Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.10 Pretoria Out. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i10.678
RESEARCH
Evaluation of the accuracy of the Asanté assay as a point-of-care rapid test for HIV-1 recent infections using serum bank specimens from blood donors in South Africa, July 2018 - August 2021
B SinghI; J MthombeniII; G OlorunfemiIII; M GoosenIV; E CutlerV; H JuliusVI; Z BrukweVII; A PurenVIII, IX
INDip (Med Tech), MTech; Department of Biomedical Sciences, Faculty of Health Sciences, University of Johannesburg, South Africa
IINDip, MPH; Department of Biomedical Sciences, Faculty of Health Sciences, University of Johannesburg, South Africa
IIIMBBS, FWACS; Division of Epidemiology and Biostatistics, School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVBSc (Hons); National Institute for Communicable Diseases/National Health Laboratory Services, Johannesburg, South Africa
IXMB BCh, PhD; Division of Virology, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VBSc (Hons), MSc; National Institute for Communicable Diseases/National Health Laboratory Services, Johannesburg, South Africa
VIMPH; National Institute for Communicable Diseases/National Health Laboratory Services, Johannesburg, South Africa
VIINDip (Biomed Tech); National Institute for Communicable Diseases/National Health Laboratory Services, Johannesburg, South Africa
VIIIMB BCh, PhD; National Institute for Communicable Diseases/National Health Laboratory Services, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Point-of-care (POC) rapid recency testing can be used as a cost-effective tool to identify recently infected individuals (i.e. infected within the last 12 months) in near-real time, support epidemic control and identify hotspots for transmission as part of recent infection surveillance
OBJECTIVE: To evaluate the performance of the Asanté (HIV-1) rapid recency assay as a POC rapid test among blood donors in South Africa (SA
METHODS: The study was a cross-sectional and validity study of the Asanté HIV-1 Rapid Recency Assay performed on 715 consecutively archived plasma donor specimens from the SA National Blood Services to determine their recency and established HIV infection status. ELISA and rapid assays for HIV antibody detection were used as the reference-testing standard for confirming an infection, while the Maxim HIV-1 limiting antigen (LAg) avidity assay was used as a reference for comparing HIV recency status. Validity tests (sensitivity, specificity, negative and positive predictive values) and Cohen-Kappa tests of the agreement were conducted to compare the Asanté HIV-1 rapid recency assay results with the reference tests
RESULTS: Of the 715 studied blood samples, 63.1% (n=451/715) were confirmed to be HIV-positive based on the reference standard. The sensitivity and specificity of the Asanté HIV-1 rapid recency assay in diagnosing established HIV infection compared to the ELISA were 98.4% (95% CI 96.7 - 99.3) and 99.6% (95% CI 97.6 - 100), respectively. Compared with HIV rapid assay, the sensitivity and specificity of the Asanté HIV-1 rapid recency assay was 98.7% (95% CI 97.0 - 99.4) and 99.2% (95% CI 97.1 - 100), respectively. Of the 451 HIV-positive blood samples, 43% were confirmed as recent HIV infections by the Maxim HIV-1 LAg avidity assay. There was high agreement between the Asanté HIV-1 rapid recency assay and the Maxim HIV-1 LAg avidity assay (94.1%, k=0.879, p<0.0001). The sensitivity and specificity of the Asante HIV-1 assay was 89.4% (95% CI 84.0 - 93.0) and 97.7% (95% CI 94.8 - 99.0), respectively
CONCLUSION: The Asanté HIV-1 rapid recency assay test results demonstrated high accuracy (>90%) compared with the HIV ELISA and rapid assays for determining established infection and the Maxim HIV-1 LAg avidity assay for classifying recent HIV-1 infections. The assay's sensitivity for established infections was below the World Health Organization criteria (<99%) for POC devices. The Asanté HIV-1 rapid recency assay can be used to distinguish between recent and long-term infections, but may not be considered a POC test for determining HIV infection
The Joint United Nations Programme on HIV/AIDS (UNAIDS) estimates that the number of people living with HIV infection in South Africa (SA) is 7 500 000 (95% CI 7 000 000 - 8 200 000), and HIV incidence in SA is reported to be 4.19% (95% CI 3.74 - 4.67).[1,2] UNAIDS announced the 90-90-90 strategy in 2014, which has now been revised to the 95-95-95 strategy, to end the AIDS epidemic by 2030 by achieving a target of 95% diagnosis among all people living with HIV, with 95% of those who have been diagnosed receiving antiretroviral treatment (ART) and 95% of those on treatment to be virally suppressed.[3,4] SA's progress towards these targets has shown that it is possible to estimate the number of HIV-positive adults diagnosed by linking HIV testing data from numerous sources.[5] Approximately 94% of people living with HIV in SA know their status.[1]
SA has the largest antiretroviral (ARV) treatment programme globally and has made tremendous improvements in encouraging people to test for HIV.[6] The benefits of early ART include reduction in transmission of new HIV infections, reduction in mother-to-child transmission, and accelerating the attainment of 95-95-95 goals.[6] The prevalence of HIV in SA is relatively stable, and it is, therefore, possible to obtain the incidence (rate of new HIV infections) of HIV.[7-9] Incidence is useful for determining the effectiveness of current national and global preventive interventions.[9] However, monitoring HIV incidence has become complex, using conventional cross-sectional serological tests for recent infections (TRI).[10] The laboratory-based HIV incidence testing provides only population-level and not individual-level data. However, several assays were developed to measure HIV incidence.[11-13] The Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA) demonstrated that no single TRI assay satisfied the target product profiles for incidence testing from their evaluation data.[13]
The Centers for Disease Control (CDC) developed the Calypte IgG-capture enzyme immunoassay (BED-CEIA) and the HIV-1 LAg avidity enzyme immune assay (EIA) specifically for incidence testing.[14[ However, there were many concerns that the BED-CEIA assay overestimated HIV-1 incidence and incidence estimates provided false recent rates (FRR).[15,16] To address these limitations, the CDC developed a multi-subtype gp 41protein that covered diverse sequences from all major subtypes of HIV (rIDR-M) and used the rIDR-M in two avidity assays.[16,17] The avidity-based assays were based on the principle of avidity binding strength of developing HIV-1 antibodies, which were less likely to be affected by disease states such as low CD4 count or viral load.[14,16,18] The format of the LAg EIA is a one-well avidity assay using limiting amounts of antigen.[13,14] The use of a recombinant protein, rIDR-M, permitted broader use of the avidity-based assays in populations with varying HIV-1 subtypes[1,13] and guaranteed equal performance across different HIV-1 subtypes (A, B, C, D and AE).[15,16] However, the application of laboratory-based avidity tests for recent infections has been limited, because these assays are conducted in a laboratory, and results may take several days or weeks to become available.[19] Identifying early infections (i.e. within 12 months of infection) could lead to earlier treatment and reduce HIV transmission, support early index case finding and advise policy for possible prevention interventions in high-incidence hotspots.[20] Therefore tools such as POC tests are needed to detect recent infections in close to real time in POC settings. Therefore, the development of a rapid test for recent infection (RTRI) allows one to perform HIV recency testing in a routine HIV programme in real time, improving access to testing and data utilised for targeted prevention.[19] The CDC developed a POC, HIV-1 rapid recency test for this purpose, which is now commercialised by Sedia Biosciences (Beaverton, Oregon, USA) as the Asanté HIV-1 rapid recency assay. The Asanté HIV-1 rapid recency assay is a rapid lateral flow-type format of the avidity assay and can be used to simultaneously detect HIV-1 infection and HIV-1 recency.[21] The test is based on the antigen-binding strength or avidity as HIV infection progresses. Therefore, recent infections have antibodies with low avidity (within the last 6 months post sero-conversion), while long-term (LT) infections have antibodies with high avidity (>12 months).[21] Results are obtained in 20 minutes. Rapid early infection detection can also support surveillance programmes in real time in terms of identifying infection hotspots and aiding the appropriate mobilisation of interventions and resources.[20,22] The Asanté HIV-1 rapid recency assay POC testing is therefore potentially useful in the context of the HIV epidemic control in SA, and such testing could easily be introduced at a programme level.[20,22] However, limited evidence exists on the diagnostic value of the Asanté HIV-1 rapid recency assay in measuring recent infections among the SA population. For this reason, the diagnostic performance of the Asanté HIV-1 rapid recency assay was evaluated. The objectives of the study were to assess (i) the accuracy of the Asanté HIV-1 rapid recency assay for established HIV infection by comparing ELISA and HIV rapid tests as the reference standard; and (ii) the accuracy of the Asanté HIV-1 rapid recency assay to detect HIV-1 recent infections using the Maxim HIV-1 LAg avidity EIA as the reference standard.
Methods
Study design and setting
A retrospective cross-sectional and validity study of adult donor blood samples (>16 years) collected by the SA National Blood Services (SANBS) was conducted across eight provinces in SA between July 2018 and August 2021. The blood samples were obtained from Gauteng, KwaZulu-Natal, Limpopo, North West, Mpumalanga, Free State, Northern Cape and Eastern Cape provinces. The HIV Sero-Molecular Laboratory conducted the testing for the study at the National Institute for Communicable Diseases (NICD).
Study population and sampling
Archived donor blood specimens (n=715), HIV-1 positive (n=451), HIV-1 negative (n=230), and p24 antigen positive (n=34) from the SANBS were used to assess the accuracy of the Asanté HIV-1 rapid recency assay. A convenience sampling method was used to select the samples that were evaluated. Only confirmed HIV-positive and negative blood specimens were obtained from SANBS. Of the 451 positive specimens obtained from SANBS, 71 specimens were known to have an HIV-1 recent infection. All specimens were provided as plasma packs and identified with a unique SANBS number anonymised and de-linked from the donor.
Assay procedure
The Standards for Reporting Diagnostic accuracy studies approach (STARD)[23] was utilised in this study to evaluate the ability of the test device to correctly classify the target population as either being recently infected or having an established HIV infection.[23] The plasma specimens were converted to serum through defibrination (removal of fibrin) and re-calcification (clotting) technique before testing.[24,25]
Reference tests
The specimens were characterised for the presence or absence of HIV antibodies[26,27] using the third-generation Genscreen HIV-1/2 V2 (Bio-Rad Laboratories, France) and Murex HIV 1.2.0 (DiaSorin, UK) assays, followed by the fourth-generation Bio-Rad Genscreen ULTRA HIV Ag-Ab (Bio-Rad Laboratories, France), Abbott Architect HIV Ag-Ab Combo (Abbott, Germany) and Diasorin Murex HIV Ag/Ab Combination (DiaSorin, UK) assays that detect antibody and antigen. Additionally, the specimens were tested on the third-generation Abon HIV 1/2/O tri-line rapid assay (Abon Biopharm (Hangzhou) China) and first response HIV1-2 O card test (Premier Medical Corporation Private Limited, India) to confirm the presence or absence of antibodies to HIV.[27] The confirmed HIV-1 positive specimens that were reactive on all tests (n=451) were tested on the HIV-1 LAg (LAg) avidity assay (Maxim Biomedical Inc., USA) to classify specimens as either HIV recent or long term (LT). The assay defines specimens as LT or recent based on their normalised optical density (ODn) reading where a specimen with an ODn >1.5 cut-off is classified as recent and a specimen with an OD >1.5 cut-off is classified as LT. The HIV-1 positive specimens consisted of 194 recent (71 had a known recent result confirmed by SANBS using the Sedia HIV-1 limiting antigen avidity EIA) and 257 LT as confirmed by the HIV-1 LAg avidity EIA. All reference tests were carried out according to the manufacturer's instructions. The p24 antigen positive specimens were added to the negative pool (n=264) as they tested negative on the antibody assays. These specimens yielded false recent HIV results on the Maxim HIV-1 LAg avidity . The LAg avidity EIA is only intended for use in individuals who have a confirmed HIV-1 serostatus and not for seronegative individuals or those with acute infections (p24 antigen/ RNA positive but antibody negative).
Index test
The Asanté HIV-1 rapid recency assay was performed according to the manufacturer's instructions. The test was conducted between 15°C and 37°C. The sample buffer tube was labelled with the specimen identification number for all controls and specimens. A precision pipette (Thermo Fisher Scientific, USA) transferred 5 uL of each control and specimen into the corresponding buffer tube containing 0.5 mL of buffer. Each specimen was mixed with the buffer by gentle agitation of the buffer tube. The test strip was removed from its foil pouch and labelled with the specimen number. The test strip was inserted into the corresponding labelled buffer tube and incubated for 20 minutes. Each test strip was placed onto an absorbent paper towel to drain excess liquid. The results were read visually by the tester, and a second competent operator verified the results independently for correctness. Discrepant results were repeated. The results were recorded on the laboratory results worksheet.
Interpretation of results
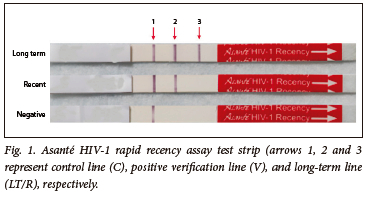
Each test strip was validated by ensuring that the built-in procedural control met the test validation criteria. If a reddish-purple line appeared in the control (C) area, regardless of whether the verification line (V) or LT/recent (R) line gave a reactive or non-reactive result, this indicated that the test was valid. All bands observed, even a faint band, were recorded as being present/reactive. The test was invalid if a line did not appear in the C area. If the test was invalid, a result could not be interpreted, and the test was repeated.
A specimen was considered LT when all three reactive lines appeared, i.e. the C line, the V line, and the LT/R line (Fig 1). A specimen was considered a recent infection when only the C and the V lines were visible. A specimen was considered an unconfirmed negative (discrepant with routine HIV testing algorithm) when only the C line appeared.
Data analysis
The data collected were entered into Excel 2016 (Microsoft, USA), which was kept secure at the NICD and only accesible to the authors of this publication. The diagnostic accuracy of the Asanté HIV-1 rapid recency assay test, as compared with the result obtained from the Maxim HIV-1 LAg avidity assay, was evaluated by calculating the Cohen's kappa test of agreement. Cohen's kappa test of agreement values were characterised as moderate agreement (0.40 - 0.59), substantial agreement (0.60 - 0.79) and strong agreement (0.80 -0.90).[28] A two-by-two contingency table was used to determine the performance of the Asanté HIV-1 rapid recency assay compared with the ELISA, Rapid and Maxim HIV-1 LAg avidity assays.[12,29] Sensitivity, specificity and agreement for the Asanté HIV-1 rapid recency assay test results compared with the results obtained from ELISA and the rapid assays (reference methods) were determined using XLSTAT, version 2021.1 software (Addinsoft, France). The calculation for Cohen's kappa and 95% confidence intervals were determined using the statistical software for Excel (XLSTAT).
Ethical approval
The Research and Ethics Committee of the Faculty of Health Sciences, University of Johannesburg approved this study (reg. no. REC 241112-035). Ethical approval was obtained from the data gatekeeper to utilise blood samples from the SANBS NPC Human Research Ethics Committee, clearance certificate number 2019/0480. All blood donors signed informed consent for their blood samples to be utilised for research purposes.
Results
Of the 715 donor specimens analysed, 63% (451/715) were confirmed to be HIV-positive, and 37% (264/715) were HIV-negative by HIV ELISA. When tested on HIV rapid assays, 62.8% (449/715) were confirmed positive and 37.2% (266/715) were confirmed negative. From the 715 specimens, 5% (34/715) were known p24 antigen-positive specimens. These specimens tested reactive on the HIV ELISA antigen/antibody tests but negative on the antibody-only tests. The known p24 antigen-positive specimens were included in the negative specimen pool as they were HIV antibody-negative specimens. The total number of HIV-negative specimens tested and analysed was 37% (264/715) (Fig 2).

Compared to the HIV ELISA reference test, the sensitivity of the Asanté HIV-1 rapid recency assay was 98.4% (95% CI 96.7 - 99.3), and the specificity was 99.6% (95 CI 97.6 - 100). Agreement between the two assays (Asanté HIV-1 rapid recency assay and ELISA) was 98.9% (95% CI 98.1 -99.7) (Table 1).
Compared to the HIV rapid reference tests, the sensitivity and specificity of the Asanté HIV-1 rapid recency assay was 98.7% (95% CI 97.0 - 99.4) and 99.2% (95% CI 97.1 - 100), respectively.
Agreement between the two assays (the Asanté HIV-1 rapid recency assay and rapid assay) was 98.9% (95% CI 98.1 - 99.7) (Table 2).
Of the 451 confirmed HIV-positive specimens, 250 were classified as LT by both Asante and LAg-avidity EIA while 168 (including 71 known recent specimens) were classified as recent by both assays. Of the LT specimens, 2% (6/257) and 0.4% (1/257) were recent and negative, respectively, on the Asante assay. Of the recent specimens, 10% (20/194) and 3% (6/194) were LT and negative, respectively, on the Asanté assay. Seven specimens that tested negative after repeat testing were excluded from the analysis because the Asanté HIV-1 rapid recency assay is only intended for confirmed positive specimens. The total number of positive specimens analysed was therefore 444 (Fig. 3). The agreement between the Asanté HIV-1 rapid recency assay and the Maxim HIV-1 LAg avidity EIA in classifying LT/recent HIV infections was 94.1% 95% CI 92.0 - 96.3); while Cohen's kappa score was 0.879 (Table 3).
Discussion
The gp41 multi-subtype protein is used by both the Asante assay and Maxim LAg-avidity EIA, which are based on the same principle of employing limiting antigen concentration to distinguish between recent and LT infections.[20] However, the Asanté assay is a lateral flow rapid test that can be carried out at an HIV clinic, healthcare facility or laboratory. In contrast, the HIV-1 LAg avidity is a laboratory-based assay that needs specific equipment and highly skilled professionals. To evaluate the performance of incidence assays, the mean duration of recent infection (MDRI) and false recent rate of the assay are characterised instead of sensitivity and specificity, which are used for evaluation of diagnostic assays.[16.30] The Asanté assay performs similarly to the LAg assay at a normalised optical density (OD-n) cut-off of 2.0, corresponding to a MDRI of 6 months. [20] The interpretation of the Asanté assay is through visual reading. However, this can be subjective and cause inter-operator variability. Therefore, when introducing and implementing recent infection surveillance, it is strongly advised that those doing RTRIs have both training and certification, as well as ongoing quality improvement and monitoring.
The Maxim LAg EIA assay includes only recency testing, and therefore requires further confirmation that specimens are HIV-positive.[16] For this purpose, specimens with an ODn <0.4 require further confirmatory testing to re-confirm HIV infection status.[20] This is to rule out false positive specimens, which can be misclassified as recent.[16,20] The Asanté assay, on the other hand, includes a verification line that confirms HIV-positive status and simultaneously classifies specimens as either recent or LT. The results from our study are encouraging and show good agreement between both assays in classifying recent infections. Our laboratory evaluation showed that the sensitivity of the Asanté HIV-1 rapid recency assay in diagnosing established HIV infections compared with ELISA and rapid assays to be 98.4% and 98.7%, respectively, which is lower than the WHO target product profile (TPP) for qualitative testing (>99%).[31] The specificity of the Asanté HIV-1 rapid recency assay, when compared with the ELISA and rapid assays, was 99.6% and 99.2%, respectively, which met the WHO guidelines (>98%).[31] The agreement for HIV established infection between the Asanté HIV-1 rapid recency assay and ELISA, and the Asanté HIV-1 rapid recency assay and HIV rapid was 98.9% (95% CI 98.1 - 99.7) and 98.7% (95% CI 98.1 - 99.7), respectively, which is higher than the criteria (>80%) defined by McHugh et al.[28]
Compared with the CDC assessment of the Asanté HIV-1 rapid recency assay conducted in 2017, our study had a lower sensitivity for detecting established infection than the CDC assessment, with a sensitivity of 99.1%.[19,20] The specificity of test results from the present study was higher when compared with 98.9% observed in the CDC study.[19,20] The CDC assessment was done using a combination of ELISA and Western blot tests, while our study used ELISA and rapid assays only. Western blot assays, a reference/ gold standard for HIV testing, are used as confirmatory tests for serology because of the high specificity of the assay.[27] The Western blot in combination with the ELISA assay will be more sensitive and specific compared with ELISA and HIV rapid test only. A combination of ELISA and Western blot is also less likely to produce false positive results than rapid tests performed on their own.[32,33] This may account for the lower sensitivity obtained from the current study compared with the CDC study.[27,32,33]
Furthermore, the differences between the sensitivity and specificity of our study and the report from the CDC may be due to the differences in diversity of subtypes and sample size. The CDC utilised a world-wide panel from Kenya, Uganda, Cameroon, Côte d'Ivoire, Sierra Leone, SA, Thailand and the USA consisting of 1 500 (920 confirmed HIV-negative and 580 confirmed HIV-positive) samples, while our study utilised SA samples with a lower sample size of 715 (264 confirmed HIV-negative and 451 confirmed HIV-positive). Additionally, HIV viral subtype diversity of the CDC panel may have contributed to the observed differences. Although the gp 41 is highly conserved across subtypes and the assay unlikely to be affected by subtype, other components specific to subtype such as structural conformation may play a role in antibody recognition. These differences could also be attributed to the use of an automated rapid reader that was confirmed by visual interpretation in the CDC study, whereas our study results were based on visual interpretation only.[19,20] The testing agreement of 99.0% achieved by the CDC study was the same as that we achieved when comparing performance with the ELISA and rapid assays.[19,20]
The performance of the HIV LT/recent infection line of the Asanté HIV-1 rapid recency assay compared with the HIV-1 LAg avidity assay showed a high agreement of 94.1% (95% CI 92.0 - 96.3); with Cohen's kappa of 0.879.[28] The kappa value showed strong agreement (0.80 - 0.90).[28] The agreement between the Asanté HIV-1 rapid recency assay and the HIV-1 LAg avidity assay to identify HIV recent infections correctly met the agreement requirements for diagnostic accuracy. The sensitivity and specificity to differentiate recent from LT infection was 89.4% (95% CI 84.0 - 93.0) and 97.7% (95% CI 94.8 - 99.0), respectively, in the current study. Compared with the 2017 CDC evaluation data for visual analysis, where the agreement was 91.7% (95% CI 89.1 - 93.7) and the kappa value was 0.722, our study data showed a higher agreement.[20] The CDC study did not report sensitivity and specificity for recent and LT infections.[20] While the cause of the difference between the agreement is not known, we speculate that it could be due to a difference in the sample number where specimens from various countries were tested in the CDC evaluation, whereas our study was confined to SA specimens only.[19,20]
In another validation study of the Asanté HIV-1 rapid recency assay conducted in Uganda in 2021 using archived specimens, the agreement between two different laboratories and two different testers was 72% and 80%, respectively.[34] The agreement varied substantially as results were read visually. This could be due to inconsistent training, subjective interpretation of test bands, technical errors, problematic test devices and a smaller number (<50) of specimens used in the comparison.[32] The agreement achieved in the Ugandan study was lower than the performance of our study.[34] In contrast, the current study results were also read through visual observation of the test bands, suggesting that reliable results could be achieved in our setting.
Although the Ugandan and the present study utilised archived specimens, the Ugandan study reported higher sensitivity of the Asanté HIV-1 rapid recency assay to determine established infection of ~99.2% (803/809).[32] However, our study reported a sensitivity of 98.4% (444/451) and 98.2% (443/451) when compared with the ELISA and rapid tests, respectively, which was also below the WHO criteria of 99%.[31] The Ugandan study suggested that using an automated reader could improve the sensitivity of the Asanté HIV-1 rapid recency assay. However, more data on using an automated reader to interpret the Asanté HIV-1 rapid recency assay test results are needed to explore further the utility of the automated reader v. visual reading in the Ugandan study. Nonetheless, using the automated reader may not be cost-effective for deployment in POC settings.
Limitations
This study had limitations. Firstly, we utilised specimens from SANBS, which represented eight of nine provinces in SA, as we could not obtain specimens from the Western Cape Blood Services. Thus, our study cannot be generalised to Western Cape Province. Secondly, compared with the CDC evaluation, the CDC study contained a larger evaluation panel (1 500 specimens) and a wider range of HIV subtypes (A, B, C, D and AE) from different countries. In contrast, the present sample set was confined to specimens obtained from SANBS, SA, where only subtype C was possibly predominant.[35] Lastly, as the sampling technique was convenience (non-probabilistic), the conclusion of the study may not be generalisable to the general population and is limited to just SANBS donor resources. A higher proportion of HIV-positive specimens were intentionally included in the study as the Asanté assay is only intended for confirmed HIV-positive specimens. In order to determine whether these infections were recently acquired or >12 months, it was crucial to include a greater proportion of HIV-positive specimens.
Conclusion
Although the sensitivity of the Asanté HIV-1 rapid recency assay relative to the ELISA or rapid kits was high (>90%), our result did not meet the WHO recommendation of >99% for established infection[31] Nevertheless, the number of discrepant results between routine HIV testing and the Asanté HIV confirmation will likely be limited and can be resolved by further testing. Compared with the Maxim HIV-1 LAg avidity assay, the Asanté HIV-1 rapid recency assay demonstrated a good agreement (>80%). These findings indicated a high level of agreement between the two tests. Rapid testing and identification of new HIV infections are key to controlling infectious diseases, identifying those who are difficult to reach, and interrupting further transmission.[11] Therefore, applying the Asanté HIV-1 rapid recency assay at a POC site as a tool for determining recency infections will provide informed decisions in these areas, e.g. identification of HIV 'hot spots' that require enhanced responses. For this reason, we propose using the Asanté HIV-1 rapid recency assay in a POC HIV testing services (HTS) algorithm for determination of HIV-1 recent infections among HIV confirmed individuals.
The Asanté HIV-1 rapid recency assay is recommended as a POC tool for the determination of HIV-1 recent infections. Until larger studies or a systematic review of all available evidence is conducted, the Asanté HIV-1 rapid recency assay may not be recommended as a POC test for established HIV-1 infections. Recommendations for future studies are: (i) to address the current study's limitations, we propose that the evaluation of a diverse set of panels be included in future studies where specimens are collected from the nine provinces in SA and not confined to blood donor services. These specimens could be obtained from archived specimens evaluated in SA surveillance studies that represent the general population; and (ii) we suggest that whole blood specimens, the representative sample type, if required for the study design, be obtained from routine HIV testing sites and in real time.
Declaration. This study was required for a Master's degree for BS. The formal qualification is a Master in Technology (Biomedical Technology) and was awarded in September 2022.
Acknowledgements. The authors would like to thank the staff of the NICD, HIV Sero-Molecular Laboratory, who provided technical assistance, processed samples and supported the laboratory testing phase. We acknowledge the contributions of Ushmita Patel, Sara Hloma, Rivashni Jagaroo, Candice Subramunian, Bongiswa Tshewula, Emma Goetsch, Deirdre Greyling, Nicolas Tagliatti and Olwetu Tuswa to this research. We would also like to thank Andrew Saville from the SANBS, who provided archived donor blood, documentation for the application for ethics approvals, and donor demographics. Special thanks to Bharat Parekh, Ernest Yufenyuy and Xiaojuan Tan from the CDC, ILB Branch, Atlanta, USA, for their technical support throughout this process.
Author contributions. The concept/design of the work was carried out by BS, AP, GO and JM. AP, GO and JM oversaw the project. BS conducted the testing on the Asanté HIV-1 rapid recency assay. Data analysis and interpretation were carried out by GO, MG and HJ. Drafting the article was carried out by BS, AP and GO. Critical revision of the article was done by AP, JM, GO, EC, ZB, MG and HJ. Final approval of the version to be published was completed by all authors.
Funding. NICD.
Conflicts of interest. None.
References
1. Joint United Nations Programme on HIV and AIDS. Country Fact Sheets South Africa 2021. https://www.unaids.org/en/regionscountries/countries/southafrica (accessed 5 August 2022). [ Links ]
2. Joint United Nations Programme on HIV and AIDS. Fact Sheet 2022 https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pi (accessed 5 August 2022). [ Links ]
3. Joint United Nations Programme on HIV and AIDS. 90-90-90 An ambitious treatment target to help end the AIDS Epidemic. Geneva, 2014 UNAIDS/JC2684. https://www.unaids.org/en/resources/documents/2017/90-90-90 (accessed 11 February 2022). [ Links ]
4. Joint United Nations Programme on HIV and AIDS. Understanding Fast-Track. Geneva, Switzerland. 2015. https://www.unaids.org/en/resources/documents/2015/201506_JC2743_Understanding_FastTrack (accessed 13 September 2021). [ Links ]
5. Johnson CC, Dalal S, Baggaley R, Taegtmeyer M. A public health approach to addressing and preventing misdiagnosis in the scale-up of HIV rapid testing programmes. J Int AIDS Society 2017;20(Suppl 6):S22190. https://doi.org/10.7448/IAS.20.7.22190 [ Links ]
6. National Department of Health. National HIV Testing Services: Policy 2016, Republic of South Africa. Pretoria, South Africa: National Department of Health, 2016. https://sahivsoc.org/Files/HTS%20Policy%2028%20July%20final%20copy.pdf (accessed 6 March 2022). [ Links ]
7. Simbayi LC, Zuma K, Zungu N, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017. Cape Town, HSRC Press, 2019. http://www.hsrc.ac.za/uploads/pageContent/10779/SABSSM%20V.pdf (accessed 7 March 2022). [ Links ]
8. Mabunda SA, Sigovana K, Chitha W, Apalata T, Nomatshila S. Socio-demographic associations of HIV among women attending antenatal care in selected rural primary care facilities in South Africa's Eastern Cape province. BMC Infect Dis 202131(1):61. https://doi.org/10.1186/s12879-020-05744-7 [ Links ]
9. Incidence Assay Critical Path Working Group. More and better information to tackle HIV epidemics: Towards improved HIV incidence assays. PLoS Med 2011;8(6):e1001045. https://doi.org/10.1371/annotation/0931760c-95fa-441c-a797-7b410a4c9326 [ Links ]
10. Mastro TD, Kim AA, Hallett T, et al. Estimating HIV Incidence in populations using tests for recent infection: Issues, challenges and the way forward. J HIV AIDS Surveil Epidem 2010;2(1):1-14. [ Links ]
11. Kim AA, Behel S, Northbrook S, Parekh BS. Tracking with recency assays to control the epidemic: Real-time HIV surveillance and public health response. Aids 2019;33(9):1527-1529. https://doi.org/10.1097/QAD.0000000000002239 [ Links ]
12. World Health Organization, AIDS JUNPoHa. WHO Protocol for Performance Laboratory Evaluation of HIV Serology Assays. Geneva: WHO, 2017. https://www.who.int/diagnostics_laboratory/evaluations/alter/171219_protocol_pqdx_030_v10_hiv_serology.pdf (accessed 15 March 2022). [ Links ]
13. World Health Organization. Estimating HIV incidence using HIV case surveillance. Geneva: WHO, 2015. https://www.who.int/publications/i/item/WHO-HIV-2017.03 (accessed 6 March 2022). [ Links ]
14. World Health Organization. When and how to use assays for recent infection to estimate HIV incidence at a population level. Geneva: WHO, 2011. https://www.who.int/diagnostics_laboratory/hiv_incidence_may13_final.pdf (accessed 6 March 2022). [ Links ]
15. Bárnighausen T, Wallrauch C, Welte A, et al. HIV incidence in rural South Africa: Comparison of estimates from longitudinal surveillance and cross-sectional cBED assay testing. PloS One 2008;3(11):e3640. https://doi.org/10.1371/journal.pone.0003640 [ Links ]
16. Duong YT, Qiu M, De AK, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: Potential for HIV-1 incidence estimates and avidity maturation studies. PloS One 2012;7(3):e33328. https://doi.org/10.1371/journal.pone.0033328 [ Links ]
17. Wei X, Liu X, Dobbs T, et al. Development of two avidity-based assays to detect recent HIV type 1 seroconversion using a multisubtype gp41 recombinant protein. AIDS Res Hum Retroviruses 2010;26(1):61-71. http://doi.org/10.1089/aid.2009.0133 [ Links ]
18. Centers for Disease Control and Prevention. Terms, definitions, and calculations used in CDC HIV surveillance publications. Atlanta: CDC, 2016. https://www.cdc.gov/hiv/statistics/surveillance/terms.html (accessed 7 February 2022). [ Links ]
19. Yufenyuy EL, Detorio M, Dobbs T, et al. Performance evaluation of the Asante Rapid Recency Assay for verification of HIV diagnosis and detection of recent HIV-1 infections: Implications for epidemic control. PLOS Glob Pub Health 2022;2(5):e0000316. https://doi.org/10.1371/journal.pgph.0000316 [ Links ]
20. Parekh B, Detorio M, Shanmugam V, et al. Performance evaluation of Asante Rapid Recency Assay for HIV diagnosis and detection of recent infection: Potential for surveillance and prevention. Paris: Centers for Disease Control, 2017. [ Links ]
21. Granade TC, Nguyen S, Kuehl DS, Parekh BS. Development of a novel rapid HIV test for simultaneous detection of recent or long-term HIV type 1 infection using a single testing device. AIDS Res Hum Retroviruses 201339(1):61-67. http://doi.org/10.1089/aid.2009.0133 [ Links ]
22. Yufenyuy EL, Detorio M, Tan X, et al. Evaluation of rapid tests for recent HIV Infection: Implications for real-time surveillance and epidemic control. Atlanta: Centers for Disease Control, 2019. https://hivtestingconference.org/wp-content/uploads/2019/04/42-Parekh.pdf (accessed 8 March 2022). [ Links ]
23. Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016;6(11):e012799. https://doi.org/10.1136/bmjopen-2016-012799 [ Links ]
24. Castro AR, Kikkert SE, Fears MB, Pope V. Defibrination of blood plasma for use in serological tests for syphilis. Clin Diagnostic Lab Immunol 2002;9(6):1376-1378. https://doi.org/10.1128/cdli.9.6.1376-1378.2002 [ Links ]
25. World Health Organization. WHO manual for organising a national external quality assessment programme for health laboratories and other testing sites Geneva: WHO, 2016. https://apps.who.int/iris/handle/10665/250117 (accessed 6 March 2022). [ Links ]
26. World Health Organization. HIV assays: Operational characteristics (phase 1), report 15, antigen/ antibody ELISAs. Geneva: WHO, 2004. https://www.who.int/diagnostics_laboratory/publications/en/HIV_Report15.pdf (accessed 26 February 2022). [ Links ]
27. Parekh BS, Ou CY, Fonjungo PN, et al. Diagnosis of human immunodeficiency virus infection. Clin Microbiol Rev 2019;32(1):e00064-18. https://doi.org/10.1128/CMR.00064-18 [ Links ]
28. McHugh ML. Interrater reliability: The kappa statistic. Biochemia Medica 2012;22(3):276-282. [ Links ]
29. Pereira P. Basic validation of qualitative tests. Madison: Westgard QC. 2016. https://www.westgard.com/basic-method-validation.htm (accessed 13 September 2021). [ Links ]
30. Duong YT, Kassanjee R, Welte A, et al. Recalibration of the limiting antigen avidity EIA to determine mean duration of recent infection in divergent HIV-1 subtypes. PLoS One 2015;10(2):e0114947. https://doi.org/10.1371/journal.pone.0114947 [ Links ]
31. World Health Organization. HIV assays operational characteristics: HIV rapid diagnostic tests (detection of hiv 1/2 antibodies) report 17. Geneva: WHO, 2013. https://apps.who.int/iris/handle/10665/93679 (accessed 6 March 2022). [ Links ]
32. Mehra B, Bhattar S, Bhalla P, Rawat D. Rapid tests versus ELISA for screening of HIV infection: Our experience from a voluntary counselling and testing facility of a tertiary care centre in North India. ISRN AIDS 2014;729684. https://doi.org/10.1155/2014/296840 [ Links ]
33. Augusto ÂDR, Iriemenam NC, Kohatsu L, et al. High level of HIV false positives using EIA-based algorithm in survey: Importance of confirmatory testing. PLoS One 2020;15(10):e0239782. https://doi.org/10.1371/journal.pone.0239782 [ Links ]
34. Galiwango RM, Ssuuna C, Kaleebu P, et al. Validation of the Asante HIV-1 Rapid recency assay for detection of recent HIV-1 infections in Uganda. AIDS Res Hum Retroviruses 2021;37(12):893-896. https://doi.org/10.1089/AID.2020.0279 [ Links ]
35. Giovanetti M, Ciccozzi M, Parolin C, Borsetti A. Molecular epidemiology of HIV-1 in African countries: A comprehensive overview. Pathogens 2020;9(12):1072. https://doi.org/10.3390/pathogens9121072 [ Links ]
 Correspondence:
Correspondence:
B Singh
beverleys@nicd.ac.za
Accepted 3 August 2023














