Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.11 Pretoria Nov. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i11.885
RESEARCH
Trisomy 21 screening with alpha software and the Fetal Medicine Foundation algorithm
L PistoriusI, II; C A CluverI, II; I BhoratIII; L GeertsI
IPhD; Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIPhD; Panorama Perinatology, Mediclinic Panorama, Cape Town, South Africa
IIIPhD; Department of Obstetrics and Gynaecology, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: Screening for trisomy 21 provides pregnant women with accurate risk information. Different algorithms are used to screen for trisomy 21 in South Africa (SA). The Fetal Medicine Foundation (FMF) provides software to screen for trisomy 21 in the first trimester by ultrasound or a combination of ultrasound and biochemistry (combined screening), and requires regular and stringent quality control. With cdpha software, first trimester combined screening and screening with biochemistry alone in the first or second trimester are possible. The alpha screening requires quality control of biochemical tests, but not of ultrasound measurements. Ideally, a screening test should have a high detection and a low screen positive rate. Despite the availability of these screening programmes, only a minority of infants with trisomy 21 are detected prenatally, raising questions about the effectiveness of screening
OBJECTIVES: To determine the screen positive and detection rates of prenatal screening for trisomy 21 in the SA private healthcare system
METHODS: Data from the three largest laboratories collected between 2010 and 2015 were linked with genetic tests to assess screen positive and detection rates. Biochemical screening alone with alpha software (first or second trimester) and combined screening using either FMF or clpha software were compared
RESULTS: One-third of an estimated 675 000 pregnancies in private practice in the 6-year study period underwent screening. There were 687 cases of trisomy 21 in 225 021 pregnancies, with only 239 (35%) diagnosed prenatally. The screen positive rates were 11.8% for first trimester biochemistry, 7.6% for second trimester biochemistry, 7.3% for first trimester FMF software ultrasound alone, 3.7% for combined first trimester screening with FMF software, and 3.5% for combined first trimester screening with alpha software. The detection rates for a 5% false positive rate were 63% for first trimester biochemistry, 69% for second trimester biochemistry, 95% for combined first trimester screening with FMF software and 80% for combined first trimester screening with alpha software. Detection and confirmation rates were highest with FMF software
CONCLUSION: Screening with FMF software has a similar screen positive rate and better detection rate than screening with alpha software. The low prenatal detection rate of trisomy 21 is mainly due to a low prevalence of screening. More research is needed in the SA setting to explore why screening and confirmatory testing after high-risk results are not performed in many pregnancies
Prenatal screening for trisomy 21 has become an integral part of antenatal care.[1] The first screening method for trisomy 21 was an amniocentesis for fetal karyotyping. This was offered to pregnant women of advanced maternal age. Subsequently, biochemical tests were developed that could be done in the first or second trimester (biochemical screening) to identify pregnancies at high risk of fetal trisomy 21. Ultrasound markers on their own (ultrasound screening), and in combination with biochemical tests (combined screening), were then shown to improve the accuracy of screening. Screening with first trimester ultrasound markers (including the nuchal translucency thickness) is more accurate than second trimester ultrasound markers.[2] Non-invasive prenatal testing (NIPT) is the most recent development. NIPT measures the free fetal DNA circulating in maternal blood to detect trisomy 21. NIPT is the most sensitive and specific screening test for trisomy 21. The development of more sophisticated screening tests has increased the accuracy, but also the costs, of screening for trisomy 21.[1]
In South Africa (SA), as in other low- and middle-income countries, the expense precludes the general availability of NIPT. '3- In the SA public health sector, screening is largely prompted by maternal age, with ultrasound used in selected patients.[4] In the private sector, biochemical and combined first trimester screening are available.'5- NIPT has also recently become available.
Ideally, the obstetrical caregiver (obstetrician, general practitioner or midwife) discusses the possibilities of screening with the pregnant woman early in her pregnancy. The woman can then choose whether or not she would prefer screening for trisomy 21. Depending on personal preference, financial situation and other factors, she can choose between the different options.[5]
The SA Society of Obstetricians and Gynaecologists (SASOG) has prepared detailed patient information leaflets on screening for trisomy 21 in many official languages (https://www.sasuog.org.za/prenatal-tests-1). The cost of screening is funded partially by private health insurance and partially by the woman herself. If the risk of trisomy 21 is higher than 1:300 (in the first trimester), she is deemed high risk. This cut-off was selected as it has an 85% detection rate for Down syndrome with a 5% false positive rate. For second trimester biochemical screening alone, a risk of 1:270 is deemed high risk. This is equivalent to the risk of 35-year-old women having a pregnancy with trisomy 21 in the midtrimester. Further testing (karyotyping of fetal cells obtained by chorionic villus sampling, amniocentesis, or fetal blood sampling) is usually offered. NIPT can be offered as first-line or as second-line screening, especially if the risk of trisomy 21 is between 1:100 and 1:1 000).[5]
A screening test should ideally have a high detection rate (the percentage of affected individuals that is detected by screening) and a low screen positive rate (number of women who screen high risk). Efficient screening for trisomy 21 should be feasible in the private sector in SA, with the availability of different screening options. However, an audit demonstrated a low prenatal detection rate (39%) for trisomy 21 in private healthcare.[6] It would be important to know whether this low yield of screening is due to a low sensitivity of the screening programme, or a low uptake of screening.
Two algorithms are available in SA to determine the risk of trisomy 21 with combined screening. To make use of the Fetal Medicine Foundation (FMF, UK) algorithm, ultrasound practitioners have to undergo training, pass an examination and pass a strict annual audit.[7] In contrast, risk evaluation with cdpha software (Logical Medical Systems, UK) is freely available to any ultrasound practitioner. Usually the provider will supply ultrasound measurements, including the crown rump length and nuchal translucency (NT) measurements, to a pathology laboratory, which then calculates the risk, without any quality control of the ultrasound measurements.[8]
Until now, it has been impossible to do an accurate assessment of the sensitivity of the different algorithms, as there is no centralised screening database. A patient could have a screening test done by one laboratory, but confirmatory karyotyping could be done by a different laboratory.
We therefore aimed to determine the percentage of pregnant women undergoing screening, and to determine the sensitivity and screen positive rates by linking screening and confirmatory data from different biochemical laboratories and FMF accredited practitioners.
Methods
Ethical approval was obtained from the Health Research Ethics Committee, Stellenbosch University (ref. no. N16/08/098). After ethical approval was obtained, a retrospective audit of SA women receiving private healthcare was conducted. The lead authors approached the three major laboratories performing prenatal screening (Ampath, Lancet and PathCare) and practitioners on the FMF database. The laboratories provided data on prenatal screening throughout SA for fetal aneuploidy between January 2011 and December 2015, and pre- and postnatal genetic testing between January 2011 and December 2016. The SA FMF practitioners provided data for the same time period. A waiver of patient consent was obtained.
For screening tests, the laboratories and practitioners both provided information on the patients' date of birth, medical insurance number, date and details of the ultrasound examination, the gestational age, the levels and multiples of the median (MoM) of first or second trimester biochemical markers, the risk of trisomy 21 by maternal age, biochemical screening or combined first trimester screening, as well as genetic test results (date, rapid testing or full karyotyping). Postnatal genetic tests that were done up to 1 year of age were included. The laboratories provided the data in a spreadsheet (as requested) or as text files. The FMF practitioners provided the data as spreadsheets. A data analyst consolidated the data and linked the screening and genetic tests by means of the medical insurance numbers. The medical aid numbers were subsequently removed to ensure anonymity. The link between screening and genetic data was validated by ensuring the absence of illogical date combinations.
We compared 5 groups: the results of first trimester alpha software using only biochemical markers, the results of alpha combined screening using first trimester biochemical and ultrasound markers, the results of FMF software using only ultrasound markers, the results of combined FMF screening using first trimester biochemical and ultrasound markers, and lastly the results of alpha software using second trimester biochemistry markers.
Calculated risks were considered high if >1:300 in the first trimester or >1:270 in the second trimester. The number of women using private antenatal healthcare was estimated using birth data from Statistics SA (https://www.statssa.gov.za/). The number of trisomy 21 diagnoses, the prevalence of screening and the screen positive and detection rates were calculated for the different tests.
Normally distributed continuous data were compared using standard parametric testing, categorical data were compared using Fisher's exact test or the χ2 test. We considered p<0.05 to be significant. Data preparation and analysis were performed with SAS University Edition Version 9.3 (SAS, USA), Excel 365 (Microsoft Corp., USA) and SPSS version 27 (IBM Corp., USA).
Results
Data on screening and linked genetic tests are presented in Fig. 1. In the study period (January 2011 - December 2016), the number of screening tests (225 036) represented 33.3% of the estimated 675 000 deliveries in private healthcare (95% confidence interval (CI) 33.2 - 33.4).
Calculated trisomy 21 risk results were available for 197 199 pregnancies (87.6% of those undergoing screening) (Fig. 1), with significant differences in patient characteristics for the different tests (Table 1). Younger women were more likely to be screened using alpha screening. Older women and women with a previous pregnancy affected by trisomy 21 were more likely to be screened using combined first trimester screening with FMF software. Background risk for trisomy 21 based on age was similar using first trimester methods, but lower for second trimester biochemistry. The expected prevalence of trisomy 21 according to the adjusted risk of the different screening tests was lower in the alpha combined first trimester screening group and higher in the FMF ultrasound-only group.
The distribution of NT measurements in the alpha group was significantly lower than in the FMF group (Fig. 2A). This difference remained when corrected for crown rump length (delta NT = measured NT minus the expected mean NT for the crown rump length[9] with a difference of 0.7 standard deviation (SD) (p<0.001)). The logarithmic MoM distributions were higher for free beta human chorionic gonadotropin (hCG) and lower for pregnancy-associated plasma protein A (PAPP-A) with alpha software compared with FMF software, but absolute differences were small (Figs 2B and C). The difference in free beta-hCG and PAPP-A values was confirmed in a set of 100 randomly selected women for whom the beta-hCG and PAPP-A MoM values were calculated with both software programs based on patients' characteristics (parity, weight, smoking, diabetes, in vitro fertilisation, previous trisomy 21) and absolute serum biochemical levels.
The screen positive rate using combined first trimester screening was similar for combined alpha and FMF software (3.5% and 3.7%, respectively), but was significantly higher with second trimester biochemistry (7.7%) and FMF ultrasound-only (7.3%). The screen positive rate was highest with first trimester biochemistry only (11.8%) (Table 2).
In women aged <35 years, the screen positive rate with alpha combined software was significantly lower than FMF combined first trimester screening (1.7% v. 2.4%; p<0.01). In women >35 years, alpha combined first trimester screening had a significantly higher screen positive rate than FMF combined first trimester screening (11.5% v. 7.2%; p<0.01).
Data were available on 6 039 genetic analyses. More than half (55.2%) could not be linked to prenatal screening. Of the 687 trisomy 21 cases, 212 (30.7%) could be linked to prenatal screening and 239 (35%) were prenatal samples (Table 3). Twenty-four diagnoses followed screening with FMF ultrasound-only, with 23 following a high-risk screening result. The positive predictive value and detection rate could not be calculated for these, as medical insurance numbers were not available, unless an invasive procedure was performed (233 of 5 491 cases (4.2%), including 24 confirmed trisomy 21 cases, compared with the 18 expected based on the age distribution).
The positive predictive value for trisomy 21 was significantly higher for FMF combined first trimester screening (21.1% (94/455)) when compared with other screening methods. It was 7.4% (15/203) with first trimester biochemistry, 9.6% (49/510) with alpha combined first trimester screening and 4.5% (38/854) with second trimester screening). alpha combined first trimester screening was significantly better than second trimester alpha screening (p<0.01).
In women >35 years, the positive predictive value of FMF combined first trimester screening was significantly higher (31.2%) than all other methods (p<0.01). The positive predictive value of combined first trimester alpha screening was also significantly higher than second trimester screening (11.7% v. 5.7%; p<0.01) (Table 2). In women <35 years, FMF combined first trimester screening had a significantly higher positive predictive value than alpha combined first trimester screening (13.0% v. 6.7%; p=0.02) and a significantly higher positive predictive value than second trimester screening (2.4%; p<0.01 and p=0.02, respectively) (Table 2).
Only 35% of trisomy 21 diagnoses (n=239) were made prenatally, and only 22% followed screening. The detection rate for high-risk results was higher for combined first trimester FMF software compared with combined first trimester alpha screening (94% v. 79%; p<0.01) and second trimester screening (74.5%; p<0.01). A similar pattern was seen for women <35 years (detection rate varying from 42% to 86%), but the differences were minimal for women >35 years (detection rate varying from 94% to 100%; all comparisons not significant).
The detection rate for a fixed screen positive rate of 5% was significantly higher for combined FMF first trimester screening (95.0%) than any other method across all ages (p<0.01) and in women <35 years (89.2%; p<0.01). Detection rates for a fixed screen positive rate of 5% for the different screening tests with alpha software (62.5% for biochemistry alone, 80.1% for combined first trimester and 68.6% for second trimester) did not differ significantly, but the study had limited power to detect differences between the alpha modalities at a significance level of p=0.05 (Table 2).
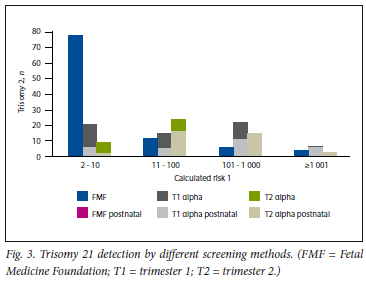
The distribution of the calculated risk of trisomy 21 cases differed significantly between the screening methods (Fig. 3). A very high-risk result (>1:10) was seen in 78% with FMF combined first trimester screening, 32% with alpha combined first trimester screening and 18% with second trimester screening.
For the 448 postnatal diagnoses of trisomy 21, serum screening was not performed in 385 (86.0%) cases, while 28 (6.3%) followed first trimester risk assessment with alpha software (9 with a low-risk result, 21 with a high-risk result), 2 (0.4%) with FMF software (both high-risk) and 36 (8.0%) second trimester risk assessment (13 low-risk, 23 high-risk). Only 22 postnatal diagnoses (4.9%) followed a false low-risk result. A total of 46 cases (10.3%) did not undergo invasive testing for confirmation after a high-risk result (Table 3).
Prenatal invasive confirmatory testing after any high-risk result was more common after FMF combined first trimester screening than after alpha combined first trimester screening (97.9% v. 63.3%; p<0.01) or second trimester screening (39.5%; p<0.01) (Table 3).
Prenatal confirmation rates for very high-risk results (risk >1:10) were also significantly higher in the FMF group (98,7%; p<0.01) but with no significant difference between alpha combined first trimester screening and second trimester alpha screening (75% v. 77.8%).
Discussion
The prenatal detection rate of trisomy 21 in this study is similar to the rate found in an audit from 2008 in women accessing private healthcare in SA.[6] This is mainly due to a low prevalence of screening, as screen positive and detection rates for the different screening tests were similar to those in the reported literature.[2] Most screening was performed using inferior tests, and there was a low uptake of confirmatory invasive testing after a high-risk result when screened by non-FMF accredited practitioners.
The reasons for the low prevalence of screening need to be explored further. Are women declining screening? Are they presenting too late for prenatal care? Is screening not being offered? Is screening with NT without biochemistry by non-FMF accredited practitioners perhaps performed on a large scale?
Second trimester screening and invasive testing without screening are still prevalent, despite the low diagnostic yield of these approaches. The reasons for using second trimester serum screening may include late initiation of antenatal care, early antenatal care provided by general practitioners (or older obstetricians who are less familiar with newer screening options), the lower cost of second trimester serum screening, or the advantage of also screening for neural tube defects for women who do not have access to fetal anomaly scans. This should also be explored further.
Combined first trimester screening using alpha software significantly underestimates the risk of trisomy 21 in spite of its systematically allocating higher MoM values for the same free-beta hCG levels, lower MoMs for PAPP-A levels and a higher age-based risk than the FMF programme, resulting in a higher biochemistry-based risk. This is also as evidenced by the high screen positive rate and higher background risk in those screened with alpha software despite a lower age distribution. This is likely an effort to compensate for the significant left-shift of the NT distribution by non-FMF-accredited practitioners, leading to a substantially lower screen positive rate for combined first trimester alpha screening compared with biochemistry-only screening. The lower mean NT measurement resulting in a significantly lower detection rate and positive predictive value for the combined first trimester alpha screening confirms the tenet of the FMF that strict adherence to protocol and regular audit are needed to maintain accurate measurements and risk assessment.[10] Results are compromised when there is a 0.02 change in SD, and in this study, the difference between FMF and alpha distributions was 0.03.
Our results confirm the superior performance of screening with FMF software. Screen positive rates by FMF operators were higher when ultrasound-based risk was not combined with biochemistry. It is possible that this represents an overestimation of true risk, but this group did contain more confirmed trisomy 21 cases than predicted by age, suggesting that there may have been non-captured reasons to forego biochemistry testing, such as abnormal ultrasound findings.
Combined first trimester screening with FMF software was superior to all other tests, and it should therefore be recommended when NIPT is not affordable. It does not over- or underestimate the risk of trisomy 21, and the detection rate and positive predictive value were significantly higher than for all other tests. In spite of only representing 18% of all screening tests, the FMF software made the largest contribution (65.8%) to all prenatal diagnoses of trisomy 21. A total of 94% of these diagnoses followed a high-risk result, and 78% a risk >1:10. Expanding the number of practitioners with FMF-accreditation should become a priority for SA, and combined first trimester FMF screening should be recommended over ultrasound-only screening.
The low rate of prenatal confirmatory testing in women having a high-risk result with cdpha software should be investigated further. It may be related to the higher risks yielded by FMF software, as uptake was considerably higher with risks >1:10. It could reflect suboptimal pre-screen counselling in a population where trisomy 21 is better accepted than in other societies (limiting the acceptance of any procedure-related risk to confirm the diagnosis), or perhaps because financial barriers limited access to genetic testing. It may also be due to bias, with partial preselection for FMF screening based on a perceived or real increased risk.
Strengths of this study include large numbers, the collation of data and a representative sample of laboratories (covering >90% of pregnant women using private antenatal care in SA) and the inclusion of all except one of FMF operators in SA active during the time of the study. Limitations include some missing data and a small possibility of inadequate matching and linking. Collating the data for this study was time consuming and labour intensive. This process was essential to compare the accuracy of different screening tests. Hopefully, the results can counter the equanimity with which obstetrical care givers and laboratories providing prenatal screening in SA have ignored the calls for accreditation and regular audit of NT measurements. User-friendly audit systems should be developed and implemented, as evaluating screen positive and detection rates should be straightforward and performed at least annually.
Conclusion
A third of women accessing private healthcare in SA from 2011 until 2016 underwent prenatal screening for trisomy 21. Only 35% of trisomy 21 diagnoses were made prenatally, with 22% following screening. Most screenings were performed with inferior tests. If prenatal detection is to be improved, more effective screening methods such as combined first trimester screening using FMF software or NIPT should be used. Research exploring reasons for not having screening, or not proceeding with confirmatory testing after a screen positive result, is needed.
Declaration. None.
Acknowledgements. We would like to thank the FMF-accredited operators and the personnel from Ampath Laboratories (Pretoria), Lancet Laboratories (Cape Town) and PathCare laboratories (N1 City, SA) for providing data, Sonja Schell and Lucy Brink for data management and Richard Hiscock for help with statistical analysis.
Author contributions. LP and CC designed the study and acquired the data. LP analysed the data. All authors interpreted the data, revised the manuscript, approved the final version and are accountable for all aspects of the work.
Funding. This study was funded by SASUOG (SA Society for Ultrasound in Obstetrics and Gynaecology) and SASOG (SA Society of Obstetricians and Gynaecologists).
Conflicts of interest. CC, IB and LP are accredited with the FMF, have provided FMF-accredited data for this study and perform fee-for-service FMF combined first trimester screening.
References
1. Hui L. Noninvasive approaches to prenatal diagnosis: Historical perspective and future directions. Methods Mol Biol 2019;1885:45-58. https://doi.org/10.1007/978-1-4939-8889-1_3 [ Links ]
2. Cuckle H, Maymon R. Development of prenatal screening - a historical overview. Semin Perinatol 2016;40(1):12-22. https://doi.org/10.1053/J.SEMPERI.2015.11.003 [ Links ]
3. Minear MA, Lewis C, Pradhan S, Chandrasekharan S. Global perspectives on clinical adoption of NIPT. Prenat Diagn 2015;35(10):959. https://doi.org/10.1002/PD.4637 [ Links ]
4. Geerts L. Prenatal diagnosis of chromosomal abnormalities in a resource-poor setting. Int J Gynecol Obstet 2008;103(1):16-21. https://doi.org/10.1016/JJJGO.2008.05.028 [ Links ]
5. Bhorat I, Chauke L, Coetzee E, et al. Challenges and controversies in prenatal genetic screening in the South African context. Obstet Gynaecol Forum 2018;28(1):33-36. https://doi.org/10.10520/EJC-cebec9ee9 [ Links ]
6. Urban M, Geerts L. Prenatal diagnostic services and prevention of birth defects in South Africa. In: Kumar D, ed. Genomics and Health in the Developing World. Oxford: Oxford University Press, 2014:547-567. [ Links ]
7. Nicolaides KH. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat Diagn 2011;31(1):7-15. https://doi.org/10.1002/pd.2637 [ Links ]
8. Wald NJ, Radeck C, Hackshaw AK, et al. First and second trimester antenatal screening for Down's syndrome: The results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen 2003;10(2):56-104. https://doi.org/10.1258/096914103321824133 [ Links ]
9. Wright D, Kagan KO, Molina FS, Gazzoni A, Nicolaides KH. A mixture model of nuchal translucency thickness in screening for chromosomal defects. Ultrasound Obstet Gynecol 2008;31(4):376-383. https://doi.org/10.1002/UOG.5299 [ Links ]
10. Cuckle H. Monitoring quality control of nuchal translucency. Clin Lab Med 2010;30(3):593-604. https://doi.org/10.1016/J.CLL.2010.04.012 [ Links ]
 Correspondence:
Correspondence:
C A Cluver
cathycluver@sun.ac.iza
Accepted 29 September 2023














