or diagnosis if no KPE performed, address); (ii) diagnosis (presenting signs and symptoms, other congenital abnormalities, diagnostic and laboratory investigations, intraoperative cholangiogram, type of BA); (iii) management (KPE performed, no KPE performed, evaluated for LT, LT done); and (iv) outcome (short-, medium- and long-term complications, alive or dead, LTFU).
Pilot study
The first 10 patients included in the study were used for a pilot study. No changes were made to the data collection sheet, and all 10 patients were included in the main study.
Data analysis
Data were captured on a Microsoft 365 Excel spreadsheet (version 2307, Microsoft Corp., USA) by the researchers. Statistical analysis was done by the Department of Biostatistics, Faculty of Health Sciences, University of the Free State (UFS), using SAS version 9.4 (SAS Institute, USA). Descriptive statistics, namely medians and percentiles, were calculated for numerical variables. Frequencies and percentages were calculated for categorical data.
Ethical considerations
The protocol was approved by the Health Sciences Research Ethics Committee, Faculty of Health Sciences, UFS (ref. no. UFS-HSD2020/0654/3006). Permission to conduct the study was obtained from the Free State Department of Health and the Head of Paediatric Surgery and Neonatology at UAHC. Identifying patient details were not recorded on any of the collection sheets. Data were handled confidentially and captured and stored on a password-protected computer.
Results
Patient profile
In total, 67 patients were included in the study population, with a median age of 107 days. Most patients were female (74.6%, 3:1 female/ male ratio) and of black African ethnicity (86.6%), and originated from the Free State (61.2%). While 26.9% were exposed to HIV, only 1 (1.5%) tested HIV positive (Table 1).
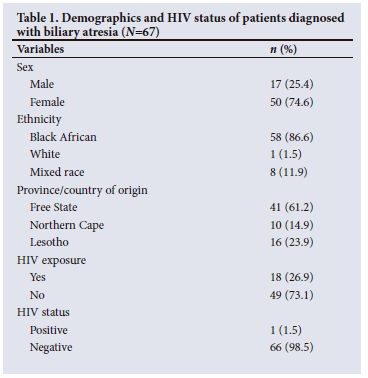
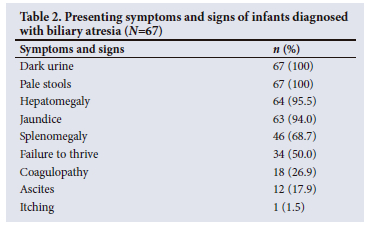
Table 2 summarises the patients' signs and symptoms. All the patients presented with pale stools and dark urine, 94.0% with jaundice and 95.5% with hepatomegaly. No patient presented with an upper gastrointestinal tract bleed.
With regard to the type of BA, 42 patients (62.7%) had IBA and 2 (3.0%) had BASM. In 23 (52.3%) of those tested (n=44), CMV IgM was positive. In addition to the presenting signs and symptoms, 15 patients (22.4%) had one or more other congenital abnormality or abnormalities. These included umbilical hernia (n=11), cardiac lesions (n=3), polysplenia (n=2), malrotation (n=2), situs inversus (n=1), intestinal atresia (n=1) and inguinal hernia (n=1), while 5 patients had other abnormalities. No patient presented with asplenia, interrupted inferior vena cava, anorectal malformation, preduodenal portal vein, syndromes, or renal, limb or spinal abnormalities.
Table 3 presents the median, minimum and maximum results from the preoperative and/or diagnostic tests.
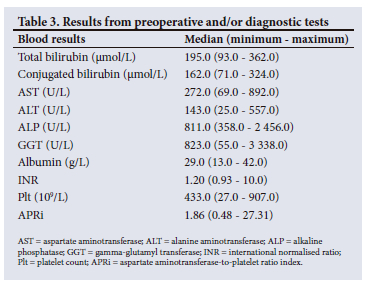
Table 4 summarises the findings of diagnostic investigations performed before KPE or at the time of diagnosis if no KPE was done. Twenty-three patients (34.9%) tested CMV IgM positive, 21 (31.8%) tested negative, and 22 (33.3%) were not tested. Results for 1 patient were missing.
Histological findings
A liver biopsy with histological evaluation was done in 61 patients (91.0%), of whom 30 (49.2) had a METAVIR scored of F2 (periportal fibrosis), 11 (18.0%) a score of F3 (septal fibrosis), and 15 (24.6%) a score of F4 (cirrhosis). The median portal plate ductule diameter in those who received a KPE was 102.5 μm (range 0 - 750 μm).
Ultrasound findings
An ultrasound scan was done in 63 out of 66 patients (95.5%). An absent/small gallbladder was observed in 51 patients (81.0%), in 28 (44.4%) the common bile duct was not visualised, and in 6 (9.5%) a triangular cord sign was seen. The ultrasound examination was inconclusive for BA in 12 patients (19.0%).
Management
KPE was performed in 32 patients (47.8%), while 35 (52.2%) did not have the procedure. Five patients (15.6%) had an intraoperative cholangiogram; in the rest, BA was evident visually. Twenty-seven (84.4%) of the patients who had a KPE passed pigmented stool during the early postoperative period (<30 days). Five patients (15.6%) had no evidence of bile flow postoperatively. Only 3 patients (9.4%) had jaundice clearance (total bilirubin <20 μmol/L).[43] The procedures were performed by a senior consultant in 20 cases (62.5%) and by a junior consultant in 12 (37.5%).
Table 4 compares the data on the patients who received KPE and those who did not. Statistically significant differences between the two groups were only found for age (p<0.0001), CMV IgM positive (p=0.025), aspartate aminotransferase-to-platelet ratio index (p=0.0002), METAVIR score (p<0.0001), and type of BA (p=0.009).
Five patients (7.5%) were referred for evaluation for an LT. Of these, 2 (3.0% of the total study population) received an LT. For the 62 patients (92.5%) who were not referred for evaluation for an LT, the following reasons were cited (in some cases, more than one reason applied): family and social problems (n=18; 29.0%), patients from Lesotho without medical aid (n=15; 24.2%), LTFU (n=10; 16.1%), end-stage liver disease (n=9; 14.5%), financial problems (n=8; 12.9%), unreliable and regularly misses clinic visits (n=6; 9.7%), and other (n=7; 11.3%).
Outcome
Intraoperative and postoperative complications after KPE
• No patient had any intraoperative complications. The postoperative complications were divided into short, medium and long term. Some patients had more than one medium- or long-term complication.
• Ten patients (31.3%) had short-term complications (<30 days): cholangitis (n=7), cessation of bile flow (n=1), postoperative bleeding (n=1) and surgical site infections (n=1).
• Fourteen patients (43.8%) had medium-term complications (30 days - 6 months): cholangitis (n=6), cessation of bile flow (n=10) and portal hypertension (n=6).
• Twenty-six patients (81.3%) had long-term complications (>6 months): cholangitis (n=3), portal hypertension (n=9) and liver failure (n=23).
Overall mortality
Of the 55 patients for whom the outcome was known (12 patients (17.9%) were LTFU, and their outcome is unknown), 52 (94.5%) died after receiving a KPA or palliative treatment only, and 3 (5.5%) were alive at the end of the study period. Of the three survivors, one had a KPE (1-year NLSR), and 2 had LTs (without a prior KPE) (1 - 5-year survival 100%).
Of the patients who received a KPE and for whom the outcome is known (n=26), 25 (96.2%) died and 1 (3.8%) was alive. Unfortunately, 6 patients (18.8%) were LTFU. Of the patients who did not receive a KPE and for whom the outcome is known (n=29), 27 (93.1%) died and 2 (6.9%) were alive. There were 6 patients (20.7%) LTFU in this group.
Discussion
Patient profile
The median age at presentation in this study was 107 days, which is notably higher than in countries where screening programmes with stool colour charts (China, Taiwan and Japan)[44] and educational programmes (UK)[16,45] have been implemented, where the age at presentation is often <60 days. The median age at presentation in this study is also higher than in other SA studies: 70 days at RXWMCH[36] and 89 days at Tygerberg Hospital (TBH).[46] However, the results do align with SA studies at Steve Biko Academic Hospital (SBAH) (112 days)[40] and an earlier survey at Chris Hani Baragwanath Academic Hospital (95.5 days).[41] These results could be explained by the late presentations of our study cohort due to poor screening or lack of knowledge about the disease. Furthermore, the trans-border and trans-provincial referrals involving long distances may also delay presentation at UAHC.
Concerning HIV, only one patient in the present study tested positive, while a quarter (26.9%) were HIV exposed. These results differ markedly from the TBH survey, where only 7.5% of the BA patients were exposed.[37] This is probably due to a higher HIV infection rate in the areas served by UAHC (13.9 - 50%)[47] than in those served by TBH, which is in Western Cape Province (12.6%).[47] The higher HIV exposure rate in our patient cohort may contribute to the poor outcome in the present study in comparison with areas where the HIV exposure rate is lower. Research on the immunosuppressive effect of HIV infection or exposure and the use of prophylactic antiretroviral drugs on BA is needed.
Of the patients tested in this study, 52.3% had CMV-associated BA. One-third of the patients were not tested, especially those in the earlier study period when CMV-associated BA had not yet been subclassified.[19] The prevalence may therefore be even higher. The findings in this study differ markedly from figures reported in research from European countries (<10%).[19,21] However, they are similar to those from China (60%)[48] and TBH (51%),[37] with a high rate of severe early liver damage in these patients. This difference could be explained by the fact that only 1% of neonates are CMV infected in high-income countries, while up to 90% CMV IgG positivity has been demonstrated in LMICs and LICs.[37] The empirical use of antiviral therapy (AVT) (ganciclovir) has been suggested for CMV IgM-positive BA patients to improve the prognosis. However, studies in this regard are limited.[12,20] There is insufficient evidence to support adjuvant AVT for this subgroup, and the decision currently depends on the clinicians.[12,20] Although there is no clear consensus, our patient population in this subgroup may benefit from empirical AVT therapy.
Since at presentation just over half (52.2%) of this study population already had features of incipient or established cirrhosis or were of advanced age (>120 days), no KPE was offered to them. These patients comprised a higher proportion than those in other SA (RXWMCH 2.5%,[36] TBH 37.8%,[46] SBAH 37%[40]) and international studies (<10%).[16-21,33] This relatively high figure could be explained by the late presentation of patients to UAHC. In addition, the faster progression of liver disease could also be attributed to the high prevalence of HIV exposure and the high proportion of CMV IgM-positive patients in this study.
Management
Almost half (47.8%) of the patients in the present study had a KPE procedure, at a median age of 76 days. These results contrast with those from both the West and the East, where almost all patients received a KPE well before 70 days.[16-21,33,44,45] Our findings are also less favourable than those from RXWMCH (77.5% operated on, at a median age of 68 days)[36] and TBH (62.2% operated on, at a median age of 76 days).[46] However, they are slightly better than those from SBAH (47.7% operated on, at a median age of 91 days).[40] According to a study by Davenport et al.,[49] a third of patients who undergo a KPE after 100 days of life may benefit. The decision not to perform a KPE should therefore not solely be determined by the patient's age. More patients in our cohort would have had the advantage of receiving a KPE at an early age if they had presented to our unit sooner.
More than 80% of the patients in the present study passed pigmented stools after KPE, which is similar to international findings (~75%),[18-20,33,34,44,45] despite portal plate ductule diameters (median 102.5 μm) that were smaller than the optimal size for bile drainage (>150 μm).[50] The same two paediatric surgeons did the procedures, with a senior consultant performing two-thirds. These results show that the technical expertise to perform the KPE is on par with international standards. Additionally, adjuvant therapy comprising steroids, ursodeoxycholic acid (when available), phenobarbitone, fat-soluble vitamins and prophylactic antibiotics is given to all post-KPE patients. Despite these treatments, only ~10% had jaundice clearance. Furthermore, the short- to medium-term jaundice clearance rate in the present study is worse than rates in international (~55%)[19,20,33,34,44,45] and local studies (14 - 39%).[36,40,41] Possible explanations for the poor jaundice clearance in our cohort may be the high post-KPE cholangitis rate and the high number of patients with CMV-associated BA, both poor prognostic factors.[12,19,20,37,51]
In the present study, only a minority (7.5%) of the patients were referred for evaluation for a possible LT, of whom 2 (3.0% of the total cohort) received one. These results are very dissatisfying. In international studies (42 - 88%),[2,26-29] and studies from RXWMCH (22.2%)[36] and SBAH (29%),[40] a much higher proportion received an LT. The main reasons for not being referred for evaluation in the present study were socioeconomic factors, Lesotho citizenship, LTFU, and non-compliance with treatment and follow-up visits. These findings are in line with those from other SA studies.[36,40]
Outcome
Approximately half of the patients in the present study suffered from cholangitis after KPE, despite postoperative adjuvant therapy, as mentioned earlier. This burden is in line with international and local studies (40 - 90%).[36,46] Cholangitis is a dreaded complications of KPE because it promotes ongoing fibrosis and obstructs bile flow, significantly worsening the NLSR and overall survival rate (OSR).[12,51] It most commonly occurs in the first 2 years after KPE. The mechanism is probably ascending cholangitis via the Roux loop into the intrahepatic duct system. Additional factors include a change in the gut microbiome with bacterial overgrowth, translocation, and haematogenous spread via the portal vein.[12] Prophylactic antibiotic practices to try to reduce the incidence of cholangitis are variable, with no consensus regarding the duration of treatment needed or how beneficial it truly is.[12] The most common combination in European practice is intravenous piperacillin-tazobactam, gentamicin and oral co-trimoxazole.[12] Changing our regimen to the above drugs in the interim until we have determined the bacterial profile and antibiotic sensitivity for cholangitis in our patient population, and lengthening the duration of treatment with prophylactic antibiotics for up to 1 year, could be beneficial.
By the end of the study period, only 3 patients were alive; 1 still had a native liver, and 2 had received an LT. This 5-year OSR of 5.5% contrasts sharply with international and local studies (55 - 95%).[31,36] The poor outcomes in this study need urgent improvement by addressing the detrimental factors identified. This action should include implementing screening and education programmes in the primary health sector to timeously identify and refer patients to UAHC for KPE to benefit from an improved NLSR. Furthermore, prevention of cholangitis in the post-KPE period and support systems (including socioeconomic) to improve patient compliance should be a priority.
Study limitations
Owing to its retrospective design, this study is limited by the lack of information in the patient files. Certain details were not documented, and specific investigations were not done in all the patients, as would have been the case in a prospective study. It was impossible to determine the outcome of patients who were LTFU. Prospective studies could address these limitations, including all the necessary special investigations (e.g. CMV work-up) and patient follow-up to better determine the effect of risk factors on outcome. Despite these limitations, the cohort was large enough to highlight essential observations (a high HIV exposure rate and a high prevalence of CMV IgM-positive cases) and shortcomings (late patient presentation, high cholangitis rate, socioeconomic burdens, LTFU) and make practical recommendations (change antibiotic drugs, extend the antibiotic prophylaxis period, and determine the local bacterial profile and sensitivity). We suggest that future studies focus on the effect of HIV infection or exposure and antiretroviral drug use on BA. The high CMV IgM-positive rate identified by the study requires more research on adjuvant AVT in this subgroup to obtain a clear consensus on its empirical use and benefits.
Recommendations for practice
This study has highlighted some important practical implications for policymakers. The first is the need to implement screening and education programmes at the primary healthcare level to diagnose and refer BA patients timeously, as proven effective by European and Asian programmes. Secondly, there is a significant need for support systems to help socioeconomically disadvantaged patients qualify for an LT evaluation. For treating physicians, there are therapeutic implications regarding adjuvant therapy. The use of empirical AVT may be beneficial for CMV IgM-positive patients and should be considered. In addition, post-KPE antibiotics should be changed to the currently recommended antibiotic regimens (intravenous piperacillin-tazobactam, gentamicin), the duration of cholangitis antibiotic prophylaxis (oral co-trimoxazole) should be increased to 1 year, and the bacterial profile and sensitivity for post-KPE cholangitis in our patient population should be determined.
Conclusion
This study illuminates critical challenges in diagnosing and treating BA patients, encompassing issues such as delayed patient presentations, a notable prevalence of CMV-associated BA, and socioeconomic impediments hindering liver transplantation evaluations. Emphasising the significance of early screening and educational initiatives for expediting BA patient identification and referrals and tackling socioeconomic barriers emerges as a vital imperative for advancing outcomes. Moreover, the study proposes potential refinements in management, including adjustments to antibiotic prophylaxis protocols and prolonging treatment duration to prevent cholangitis. Exploring AVT for CMV IgM-positive patients is a promising avenue. Despite inherent limitations, the study's recommendations offer valuable insights for policymakers, healthcare providers and researchers striving to elevate the standard of BA patient care in SA and similar regions, potentially leading to improved prognoses and an enhanced quality of life for affected children.
Declaration. None.
Acknowledgements. The authors acknowledge K F Rivele, E S Yapi, X L Wesenaar, L A Mlungwana, S Mabalo, K C Mashego, S Sosibo and A Mabaso, undergraduate students in the Faculty of Health Sciences, University of the Free State, for writing the initial draft of the protocol and article, C van Rooyen, biostatistician, Faculty of Health Sciences, University of the Free State, for performing the initial data analysis and planning and assisting with the interpretation, and Ms T Mulder, medical editor/writer, Faculty of Health Sciences, University of the Free State, for the technical and editorial preparation of the manuscript.
Author contributions. EB supervised the study, suggested the concept, assisted with the protocol development, collected and interpreted the data, and wrote the final draft of the manuscript. SMlG assisted with the planning and data interpretation and critical evaluation of the manuscript. Both authors approved the final version of the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Davenport M. Biliary atresia. Semin Pediatr Surg 2005;14(1):42-48. https://doi.org/10.1053/j.sempedsurg.2004.10.024 [ Links ]
2. Superina R. Biliary atresia and liver transplantation: Results and thoughts for primary liver transplantation in select patients. Pediatr Surg Int 2017;33(12):1297-1304. https://doi.org/10.1007/s00383-017-4174-4 [ Links ]
3. Lin YC, Chang MH, Liao SF, et al. Decreasing rate of biliary atresia in Taiwan: A survey, 2004 - 2009. Pediatrics 2011;128(3):e530-e536. https://doi.org/10.1542/peds.2011-0742 [ Links ]
4. Wada H, Muraji T, Yokoi A, et al. Insignificant seasonal and geographical variation in incidence of biliary atresia in Japan: A regional survey of over 20 years. J Pediatr Surg 2007;42(12):2090-2092. https://doi.org/10.1016/j.jpedsurg.2007.08.035 [ Links ]
5. Livesey E, Cortina Borja M, Sharif K, et al. Epidemiology of biliary atresia in England and Wales (1999 - 2006). Arch Dis Child Fetal Neonatal Ed 2009;94(6):F451-F455. https://doi.org/10.1136/adc.2009.159780 [ Links ]
6. Chardot C, Carton M, Spire-Bendelac N, le Pommelet C, Golmard JL, Auvert B. Epidemiology of biliary atresia in France: A national study 1986 - 96. J Hepatol 1999;31(6):1006-1013. https://doi.org/10.1016/s0168-8278(99)80312-2 [ Links ]
7. Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI. International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr 2013;56(4):344-354. https://doi.org/10.1097/MPG.0b013e318282a913 [ Links ]
8. Hopkins PC, Yazigi N, Nylund CM. Incidence of biliary atresia and timing of hepatoportoenterostomy in the United States. J Pediatr 2017;187:253-257. https://doi.org/10.1016/j.jpeds.2017.05.006 [ Links ]
9. Chiu CY, Chen PH, Chan CF, Chang MH, Wu TC; Taiwan Infant Stool Color Card Study Group. Biliary atresia in preterm infants in Taiwan: A nationwide survey. J Pediatr 2013;163(1):100-3.e1. https://doi.org/10.1016/j.jpeds.2012.12.085 [ Links ]
10. Van Wessel DB, Boere T, Hulzebos CV, et al. Preterm infants with biliary atresia: A nationwide cohort analysis from the Netherlands. J Pediatr Gastroenterol Nutr 2017;65(4):370-374. https://doi.org/10.1097/MPG.0000000000001692 [ Links ]
11. Tam PKH, Yiu RS, Lendahl U, Andersson ER. Cholangiopathies - towards a molecular understanding [published correction appears in EBioMedicine 2018 Oct;36:564]. EBioMedicine 2018;35:381-393. https://doi.org/10.1016/j.ebiom.2018.08.024 [ Links ]
12. Burns J, Davenport M. Adjuvant treatments for biliary atresia. Transl Pediatr 2020;9(3):253-265. https://doi.org/10.21037/tp.2016.10.08 [ Links ]
13. Davenport M. Biliary atresia: From Australia to the zebrafish. J Pediatr Surg 2016;51(2):200-205. https://doi.org/10.1016/j.jpedsurg.2015.10.058 [ Links ]
14. Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: Current concepts. J Pediatr Gastroenterol Nutr 2003;37(1):4-21. https://doi.org/10.1097/00005176-200307000-00003 [ Links ]
15. Harpavat S, Finegold MJ, Karpen SJ. Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics 2011;128(6):e1428-e1433. https://doi.org/10.1542/peds.2011-1869 [ Links ]
16. Davenport M, Savage M, Mowat AP, Howard ER. Biliary atresia splenic malformation syndrome: An etiologic and prognostic subgroup. Surgery 1993;113(6):662-668. [ Links ]
17. Asai A, Miethke A, Bezerra JA. Pathogenesis of biliary atresia: Defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol 2015;12(6):342-352. https://doi.org/10.1038/nrgastro.2015.74 [ Links ]
18. Schwarz KB, Haber BH, Rosenthal P, et al. Extrahepatic anomalies in infants with biliary atresia: Results of a large prospective North American multicenter study. Hepatology 2013;58(5):1724-1731. https://doi.org/10.1002/hep.26512 [ Links ]
19. Zani A, Quaglia A, Hadzic N, Zuckerman M, Davenport M. Cytomegalovirus-associated biliary atresia: An aetiological and prognostic subgroup. J Pediatr Surg 2015;50(10):1739-1745. https://doi.org/10.1016/j.jpedsurg.2015.03.001 [ Links ]
20. Parolini F, Hadzic N, Davenport M. Adjuvant therapy of cytomegalovirus IgM + ve associated biliary atresia: Prima facie evidence of effect. J Pediatr Surg 2019;54(9):1941-1945. https://doi.org/10.1016/j.jpedsurg.2018.12.014 [ Links ]
21. Lakshminarayanan B, Davenport M. Biliary atresia: A comprehensive review. J Autoimmun 2016;73:1-9. https://doi.org/10.1016/j.jaut.2016.06.005 [ Links ]
22. Kaur N, Goyal G, Garg R, Tapasvi C, Chawla S, Kaur R. Potential role of noninvasive biomarkers during liver fibrosis. World J Hepatol 2021;13(12):1919-1935. https://doi.org/10.4254/wjh.v13.i12.1919 [ Links ]
23. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344(7):495-500. https://doi.org/10.1056/NEJM20010215344070 [ Links ]
24. Capparelli MA, Ayarzabal VH, Halac ET, et al. Preoperative risk factors for the early failure of the Kasai portoenterostomy in patients with biliary atresia. Pediatr Surg Int 2021;37(9):1183-1189. https://doi.org/10.1007/s00383-021-04933-y [ Links ]
25. El-Araby HA, Saber MA, Radwan NM, Taie DM, Adawy NM, Sira AM. Temporal histopathological changes in biliary atresia: A perspective for rapid fibrosis progression. Ann Hepatol 2021;21:100263. https://doi.org/10.1016/j.aohep.2020.09.007 [ Links ]
26. Chardot C, Buet C, Serinet MO, et al. Improving outcomes of biliary atresia: French national series 1986 - 2009. J Hepatol 2013;58(6):1209-1217. https://doi.org/10.1016/j.jhep.2013.01.040 [ Links ]
27. Bondoc AJ, Taylor JA, Alonso MH, et al. The beneficial impact of revision of Kasai portoenterostomy for biliary atresia: An institutional study. Ann Surg 2012;255(3):570-576. https://doi.org/10.1097/SLA.0b013e318243a46e [ Links ]
28. Kakos CD, Ziogas IA, Alexopoulos SP, Tsoulfas G. Management of biliary atresia: To transplant or not to transplant. World J Transplant 2021;11(9):400-409. https://doi.org/10.5500/wjt.v11.i9.400 [ Links ]
29. Scottoni F, Davenport M. Biliary atresia: Potential for a new decade. Semin Pediatr Surg 2020;29(4):150940. https://doi.org/10.1016/j.sempedsurg.2020.150940 [ Links ]
30. Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: A rational basis for biliary atresia screening. Pediatrics 2009;123(5):1280-1286. https://doi.org/10.1542/peds.2008-1949 [ Links ]
31. Chung PHY, Zheng S, Tam PKH. Biliary atresia: East versus West. Semin Pediatr Surg 2020;29(4):150950. https://doi.org/10.1016/j.sempedsurg.2020.150950 Taylor SA, Venkat V, Arnon R, et al. Improved outcomes for liver transplantation in patients with biliary atresia since pediatric end-stage liver disease implementation: Analysis of the Society of Pediatric Liver Transplantation Registry. J Pediatr 2020;219:89-97. https://doi.org/10.1016/j.jpeds.2019.12.023 [ Links ]
32. Nio M, Ohi R, Miyano T, et al Five- and 10-year survival rates after surgery for biliary atresia: A report from the Japanese Biliary Atresia Registry. J Pediatr Surg 2003;38(7):997-1000. https://doi.org/10.1016/s0022-3468(03)00178-7 [ Links ]
33. Davenport M, Ong E, Sharif K, et al. Biliary atresia in England and Wales: Results of centralisation and new benchmark. J Pediatr Surg 2011;46(9):1689-1694. https://doi.org/10.1016/j.jpedsurg.2011.04.013 [ Links ]
34. Davenport M, Grieve A. Maximizing Kasai portoenterostomy in the treatment of biliary atresia: Medical and surgical options. S Afr Med J 2012;102(11 Pt 2):865-867. https://doi.org/10.7196/SAMJ.6120 [ Links ]
35. Levin LN. Biliary atresia at Red Cross War Memorial Children's Hospital: A retrospective descriptive study reviewing the age of presentation, clinical course and outcome of infants presenting to RCWMCH with biliary atresia. Master's thesis. Cape Town: Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, 2016. http://hdl.handle.net/11427/22822 (accessed 30 October 2019). [ Links ]
36. Moore SW, Zabiegaj-Zwick C, Nel E. Problems related to CMV infection and biliary atresia. S Afr Med J 2012;102(11 Pt 2):890-892. https://doi.org/10.7196/SAMJ.6163 [ Links ]
37. Spearman CW, McCulloch M, Millar AJ, et al. Liver transplantation at Red Cross War Memorial Children's Hospital. S Afr Med J 2006;96(9 Pt 2):960-963. [ Links ]
38. Lala SG, Britz R, Botha J, Loveland J. Paediatric liver transplantation for children treated at public health facilities in South Africa: Time for change. S Afr Med J 2014;104(11):829-832. https://doi.org/10.7196/SAMJ.8624 [ Links ]
39. Van der Schyff F, Terblanche AJ, Botha JF. Improving poor outcomes of children with biliary atresia in South Africa by early referral to centralised units. JPGN Rep 2021;2(2):e073 https://doi.org/10.1097/PG9.0000000000000073 [ Links ]
40. Zuckerman M, Hajinicolaou C. Incidence and outcome of biliary atresia in black infants in Soweto (South Africa): Review of cases from 1993 - 1996. J Pediatr Gastroenterol Nutr 1998;26(5):587. [ Links ]
41. The French METAVIR Cooperative Study Group, Bedossa P. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20(1):15-20. https://doi.org/10.1002/hep.1840200104 [ Links ]
42. Davenport M, Caponcelli E, Livesey E, Hadzic N, Howard E. Surgical outcome in biliary atresia: Etiology affects the influence of age at surgery. Ann Surg 2008;247(4):694-698. https://doi.org/10.1097/SLA.0b013e3181638627 [ Links ]
43. Lien TH, Chang MH, Wu JF, et al Effects of the infant stool color card screening program on 5-year outcome of biliary atresia in Taiwan. Hepatology 2011;53(1):202-208. https://doi.org/10.1002/hep.24023 [ Links ]
44. Alonso EM, Ye W, Hawthorne K, et al. Impact of steroid therapy on early growth in infants with biliary atresia: The Multicenter Steroids in Biliary Atresia Randomised Trial. J Pediatr 2018;202:179-185.e4. https://doi.org/10.1016/j.jpeds.2018.07.002 [ Links ]
45. Karangwa OR. A retrospective review of the outcome of children presenting to Tygerberg Children's Hospital with biliary atresia. PhD thesis. Cape Town: Stellenbosch University, 2016. https://www.semanticscholar.org/paper/A-retrospective-review-of-the-outcome-of-children-Karangwa/bfff8b317d009a9a79a438126be21887ba3b4989 (accessed 4 October 2023). [ Links ]
46. Human Sciences Research Council. The Fifth South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017. Pretoria: HSRC, 2018. https://www.hsrc.ac.za/uploads/pageContent/9234/SABSSMV_Impact_Assessment_Summary_ZA_ADS_cleared_PDFA4.pdf (accessed 29 May 2019). [ Links ]
47. Xu Y, Yu J, Zhang R, et al. The perinatal infection of cytomegalovirus is an important etiology for biliary atresia in China. Clin Pediatr (Phila) 2012;51(2):109-113. https://doi.org/10.1177/0009922811406264 [ Links ]
48. Davenport M, Puricelli V, Farrant P, et al. The outcome of the older (>100 days) infant with biliary atresia. J Pediatr Surg 2004;39(4):575-581. https://doi.org/10.1016/j.jpedsurg.2003.12.014 [ Links ]
49. Sanghai SR, Shah I, Bhatnagar S, Murthy A. Incidence and prognostic factors associated with biliary atresia in western India. Ann Hepatol 2009;8(2):120-122. [ Links ]
50. Koga H, Wada M, Nakamura H, et al. Factors influencing jaundice-free survival with the native liver in post-portoenterostomy biliary atresia patients: Results from a single institution. J Pediatr Surg 2013;48(12):2368-2372. https://doi.org/10.1016/j.jpedsurg.2013.08.007 [ Links ]
 Correspondence:
Correspondence:
E Brits
lizettebrits@gmail.com
Accepted 18 September 2023














