Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.11 Pretoria Nov. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i11.1089
RESEARCH
Dyslipidaemia in patients with chronic kidney disease - a neglected cardiovascular risk factor
M R EssopI; F SeedatII; F J RaalIII
IMB ChB, MMed (Int Med); Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB ChB, MMed (Int Med); Department of Internal Medicine and Division of Endocrinology, Faculty of Health Sciences, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
IIIPhD, DSc; Department of Internal Medicine and Division of Endocrinology, Faculty of Health Sciences, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Atherosclerotic cardiovascular disease (ASCVD) is a major cause of morbidity and mortality in patients with chronic kidney disease (CKD). In addition, CKD itself is a coronary artery disease equivalent due to its atherogenic potential. Despite the role of CKD in ASCVD and recommendations to control lipid levels aggressively, landmark lipid studies have often excluded patients with advanced CKD. Furthermore, there is a scarcity of data on the use and efficacy of lipid-lowering therapy (LLT) in those with CKD in South Africa (SA
OBJECTIVES: To determine the prevalence and control of dyslipidaemia in a cohort of SA patients with CKD
METHODS: A retrospective, cross-sectional observational study of 250 patients with CKD attending the Charlotte Maxeke Johannesburg Academic Hospital renal clinic from 1 July 2019 to 31 July 2020 was carried out. Lipograms, the use of LLT and achievement of target lipid levels were examined
RESULTS: The median (interquartile range) age of this cohort was 58 (46 - 69) years; 50.4% were males and 64.4% black African. Dyslipidaemia was prevalent in 83.6% (n=209) of patients. A total of 169 (67.6%) patients were on LLT, and of these only 28 (16.6%) achieved the recommended low-density lipoprotein cholesterol (LDL-C) target. Of those not on LLT, 51 (63%) were eligible for LLT and almost all were classified as either very high risk (64.2%) or high risk (28.4%) for ASCVD. Of those on LLT, all were on statin therapy, of which simvastatin at a mean dose of 20 mg daily was the most commonly prescribed LLT
CONCLUSION: This cohort comprised a large proportion of patients classified as high or very high risk for ASCVD. Despite this, the use of LLT was inadequate, and <20% of patients were at target LDL-C levels. These data suggest a greater need for awareness of initiating LLT to achieve recommended target LDL-C levels in patients with CKD
Atherosclerotic cardiovascular disease (ASCVD), including cerebrovascular disease, coronary artery disease, peripheral vascular disease and aortic aneurysm,[1] contributes significantly to global morbidity and mortality, and is the leading cause of death in both developed and developing countries worldwide.[2] In 2019, ASCVD was responsible for 32% (17.9 million) of deaths globally,[2] and in South Africa (SA) in 2014, ASCVD was responsible for 215 deaths daily, second only to HIV/AIDS.[3]
Dyslipidaemia is a major risk factor for ASCVD. In a recent SA study, 67% of the rural population met criteria for dyslipidaemia, while <1% were receiving lipid-lowering therapy (LLT).[4] The Dyslipidaemia International Study (DYSIS) revealed that 50% of SA patients on statin therapy were not at low-density lipoprotein cholesterol (LDL-C) target, and 74% of these patients were at high or very high risk for ASCVD.[5] Furthermore, against the background of a severely constrained public health system where socioeconomic factors limit access to healthcare, it is likely that the extent of dyslipidaemia is underestimated. Many factors contribute to the growing burden of dyslipidaemia in SA. Increasing urbanisation has resulted in reduced physical activity and greater exposure to unhealthy diets.[6] Dyslipidaemia also co-exists with traditional risk factors for ASCVD (diabetes, hypertension, smoking, obesity and gout) as well as HIV and antiretroviral therapy (ART).[6]
Chronic kidney disease (CKD) is a risk factor for ASCVD, with a 10-fold higher prevalence of ASCVD in CKD. Moreover, the risk of ASCVD increases progressively as renal function declines.[7] Of all deaths among those with end-stage renal disease (ESRD), at least 50% are related to ASCVD.[7] Existing data show that patients with CKD have a much higher incidence, prevalence and severity of ASCVD, with the incidence of de novo cardiovascular disease in patients with CKD reported as 41% in males and 19% in females, respectively.[8] The incidence of myocardial infarction was 2.5 times higher in men with CKD, while the prevalence and severity of cardiovascular disease are increased with deterioration in renal function.[8] The Atherosclerosis in Communities Study (ARIC) highlighted the fact that the incidence of cardiovascular disease was 4.8% in stage 2 CKD, and increased to 9.3% in stage 3 - 4 CKD.[9] The ARIC study also noted an increased incidence of recurrent cardiovascular events with the decline in renal function.[9] Current guidelines recommend lipid-lowering therapy (LLT) for all patients with CKD. However, these recommendations are heterogenous across the guidelines. The SA Dyslipidaemia Consensus Statement recommends the management of dyslipidaemia based on the European Society of Cardiology (ESC) guidelines.[10] The ESC recommends initiating a statin with or without ezetimibe in all patients with stage 3 - 5 CKD that are non-dialysis dependent.[11] In dialysis-dependent patients already on a statin with or without ezetimibe, treatment should be continued.[11] LLT is not routinely advised for patients with ESRD on dialysis who have low ASCVD risk.[11] Treatment targets are based on stratification scoring systems that divide patients into categories of risk. Patients at very high risk include CKD with an estimated glomerular filtration rate (eGFR) under 30 mL/min/1.73m2.[11] The therapeutic goal is to achieve an LDL-C <1.4 mmol/L and a 50% reduction in LDL-C from baseline.[11] Patients with a eGFR between 30 and 60 mL/min/1.73m2 are considered high risk, and a reduction of LDL-C to <1.8 mmol/L and 50% reduction from baseline is recommended.[11] Patients considered to be at moderate risk include young patients with diabetes (type 1 diabetes aged <35 years and type 2 diabetes aged <50 years) with a duration of disease <10 years and no other ASCVD risk factors and a systematic coronary estimation score (SCORE) between 1% and 5% for 10-year ASCVD risk. In these patients, a target LDL-C of <2.6 mmol/L is advised. For patients at a low 10-year ASCVD risk (based on a SCORE <1%) and an LDL-C of <3 mmol/L, LLT is recommended.[11] In the 2020 SA dyslipidaemia guidelines, CKD is considered to be high risk if the eGFR is 30 - 59 mL/min/1.73m2, and very high risk if the eGFR is <30 mL/ min/1.73m2.[6] The recommended LDL-C targets are in keeping with those of the ESC. The recommended dose of statin is based on the target LDL-C, starting LDL-C level and the percentage reduction in LDL-C required in order to achieve target.[6] For patients at high to very high risk, high-intensity statins such as atorvastatin 80 mg or rosuvastatin 40 mg daily are recommended.[6] The American College of Cardiology (ACC) advises the use of a statin in all patients with CKD stage 1 - 5 not on haemodialysis, and in those who have had a renal transplant.[12] The ACC goal of LLT in patients at high risk of ASCVD is also a 50% reduction in LDL-C from baseline.[12] The ACC also recommends continuing statin treatment in those patients on dialysis who are currently receiving a statin, but advises against initiating statin therapy in patients requiring dialysis who are not already on LLT.[12] The Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend a statin for all CKD patients >50 years old and in those <50 years with the presence of an additional risk factor for coronary artery disease.[7,13] Similar to the ACC, statins are not recommended for patients with ESRD requiring dialysis unless they have already been initiated on treatment.[13] This study aimed to describe the prevalence, severity and pattern of dyslipidaemia among CKD patients in a quaternary SA hospital. Furthermore, the study aimed to determine the proportion of CKD patients on LLT, whether CKD patients on LLT were at appropriate targets and to evaluate the type and dose of LLT used to treat dyslipidaemia.
Methods
We conducted a cross-sectional retrospective review of 250 patients with CKD attending Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) renal clinic from 1 July 2019 to 31 July 2020. Criteria for inclusion included the following: documented CKD, age >18 years, and a lipogram performed within the last year. Patient records were selected at random based on fulfilment of eligibility criteria and complete clinic records. Clinic files were used to obtain patients' age, gender, race, weight, height and whether a patient was on renal replacement therapy. Haematology and biochemistry results were extracted and analysed. These included: haemoglobin, mean corpuscular volume, albumin, urea and electrolytes, creatinine, eGFR, corrected calcium, magnesium, phosphate, TC, LDL-C, high-density lipoprotein (HDL)-C, triglycerides, HbA1c and urine albumin to creatinine ratio or urine protein to creatinine ratio. Comorbidities including diabetes, hypertension, smoking, alcohol use, HIV status, obesity, dyslipidaemia and gout were noted. Complications such as stroke, peripheral vascular disease and coronary artery disease were recorded. The use of immunosuppressive agents, steroids and LLT was noted, in addition to the dose and duration of treatment.
Ethics approval was obtained from the Human Research Ethical Committee of the University of the Witwatersrand (ref. no. M200760). Consent was obtained from the head of the Department of Medicine to access clinic files. The data obtained were only made available to the researcher, statistician and supervisors. To ensure confidentiality, each data sheet was assigned a study number with no record of name or any other personal identifier.
Statistical analysis
Completed data extraction forms were entered into an Excel (Microsoft Corp., USA) spreadsheet and subsequently exported into Stata 14.2 (StataCorp, USA) for analysis. The demographic and clinical profiles of patients included were described using descriptive statistics, with frequencies and proportions for categorical data as well as medians and interquartile ranges for continuous variables.
Results
The median (interquartile range) age of the cohort of 250 patients was 58 (46 - 69) years. There was a slight predominance of males (50.4%) who were younger than their female counterparts (male median age 57.5 v. 58.5 years in females). The majority of patients were black African (64%), followed by white (23%). The leading causes of CKD were hypertension (76.4%), followed by type 2 diabetes mellitus (T2DM) (32%). Hypertension (83%), T2DM (35%) and obesity (23%) were the most common comorbidities. The majority of patients on LLT were males (72%) as opposed to females (62%). The mean eGFR was 29 mL/min/1.73m2. The most common stage of CKD was stage 4 (30%), followed by stage 3 (27%) and stage 5 (21%).
In this study, the overall prevalence of dyslipidaemia was 84%. Among those with dyslipidaemia, the majority were >50 years (p=0.015), were classified as very high risk (p<0.001) and were at a CKD stage 3 and lower (p<0.001). Dyslipidaemia was most prevalent in mixed-race patients (89%), followed by Indians (87%) and black Africans (83%). Patients not treated with LLT had a high prevalence of dyslipidaemia (84%).
Of the 250 patients, 67% were on LLT. Of those on LLT, 59% were classified as very high-risk, 32.5% as high-risk and 8.3% as low to moderate risk based on the ESC risk score (Table 1). Of those not on LLT, 62.4% were very high risk, 28.4% high risk and 7.4% low to moderate risk, respectively (Table 1). A total of 84% of patients on LLT did not meet the recommended target LDL-C for their risk group. Of the 16% whose LDL-C levels were at target, 10 participants were very high risk and 8 were high risk (Fig. 1).
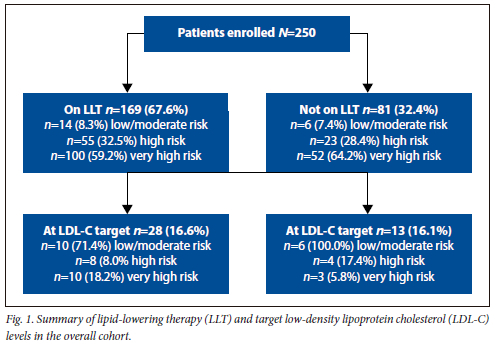
The overall median total cholesterol was 4.3 mmol/L, LDL-C 2.5 mmol/L, HDL-C 1.2 mmol/L and triglyceride 1.3 mmol/L. In the very high-risk and high-risk group, the median total cholesterol and LDL-C were elevated, while the median HDL-C was reduced. In the low- to moderate-risk group the median total cholesterol and LDL-C were lower and median HDL-C higher. Triglycerides varied in all groups, with no consistent pattern (Table 2).
Severe dyslipidaemia was most prevalent in patients considered to be at high risk for ASCVD and patients with progressive renal impairment (Table 3). Other factors such as gender, ethnicity, retroviral status and whether patients were on LLT were not statistically significant.
The most common LLT used was simvastatin (87%) at a median dose of 20 mg. Just 10% of patients received atorvastatin, at a median dose of 30 mg. No patients in this study received dual statin therapy.
Of the 250 patients enrolled in this study, 32.4% did not receive LLT. However, based on the ESC/SA guidelines, 63% of this group patients were eligible for therapy. Most of the statin-eligible patients who did not receive therapy fell into the very high-risk category.
Among the 81 patients not on LLT, 86% met criteria for the use of LLT. By risk categories, two-thirds (64%) were high risk, 28% very high risk and 7% low moderate risk for ASCVD (Table 3). In the high-risk group, just 9.6% had an LDL-C level at the appropriate target of >1.8 mmol/L, and no patients met the LDL-C target of <1.4 mmol/L. A total of 40% had an LDL-C that was between 3 and 4.9 mmol/L, and 35% had an LDL-C level between 1.8 and 2.64 mmol/L. All 6 patients at low to moderate risk were at the appropriate LDL-C target.
Although it is well documented, in particular in terms of protease inhibitors, that ART raises LDL-C, the presence of HIV or its treatment did not appear to have an impact on the degree of dyslipidaemia. In this study, 77 of the 250 patients were HIV positive, with just half of these on ART. However, as numbers were small and the majority of patients were not receiving protease inhibitors, the study may have been underpowered in this regard.
Discussion
The prevalence rate of dyslipidaemia among patients with CKD was notably high, at 83.6%. This figure is higher than previous estimates described in local and international literature, but can be at least partially explained by the lower LDL-C targets now recommended by the ESC.[11] The prevalence of dyslipidaemia in the National Health and Nutrition Examination Survey (NHANES) study was 53.9%.[14] A cross-sectional study by Madala et al.,[15] conducted in KwaZulu-Natal Province in patients with CKD, estimated the prevalence of dyslipidaemia to be 39.1%. As traditionally thought, this study revealed that dyslipidaemia was more common in males, the elderly, patients other than white and patients with HIV infection on ART. The prevalence of dyslipidaemia increased with deterioration in eGFR from CKD stage 1 to 5. Similarly, in the NHANES study, dyslipidaemia in patients with CKD increased from 45% in stage 1 CKD to 67.8% in stage 4. The high prevalence of dyslipidaemia may be attributed to infrequent screening for dyslipidaemia, advanced renal disease and an urban lifestyle that is often sedentary with a diet rich in processed foods.
This study demonstrated the following pattern of dyslipidaemia: an elevated LDL-C relative to the treatment target, and reduced HDL-C, especially in the higher-risk categories. This pattern of dyslipidaemia is similar to that in other studies examining dyslipidaemia in CKD, such as the PREPARE-2 study.[16]
The majority of statin-eligible patients who were not treated were black African, female and elderly. These constitute the usual group of patients in cardiovascular trials who fail to be treated appropriately as recommended by standardised guidelines. This failure may be a reflection of either prejudicial behaviour or a genuine but misguided belief that these are low-risk patients who do not merit therapy. A further factor that may account for the failure to prescribe appropriate statin therapy may relate to financial constraints in the public sector healthcare system that is already under severe pressure by diseases considered more pressing by policy makers, such as HIV and TB. The lack of recognition of CKD as a major risk factor of ASCVD by physician trainees may be a contributing factor, as well as the failure to practise evidence-based medicine published by the ESC and the Lipid and Atherosclerosis Society of Southern Africa (LASSA).
Encouragingly, the majority of patients enrolled in this study were treated with LLT. However, few patients had their treatment escalated to a more potent statin, and even fewer received dual therapy with the addition of ezetimibe or a fibrate. Despite the recommendations by guidelines and lipid societies that the majority of high-risk patients be treated with high-dose atorvastatin or rosuvastatin, this study revealed these agents were infrequently used in those at high or very high risk for ASCVD. This may be a reflection of the lack of availability of these agents in the state sector.
In this analysis, achievement of target LDL-C levels was much lower than in the studies previously mentioned. This may be due in part to the fact that this study was a cohort of patients with CKD as opposed to the comparative studies, none of which specifically examined patients with CKD. The safety and efficacy of high-intensity statin therapy remains a concern in patients with CKD, especially in those with ESRD.[17] Whether the failure to prescribe high-intensity statin therapy was based on physician reluctance could not be determined, although a more likely explanation would be the lack of availability of potent statins in the public sector healthcare setting of SA. A further factor impeding achievement of target LDL-C would be low patient compliance among patients with CKD. Similar to other studies, high cardiovascular risk was found to be a predictor of failure to reach target LDL-C. The reason for this would be the lower target LDL-C in higher-risk patients, making the target more difficult to achieve.
There is a reluctance among physicians to prescribe statin therapy in CKD due to concern for adverse effects, such as hepatotoxicity, myotoxicity and the development of T2DM.[18] Statin therapy has numerous drug interactions that combined with the generation of active metabolites and decreased excretion in patients with CKD may lead to increased risk of adverse side-effects, particularly in the elderly.[18]
Compliance with medical therapy among patients with ESRD in general has been previously documented to be quite low, ranging from 19 to 99%.[19] It is possible that the inability to achieve target LDL-C may also have been related to lack of compliance.
The research conducted has highlighted the severity of dyslipidaemia, even at a quaternary institution such as CMJAH. A follow-up study will be useful to determine whether clinicians are appropriately treating to target, and whether this article has had any impact on LLT and LDL-C. It may be interesting to compare the levels of dyslipidaemia and the dose of statin in patients with other forms of ASCVD, such as acute coronary syndrome, and determine whether patients in cardiology are treated more aggressively.
Study limitations
Although this analysis allowed us to make significant observations, there are some limitations to this study. This was a retrospective observational study that may have entailed certain biases in terms of patient selection and management that we may not have accounted for. Additionally, there were missing data points for patients. Although the overall number of patients included in this study was moderately large, subgroup analysis could not allow definitive conclusions because of relatively small numbers. Only single lipid estimations were obtained at one point in time, with no baseline pre-treatment lipid levels, which may affect observations with respect to eligibility for statin therapy and degree of control. Finally, compliance with statin therapy could not be accessed owing to the study design. In other studies, non-compliance with statin therapy occurred in up to 50% of patients, resulting in an underestimation of the benefits of LLT.[20]
Conclusion
In this retrospective analysis of patients with CKD, a high prevalence of dyslipidaemia was noted. Concerningly, a large proportion of CKD patients who required LLT remained untreated, and even in those on LLT, only a minority achieved recommended LDL-C targets. These findings highlight the need to improve the management of dyslipidaemia in patients with CKD in the SA public sector in order to reduce the burden of ASCVD on the morbidity and mortality in the CKD population.
Declaration. This study was conducted by MRE as part of a MMed (Int Med) degree at the University of the Witwatersrand, and awarded with distinction.
Acknowledgements. We extend our gratitude to the CMJAH renal department for granting us the use of their clinic to conduct the study.
Author contributions. MRE drafted the study protocol, collected and analysed data and wrote the manuscript. The co-authors assisted with manuscript preparation and revision.
Funding. None.
Conflicts of interest. None.
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/ APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol. J Am Coll Cardiol 2019;73(24):e285-350. https://doi.org/10.1016/j.jacc.2018.11.003 [ Links ]
2. World Health Organization. Cardiovascular diseases (CVDs). Geneva: WHO, 2021. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cv (accessed 31 August 2021). [ Links ]
3. Byrne J, Eksteen G, Crickmore C. Heart and stroke foundation South Africa cardiovascular disease statistics reference document. Vlaeberg Heart and Stroke Foundation South Africa, 2016. http://www.heartfoundation.co.za/wp-content/uploads/2017/10/CVD-Stats-Reference-Document-2016-FOR-MEDIA-1.pdf (accessed 1 December 2019). [ Links ]
4. Reiger S, Jardim TV, Abrahams-Gessel S, et al. Awareness, treatment, and control of dyslipidemia in rural South Africa: The HAALSI (Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa) study. PLoS ONE 2017;12(10):e0187347. https://doi.org/10.1371/journal.pone.0187347 [ Links ]
5. Raal FJ, Blom DJ, Naidoo S, Bramlage P, Brudi P. Prevalence of dyslipidaemia in statin-treated patients in South Africa: Results of the DYSlipidaemia International Study (DYSIS). Cardiovasc J Afr 2013;24(8):330-338. https://doi.org/10.5830/cvja-2013-071 [ Links ]
6. Ntusi N. Dyslipidaemia in South Africa. S Afr Med J 2018;108(4):256-257. https://doi.org/10.7196/SAMJ.2018.v108i4.13265 [ Links ]
7. Obialo CI, Ofili EO, Norris KC. Statins and cardiovascular disease outcomes in chronic kidney disease: Reaffirmation vs. repudiation. Int J Environ Res Public Health 2018;15(12):2733. https://doi.org/10.3390/ijerph15122733 [ Links ]
8. Jungers P, Massy ZA, Nguyen Khoa T, et al. Incidence and risk factors of atherosclerotic cardiovascular accidents in predialysis chronic renal failure patients: A prospective study. Nephrol Dial Transplant 1997;12(12):2597-2602. https://doi.org/10.1093/ndt/12.12.2597 [ Links ]
9. Kundhal K, Lok CE. Clinical epidemiology of cardiovascular disease in chronic kidney disease. Nephron Clin Pract 2005;101(2):c47-c52. https://doi.org/10.1159/000086221 [ Links ]
10. Klug EQ, Raal FJ. New cholesterol targets for patients at high or very high cardiovascular risk and the indications for PCSK9 inhibitors. S Afr Med J 2020;110(11):1059. https://doi.org/10.7196/SAMJ.2020.v110i11.15191 [ Links ]
11. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2019;41(1):111-188. https://doi.org/10.1093/eurheartj/ehz455 [ Links ]
12. Kovell L. Lipid management guidelines for adults with chronic kidney disease. Washington: American College of Cardiology, 2016. https://www.acc.org/latest-in-cardiology/articles/2016/05/31/13/00/lipid-management-guidelines-for-adults-with-chronic-kidney-disease (accessed 1 December 2019). [ Links ]
13. Kennard A, Singer R. Lipid lowering in renal disease. Aust Prescr 2017;40(4):141-146. https://doi.org/10.18773/austprescr.2017.047 [ Links ]
14. Kuznik A, Mardekian J, Tarasenko L. Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults with chronic kidney disease: An analysis of national health and nutritional examination survey data, 2001 - 2010. BMC Nephrol 2013;14:132-142. https://doi.org/10.1186/1471-2369-14-132 [ Links ]
15. Madala ND, Thusi GP, Assounga AGH, Naicker S. Characteristics of South African patients presenting with kidney disease in rural KwaZulu-Natal: A cross sectional study. BMC Nephrol 2014;15(1):61-69. https://doi.org/10.1186/1471-2369-15-61 [ Links ]
16. Voskamp PWM, van Diepen M, Dekker FW, Hoogeveen EK. Dyslipidemia and risk of renal replacement therapy or death in incident pre-dialysis patients. Sci Rep 2018;8(1):3130-3138. https://doi.org/10.1038/s41598-018-20907-y [ Links ]
17. Wong MG, Wanner C, Knight J, Perkovic V. Lowering cholesterol in chronic kidney disease: Is it safe and effective? Eur Heart J 2015;36(43):2988-2995. https://doi.org/10.1093/eurheartJ/ehv393 [ Links ]
18. Newman CB, Preiss D, Tobert JA, et al. Statin safety and associated adverse events: A scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2019;39(2):e38-e81. https://doi.org/10.1161/atv.0000000000000073 [ Links ]
19. Herselman M. Non-adherence to dietary prescriptions in chronic kidney disease. South Afr J Clin Nutr 2008;21(2):13-14. https://doi.org/10.1080/16070658.2008.11734156 [ Links ]
20. Lansberg P, Lee A, Lee ZV, Subramaniam K, Setia S. Nonadherence to statins: Individualised intervention strategies outside the pill box. Vasc Health Risk Manag 2018;2018(14):91-102. https://doi.org/10.2147/vhrm.s158641 [ Links ]
 Correspondence:
Correspondence:
M R Essop
a0041283@wits.ac.za
Accepted 28 September 2023














