Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.10 Pretoria Out. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i10.746
RESEARCH
Impact of COVID-19 lockdown on low birthweight in Soweto, South Africa
R E DrysdaleI; W SlemmingII; D MombergIII; R Said-MohamadIII; L M RichterI
IPhD; DSI-NRF Centre of Excellence in Human Development, University of the Witwatersrand, Johannesburg, South Africa
IIPhD; Division of Community Paediatrics, Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIPhD; SAMRC/Wits Developmental Pathways for Health Research Unit, Department of Paediatrics and Child Health, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Pregnant women were indirectly affected by the COVID-19 pandemic owing to heightened stress, fear of mother-to-child transmission of COVID-19 and the disruption of antenatal health services. Increased stress and lack of antenatal healthcare could result in an increase in adverse birth outcomes such as preterm birth or low birthweight
OBJECTIVES: Using a case-control design, to compare the prevalence of low birthweight among infants born before and during the pandemic in Soweto, South Africa
METHOD: Infants born before the pandemic and national lockdown were included in the control group, while infants who were in utero and born during the pandemic were included in the case group. Only infants born >37 weeks' gestation with no birth complications were included. Multivariable logistic regression was employed to determine whether the pandemic was associated with an increase in low birthweight. A birthweight <2.5 kg was classified as low birthweight
RESULTS: In total, 199 mother-infant pairs were included in the control group, with 201 mother-infant pairs in the case group. The prevalence of low birthweight was 4% in the control group and 11% in the case group, with those born during the pandemic at a higher risk of being of low birthweight
CONCLUSION: The high prevalence of low birthweight in infants born >37 weeks' gestation during the pandemic could result in an increase in child stunting and poor development. Future research should measure early child development and growth in infants born during the pandemic to assess whether there is a need to intervene and provide additional support to minimise the negative effects
In March 2020, the World Health Organization (WHO) declared the novel coronavirus (COVID-19) outbreak a global pandemic.[1] Within a few months, the virus had spread across the globe, with over 5.5 million confirmed cases and 350 000 deaths by the end of May 2020.[2] Two years later, fuelled by five variants of concern (Alpha, Beta, Gamma, Delta and Omicron),[3] there had been over 505 million confirmed cases and 6 million deaths.[4] In order to limit the spread of the virus, governments across the globe implemented lockdowns of varying severity, restricted movement within and between countries, implemented curfews, closed schools and universities and suspended a large number of formal and informal social and economic activities. Restrictions in many countries have since been eased with the introduction of vaccines, but enduring and indirect consequences of government containment efforts are becoming apparent.
In March 2020, South Africa (SA) implemented one of the strictest national lockdown policies globally, and followed a risk-adjusted strategy, with lockdown levels ranging from level 5 (the most restrictive) to 1 (the least restrictive), moving between levels depending on the risk of community transmission. There were various restrictions on movement, with travel bans, border closures and curfews, the sale of non-essential items was limited and slowly re-introduced as restrictions eased, cigarette and alcohol sales were banned, schools were closed and gathering numbers were limited, for social, economic and religious events. Despite the restrictions, SA has confirmed over 4 million COVID-19 cases and over 100 000 deaths.[5] In addition, the country has been indirectly affected, with increasing unemployment,[6] rising household and child hunger[7,8] and increasing depression associated with unemployment, hunger and loss of income.[9]
Pregnant women in particular were negatively affected by the COVID-19 pandemic, both directly and indirectly. Not only were pregnant women with COVID-19 at higher risk of experiencing severe illness and preterm births,[10,11] but the pandemic and lockdown heightened stress and anxiety[12-14] and disrupted antenatal services.[14] In addition, fear of mother-to-child transmission of COVID-19 resulted in mothers and infants being separated, and there was confusion and mixed messages around breastfeeding.[15,16] Prenatal stress and poor attendance at routine antenatal health services are high risk factors for preterm birth, low birthweight and complications at birth,[17-20] all of which can negatively impact an individual throughout their life.[21-24] In addition, evidence suggests that children conceived or in utero during an epidemic or natural disaster are more likely to experience life-long negative consequences, such as reduced education attainment and higher risk of non-communicable diseases and mental health problems.[25-28] This results from the first 1 000 days, from conception to 2 years, being a time of tremendous potential and vulnerability, when the foundations for health, growth and neurodevelopment are established. The way in which a mother and child are cared for during this time can have serious implications for future development.
Research in SA has mainly focused around birth outcomes in women with active COVID-19 infections, assessing the direct impact of the pandemic. While the outcomes vary across studies depending on the data available and the population studied, common outcomes reported included low birthweight, preterm birth and still birth.[29-33] There is, however, limited evidence available on how the COVID-19 pandemic and lockdown indirectly affected pregnant women and birth outcomes in SA. This article compares the prevalence of low birthweight among infants born before and during the COVID-19 pandemic to women without COVID-19 infection in Soweto, SA.
Methods
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation in SA and with the Helsinki Declaration of 1975, as revised in 2008, and it has been approved by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (ref. nos M170753; M170872; M170955; M181915; M200605). Permission was obtained from Chris Hani Baragwanath Academic Hospital (CHBAH) to recruit participants from the Foetal Medicine Unit and the maternity ward. Written consent was obtained from all participants, who were given a unique identifier to maintain confidentiality. A data sharing agreement was created between the principal investigators of the three studies.
Design
The study followed a case-control design and compared data from two groups of infants and their mothers. The control group were born before the COVID-19 pandemic, and were involved in two separate studies, namely the Soweto Baby Wash Study (SBW)'34 and the Healthy Pregnancy Healthy Baby Study (HPHB).'351 The case group were born during the COVID-19 pandemic and were involved in a study entitled Child Growth During a Global Pandemic (CGGP). Both primary and secondary data from the three studies were utilised.
Setting
The study is set in Soweto, the largest township in SA, located outside of Johannesburg. It has a population of 1.2 million'361 and the highest number of people living under the poverty line in the City of Johannesburg metropolitan area.'371 Recruitment procedures took place at CHBAH, where approximately 60 000 women deliver their babies in the maternity unit per year.'381 All follow-up procedures took place at the SAMRC/Wits Developmental Pathways for Health Research Unit (DPHRU), which is located in the CHBAH grounds.
Participants
The participants in the three studies were recruited from the same population group and had similar recruitment and study procedures. For each study, only women aged >18 years of age, who were planning to reside in Soweto for at least 12 months after giving birth, and had a singleton pregnancy with no fetal abnormalities detected during pregnancy or at birth, were invited to participate. Women were recruited while either attending a routine antenatal health visit during pregnancy (HPHB) or from the postnatal labour ward after giving birth (SBW; CGGP). Additional eligibility criteria for those in SBW and CGGP included no birth complications for mother or baby, and a gestational age of >37 weeks, both of which were recorded from hospital birth records. Mothers or infants in the case group with a confirmed and active COVID-19 infection at the time of recruitment were not eligible for participation.
Sample size
Participants in the control group were included in the study if data on sociodemographic characteristics and birth outcomes were complete. Those in HPHB were only included if their gestational age was >37 weeks as per their Road to Health Book (RTHB), and if they reported no complications for mother or baby at birth. This was to measure growth in otherwise healthy infants, and to match the eligibility criteria as the other two studies. In total, this made up 199 mother-infant pairs, 65 from SBW and 134 from HPHB.
The sample size for participants in the case group was calculated using length-for-age z-scores (LAZ) based on data from the SBW study.'[34] Based on a standard deviation of LAZ of 0.96, a two-sided significance level of 0.05, power of 80% and a difference in LAZ of 0.3, the desired sample size was 161. Assuming an attrition rate of 15%, a target of 185 was set. However, owing to the ongoing pandemic and the uncertainty around SA's lockdown restrictions, additional participants were recruited, to a total of 203 mother-infant pairs.
Data collection
Data were collected between January 2018 and March 2018 (SBW) and April 2019 and March 2020 (HPHB) for the control group, and November 2020 and April 2021 for the case group. All data from those in SBW and CGGP were collected during recruitment after birth, while data from HPHB were collected during an antenatal visit and a 6-week postnatal follow-up visit.
In all studies, birthweight was recorded from either the hospital records or the child's RTHB. This measurement will have been taken and recorded by hospital staff at birth. Participants were only included in the analysis if this measurement was available. Gestational age recorded from birth records or RTHB was used to determine pregnancy term. Pregnancy term was categorised as early term (37 0/7 weeks through 38 6/7 weeks), full term (39 0/7 weeks through 40 6/7 weeks), late term (41 0/7 weeks through 41 6/7 weeks) and post term (>42 0/7 weeks).[39]
Sociodemographic characteristics of the mother and their household were collected through individual questionnaires. This included the mother's age, education level, employment and relationship status. Relationship status was coded as a binary response using single or committed. Participants were reported as being committed if they were married (including traditional or customary) or if they considered themselves in a committed relationship, whether they lived together or not. In addition, information on the dwelling type, water and toilet provision, number of rooms used for sleeping and asset ownership was collected. Asset ownership included, but was not limited to, items such as a television, internet, cellphone, computer and fridge.
Data analysis
Data were captured and analysed using Stata/IC 15 (StataCorp, USA).
Descriptive data are presented as frequencies and proportions for categorical data, or mean and standard deviations for continuous data. An infant was categorised as being born with low birthweight if their birthweight was <2.5 kg. Asset ownership is measured as a cumulative score of ownership of 11 items. For categorical variables, Pearson's x2 was used to determine whether there were significant differences between the groups. For continuous variables, Student's f-test was used to determine whether there were significant differences between the means of the two groups.
The prevalence of low birthweight between the groups was compared using Pearson's x2. To confirm whether exposure to the indirect effects of the pandemic impacted fetal growth and the prevalence of low birthweight, univariate and multivariable logistic regression was conducted. The multivariable analysis controlled for sociodemographic variables ofp<0.1 in the x2 and t-test analysis, and included mother's age, relationship status and dwelling type. All statistical tests were considered significant at p<0.05.
Results
In total, data from 400 mother-infant pairs were analysed: 199 in the control group (135 from HPHB and 64 from SBW) and 201 in the case group.
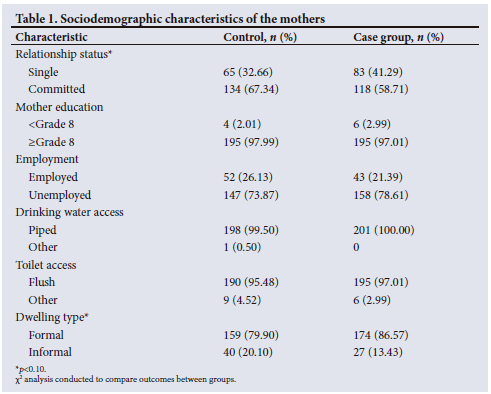
Sociodemographic characteristics
Table 1 highlights the sociodemographic characteristics of the mothers. The mean (standard deviation (SD) age of the mothers in the control group was 31 (6.92) years, while the mean (SD) age of those in the case group was 29 (6.53) years. The mean age of the mothers differed significantly between the two groups (p=0.002). For both groups of participants, the mean (SD) number of assets and rooms used for sleeping in the household were 7 (1.85) and 2 (1.13), respectively. There were no significant differences between the mothers' education, employment, drinking water or toilet access. There was some evidence to suggest a significant difference in the mothers' relationship status, with 33% of the control group being single compared with 41% of the case group (p=0.074). This was also the case for dwelling type, with 2% living in an informal dwelling in the control group compared with 13% in the case group (p=0.074).
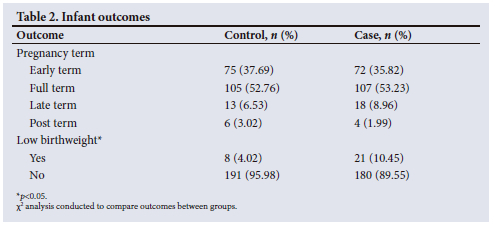
Infant outcomes
The mean (SD) gestational age of the infants in both the control and case groups was 39 (1.25) weeks. As shown in Table 2, there were no significant differences in pregnancy term in infants born >37 weeks' gestation before and during the pandemic. The majority in both groups were born full term (53%), followed by early term. The mean birthweight of those in the control group was 3.12 kg, and 3.10 kg in the case group, with no significant difference between the means. The prevalence of low birthweight was 4% in the control group and 11% in the case group. The difference between the groups was statistically significant (p=0.013). The results of the regression analysis, as shown in Table 3, indicate that those in the case group were between two and three times more likely to be born with low birthweight (p=0.014) compared with the control group.
Discussion
This paper compares the prevalence of low birthweight among infants born in Soweto, SA, before and during the COVID-19 pandemic and national lockdown. In total, 4% of infants born before the pandemic were born of low birthweight, compared with 11% born during. Results from the regression analysis indicate that infants born >37 weeks' gestation with no complications during the pandemic were between two to three times more likely to be born of low birthweight compared with those born before. This suggests that the COVID-19 pandemic and national lockdown indirectly affected fetal growth and birthweight.
The national prevalence of low birthweight in SA prior to the COVID-19 pandemic was 13%.[40] In Gauteng Province, where the study took place, this was slightly higher at 14.6%.[40] Although the prevalence of low birthweight in both groups in this study was below the national average, the figures presented by the National Department of Health (NDoH) include infants who were born prematurely and those who experienced complications at birth, both of which are strongly associated with low birthweight.[41-44] In this study, we only included infants who were born >37 weeks' gestation with no reported complications. If national statistics were to do something similar, with an estimated 12.4% of infants in SA born prematurely,[45] it is likely that the prevalence of low birthweight would be lower and possibly closer to that of the control group. The prevalence rate of 11% in the case group thus suggests that the pandemic has negatively affected in utero growth in otherwise healthy infants.
Contrary to our findings, studies in high-income countries found that the prevalence of low birthweight actually decreased during the pandemic.[46,47] It is speculated that this may be due to behaviour modifications, such as working from home removing the need and stress of a daily commute, improved social distancing and personal hygiene practices limiting the threat of infection or illness, increased sleep and rest while confined to the home, partner
presence and reduced exposure to air pollutants.[46-48] Low-resource settings and middle-income countries such as SA have different environments and individuals, particularly the poorest, and are unlikely to experience the same benefits during lockdown as those in high-income settings. For example, with approximately 58% of dwellings in Soweto considered to be overcrowded,[49] the possibility of social distancing in the home remained low. In addition, working from home is not possible for the majority in SA, with differences between racial groups, formal and informal jobs and employment sectors.[50] There is also evidence to suggest that the lockdown in SA was associated with increasing unemployment and child and household hunger.[6,7] Women in particular were disproportionally affected, as not only was unemployment and job loss higher in women, but they were also increasingly responsible for childcare duties when schools and early child development centres were closed.[51]
Further, while routine antenatal care attendance remained high throughout lockdown, evidence from SA suggests that women were delaying their first visit, with fewer attending antenatal care before 20 weeks' gestation in 2020 compared with 2019.[52] In other African countries, insufficient care and low attendance at maternal health visits were reported[53] due to lack of transportation, isolation, fear of the virus and confusion about what services were being offered.[54-56] In SA, people reportedly changed their health-seeking behaviours throughout lockdown by not seeking care when needed, and by not accessing chronic medication.[57-58] Their reasons for doing so included fear of COVID-19 infection, no money or transport available, and fear of being arrested or fined for leaving their homes.[57,58] It is possible that pregnant women delayed their first antenatal visit for similar reasons. Low attendance at routine antenatal health appointments increases the risk of poor pregnancy outcomes,[19] including low birthweight,[20] and while women appeared to attend some appointments, by delaying their first visit, they were unlikely to have attended the recommended eight visits.
Being born with low birthweight can have a significant impact on an individual throughout their life, including, but not limited to, increased risk of dying in the first year of life, higher rates of childhood illnesses, poor cognitive development and poor growth.[59-65] The high prevalence of low birthweight among infants born >37 weeks' gestation during the pandemic could result in an increase in child stunting and a higher number of children not reaching their full developmental potential.
Strengths and limitations
To our knowledge, this is one of the only studies in SA that has assessed the indirect effects of the COVID-19 pandemic on fetal growth and low birthweight. Having similar data from before the pandemic was a key strength of the study. However, because secondary data from previous studies were utilised, the variables available were limited. For instance, maternal variables that may contribute towards poor fetal growth, such as smoking, alcohol or dietary habits, were not available. That said, the results of the study do provide a useful indication of how the pandemic has indirectly affected children in SA.
Conclusion
The results of this study highlight the fact that the COVID-19 pandemic indirectly affected fetal growth and increased the risk of low birthweight in infants born during the national lockdown in Soweto, SA. With low birthweight being a risk factor for stunting and poor child development, there is a need to continuously measure early child development and growth among infants born during the pandemic to assess whether there is a need to intervene and provide additional support to minimise negative effects.
Declaration. None.
Acknowledgements. The support of the DSI NRF Centre of Excellence (CoE) in Human Development at the University of the Witwatersrand towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at are those of the authors, and are not necessarily to be attributed to the CoE in Human Development. Additional support from the SAMRC/Wits Centre for Health Economics and Decision Science - PRICELESS SA was provided to Dr Drysdale.
Author contributions. RED conceptualised the paper, analysed the data and wrote the manuscript. WS, DM, RSM and LMR provided substantial contributions and approved the final version for publication. All authors have read and approved the manuscript.
Funding. This research is funded by Saving Brains, a partner of Grand Challenges Canada, the Aga Khan Foundation Canada, the Bernard van Leer Foundation, the Bill and Melinda Gates Foundation, the ELMA Foundation, Grand Challenges Ethiopia, the Maria Cecilia Souto Vidigal Foundation, the Palix Foundation, the UBS Optimus Foundation and World Vision Canada (SB-POC-1810-19664), the DSI-NRF Centre of Excellence in Human Development (ACC2020-COVIDHD-5) (ACC2017007), DSI-NRF Centre of Excellence in Food Security (160502) and SAMRC/Wits Developmental Pathways for Health Research Unit. The funders did not play a role in the study design, collection, analysis or interpretation of the data or the writing of the manuscript.
Conflicts of interest. None.
References
1. World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. WHO, 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed 24 April 2022). [ Links ]
2. Johns Hopkins University. Coronavirus Resource Centre. Baltimore: Johns Hopkins University, 2020. https://coronavirus.jhu.edu/map.html (accessed 27 May 2020). [ Links ]
3. World Health Organization. Tracking SARS-CoV-2 variants. Geneva: WHO, 2022. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed 24 April 2022). [ Links ]
4. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Geneva: WHO, 2022. https://covid19.who.int/ (accessed 24 April 2022). [ Links ]
5. South Africa Coronavirus Portal. Update on COVID-19 (Friday 29 July 2022). Pretoria: SA Coronavirus Portal, 2022. https://sacoronavirus.co.za/2022/07/29/update-on-covid-19-friday-29-july-2022/ (accessed 20 June 2022). [ Links ]
6. Statistics South Africa. Quarterly Labour Force Survey: Quarter 1 2021, 2021. Pretoria: Stats SA, 2021. [ Links ]
7. Van der Berg S, Patel L, Bridgman G. Hunger in South Africa during 2020: Results from Wave 3 of NIDS-CRAM. National Income Dynamics Study (NIDS) - Coronavirus Rapid Mobile Survey (CRAM), 2021. https://cramsurvey.org/wp-content/uploads/2021/02/10.-Van-der-Berg-S.-Patel-L.-Bridgman-G.-2021-Hunger-in-South-Africa-during-2020-Results-from-Wave-3-of-NIDS-CRAM-1.pdf (accessed 28 May 2021). [ Links ]
8. Richter LM, Naicker SN. A data free digital platform to reach families with young children during the COVID-19 pandemic: Online survey study. JMIR Pediat Parent 2021; 4(2), e26571. https://doi.org/10.2196/26571 [ Links ]
9. Hunt X, Breet E, Stein DJ, Tomlinson M. The COVID-19 pandemic, hunger, and depressed mood among South Africans. National Income Dynamics Study (NIDS) - Coronavirus Rapid Mobile Survey (CRAM), 2021. https://cramsurvey.org/wp-content/uploads/2021/07/6.-Hunt-X.-Breet-E.-Stein-D.-_-Tomlinson-M.-2021-The-COVID-19-Pandemic-Hunger-and-Depressed-Mood-Among-South-Africans.pdf (accessed 15 July 2021). [ Links ]
10. Woodworth KR, Olsen EO, Neelam, V, et al Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions March 29 - October 14, 2020. MMWR Morb Mortal Weekly Rep 2020;69(44):1635-1640. https://doi.org/10.15585/mmwr.mm6944e2 [ Links ]
11. Zambrano LD, Ellington, S, Strid, P, et al Update: Characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22 - October 3, 2020. MMWR Morb Mortal Weekly Rep 2020;69(44):1641-1647. https://doi.org/10.15585/mmwr.mm6944e3 [ Links ]
12. Mappa I, Distefano FA, Rizzo G. Effects of coronavirus 19 pandemic on maternal anxiety during pregnancy: A prospectic observational study. J Prenat Med 2020;48(6):545-550. https://doi.org/10.1515/jpm-2020-0182 [ Links ]
13. King LS, Feddoes DE, Kirshenbaum JS, Huphreys KL, Gotlib IH. Pregnancy during the pandemic: The impact of COVID-19-related stress on risk for prenatal depression. Psychol Med 2021;53(1):1-11. https://doi.org/10.1017/s003329172100132x [ Links ]
14. Preis H, Mahaffey B, Heiselman C, Lobel M. Pandemic-related pregnancy stress and anxiety among women pregnant during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol 2020;2(3):100155. https://doi.org/10.1016%2Fj.ajogmf.2020.100155 [ Links ]
15. Tomori C, Gribble K, Palmquist AEL, Ververs MT, Gross MS. When separation is not the answer: Breastfeeding mothers and infants affected by COVID-19. Matern Child Nutr 2020;16(4):e13033. https://doi.org/10.1111/mcn.13033 [ Links ]
16. Wang L, Shi Y, Xiao T, et al Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection. Ann Transl Med 2020;8(3):47. https://doi.org/10.21037/atm.2020.02.20 [ Links ]
17. Harville EW, Xiong X, Buekens P. Disasters and prenatal health: A systematic review. Obstet Gynecol Surv 2010;65(11):713-728. https://doi.org/10.1097/ogx.0b013e31820eddbe [ Links ]
18. Ibrahim SM, Lobel M. Conceptualization, measurement, and effects of pregnancy-specific stress: Review of research using the original and revised prenatal distress questionnaire. J Behav Med 2020;43(1):16-33. https://doi.org/10.1007/s10865-019-00068-7 [ Links ]
19. Raatikainen K, Heiskanen N, Heinonen S. Under-attending free antenatal care is associated with adverse pregnancy outcomes. BMC Public Health 2008;7:268. https://doi.org/10.1186/1471-2458-7-268 [ Links ]
20. Zhou H, Wang A, Huang X, et al. Quality antenatal care protects against low birth weight in 42 poor countries of Western China. PLoS One 2019;14(1):e0210393. https://doi.org/10.1371/journal.pone.0210393 [ Links ]
21. Lambiris MJ, Blakstad MM, Perumal N, et al. Birth weight and adult earnings: A systematic review and meta-analysis. J Dev Orig Health Dis 2021;13(3):284-291. https://doi.org/10.1017/s2040174421000404 [ Links ]
22. Vogel JP, Chawanpaiboon S, Moller AB, et al. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol 2018;52:3-12. https://doi.org/10.1016/j.bpobgyn.2018.04.003 [ Links ]
23. Beauregard JL, Drews-Botsch C, Sales JM, Flanders WD, Kramer MR. Preterm birth, poverty, and cognitive development. Pediatrics 2018;141(1):e20170509. https://doi.org/10.1542/peds.2017-0509 [ Links ]
24. Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, Siahanidou T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: A systematic review and meta-analysis. J Pediatr 2019;210:69-80. https://doi.org/10.1016/j.jpeds.2019.02.041 [ Links ]
25. Almond D. Is the 1918 influenza pandemic over? Long-term effects of in utero influenza exposure in the post-1940 US population. J Polit Econ 2006;114(4):672-712. https://doi.org/10.1086/507154 [ Links ]
26. Lumey LH, Stein AD, Kahn HS, et al Cohort profile: the Dutch Hunger Winter families study. Int J Epidemiol 2007;36(6):1196-1204. https://doi.org/10.1093/ije/dym126 [ Links ]
27. Britto PR, Pérez-Escamilla R. No second chances? Early critical periods in human development. Soc Sci Med 2013;97:238-240. https://doi.org/10.1016/j.socscimed.2013.09.001 [ Links ]
28. King S, Laplante DP. Using natural disasters to study prenatal maternal stress in humans. Adv Neurobiol 2015;10:285-313. https://doi.org/10.1007/978-1-4939-1372-5_14 [ Links ]
29. Basu JK, Chauke L, Magoro T. Maternal mortality from COVID 19 among South African pregnant women. J Matern Fetal Neonatal Med 2022;35(25):5932-5934. https://doi.org/10.1080/14767058.2021.1902501 [ Links ]
30. Ramdin TD, Bandini RM, Radomsky M, Bhoora SA, Ballot DE. Clinical characteristics, maternal and neonatal outcomes of COVID-19 positive pregnant mothers at a tertiary hospital in Johannesburg, South Africa. J Pediatr Perinatol Child Health 2021;5:252-263. [ Links ]
31. De Waard L, Langenegger E, Erasmus K, et al. Maternal and neonatal outcomes of COVID-19 in a high-risk pregnant cohort with and without HIV. S Afr Med J 2021;111(12):1174-1180. https://doi.org/10.7196/samj.2021.v111i12.15683 [ Links ]
32. Budhram S, Vannevel V, Botha T, et al. Maternal characteristics and pregnancy outcomes of hospitalized pregnant women with SARS-CoV-2 infection in South Africa: An international network of obstetric survey-systems based cohort study. Gynecol Obstet 2021;155(3):455-465. https://doi.org/10.1002/ijgo.13917 [ Links ]
33. Pattinson R, Fawcus S, Gebhardt S, et al The impact of COVID-19 on use of maternal and reproductive health services and maternal and perinatal mortality. In: Health Systems Trust (ed). South Africa Health Review 2021: Health Sector Responses to COVID-19. Durban: Health Systems Trust, 2021. [ Links ]
34. Momberg DJ, Voth-Gaeddert LE, Ngandu BC, et al. Water, sanitation, and hygiene (WASH) factors associated with growth between birth and 1 year of age in children in Soweto, South Africa: Results from the Soweto Baby WASH study. J Water Health 2020;18(5):798-819. https://doi.org/10.2166/wh.2020.085 [ Links ]
35. Richter L, Slemming W, Norris SA, et al. Healthy Pregnancy, Healthy Baby: Testing the added benefits of pregnancy ultrasound scan for child development in a randomised control trial. Trials 2020;21(25). https://doi.org/10.1186/s13063-019-3924-0 [ Links ]
36. Statistics South Africa. Statistics by Place: Soweto. Pretoria: Stats SA, 2011. http://www.statssa.gov.za/?page_id=4286&id=11317 (accessed 17 November 2021). [ Links ]
37. City of Joburg. Joburg demographics and key socio economic indicators. Johannesburg: CoJ, 2020. https://www.joburg.org.za/documents_/Documents/Statistical%20Briefs/Issue%2023%20Joburg%20Demographics%20and%20Key%20Socio%20Economic%20Indicators.pdf (accessed 17 November 2021). [ Links ]
38. Chris Hani Baragwanath Hospital. The Chris Hani Baragwanath Hospital: General Information. Johannesburg: CHBH, 2021. https://www.chrishanibaragwanathhospital.co.za/ (accessed 17 November 2021). [ Links ]
39. Sprong CY. Defining the 'term' pregnancy: Recommendations from the Defining 'Term' Pregnancy Workshop. JAMA 2013;309(23):2445-2446. https://doi.org/10.1001/jama.2013.6235. [ Links ]
40. National Perinatal Morbidity and Mortality Committee. Saving babies: Triennial report on perinatal mortality in South Africa: 2014 - 2016. NPMMC, 2016. https://www.westerncape.gov.za/assets/departments/health/napemmco_triennial_report_2014-2016_saving_babies.pdf (accessed 6 December 2021). [ Links ]
41. Ahenkorah B, Sakyi SA, Helegbe G, et al. Foetal-maternal complications associated with low birth weight: A prospective multicentre study in northern Ghana. PLoS One 2022;17(4):e0266796. https://doi.org/10.1371/journal.pone.0266796 [ Links ]
42. Anil KC, Basel PL, Singh S. Low birth weight and its associated risk factors: Health facility-based case-control study. PLoS One 2020;15(6):e0234907. https://doi.org/10.1371/journal.pone.0234907 [ Links ]
43. Sema A, Tesfaye F, Belay Y, et al. Associated factors with low birth weight in Dire Dawa City, Eastern Ethiopia: A cross-sectional study. BioMed Res Int 2019:2965094. https://doi.org/10.1155/2019/2965094 [ Links ]
44. Endalamaw A, Engeda EH, Ekubagewargies DT, Belay GM, Tefera MA. Low birth weight and its associated factors in Ethiopia: A systematic review and meta-analysis. Ital J Pediatr 2018;44:141. https://doi.org/10.1186/s13052-018-0586-6 [ Links ]
45. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob Health 2019;7(1):e37-e46. https://doi.org/10.1016%2FS2214-109X(18)30451-0 [ Links ]
46. Philip RK, Purtil H, Reidy E, et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: A 'natural experiment' allowing analysis of data from the prior two decades. BMJ Glob Health 2020;5(9):e003075. https://doi.org/10.1136/bmjgh-2020-003075 [ Links ]
47. Been JV, Ochoa LB, Bertens LCM, et al. Impact of COVID-19 mitigation measures on the incidence of preterm birth: A national quasi-experimental study. Lancet Pub Health 2020;5(11):e604-e611. https://doi.org/10.1016/s2468-2667(20)30223-1 [ Links ]
48. Hedermann G, Hadley PL, Bœkvad-Hansen M, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed 2021;106(1):93-95. https://doi.org/10.1136/archdischild-2020-319990 [ Links ]
49. Nkosi V, Haman T, Naicker N, Mathee A. Overcrowding and health in two impoverished suburbs of Johannesburg, South Africa. BMC Public Health 2019;19:1358. https://doi.org/10.1186/s12889-019-7665-5 [ Links ]
50. Benhura M, Magejo P. Who cannot work from home in South Africa? Evidence from wave 4 of NIDS-CRAM. National Income Dynamics Study (NIDS) - Coronavirus Rapid Mobile Survey (CRAM), 2021. https://cramsurvey.org/wp-content/uploads/2021/05/2.-Benhura-M.-_-Magejo-P.-2021-Who-cannot-work-from-home-in-South-Africa_-Evidence-from-wave-4-of-NIDSCRAM.pdf (accessed 25 April 2022). [ Links ]
51. Casale D, Shepherd D. The gendered effects ofthe COVID-19 crisis and ongoing lockdown in South Africa: Evidence from the NIDS-CRAM Waves 1-3. National Income Dynamics Study (NIDS) - Coronavirus Rapid Mobile Survey (CRAM), 2021. https://cramsurvey.org/wp-content/uploads/2021/02/4.-Casale-D.-Shepherd-D.-2021-The-gendered-effects-of-the-Covid-19-crisis-and-ongoing-lockdown-in-South-Africa-Evidence-from-NIDS-CRAM-Waves-1-3.pdf (accessed 8 August 2022). [ Links ]
52. Pillay Y, Pienaar S, Barron P, Zondi T. Impact of COVID-19 on routine primary healthcare services in South Africa. S Afr Med J 2021;111(8):714-719. https://doi.org/10.7196/samj.2021.v111i8.15786 [ Links ]
53. Pallangyo E, Nakate MG, Maina R, Flemming V. The impact of COVID-19 on midwives practice in Kenya, Uganda and Tanzania: A reflective account. Midwifery 2020;89:102775. https://doi.org/10.1016/j.midw.2020.102775 [ Links ]
54. Aryal S, Shrestha D. Motherhood in Nepal during COVID-19 pandemic: Are we heading from safe to unsafe? J Lumbini Med Coll 2020;8(1):128-129. https://doi.org/10.22502/jlmc.v8i1.351 [ Links ]
55. Balogun M, Banke-Thomas A, Sekoni A, et al. Challenges in access and satisfaction with reproductive, newborn and child health services in Nigeria during the COVID-19 pandemic: A cross-sectional survey. PLoS One 2021;16(5):e0251382. https://doi.org/10.1371/journal.pone.0251382 [ Links ]
56. Laouan FZ. Rapid gender analysis - COVID-19: West Africa April 2020. CARE, 2020. https://reliefweb.int/sites/reliefweb.int/files/resources/CARE%20West%20Africa%20Rapid%20Gender%20Analysis%20COVID-19%20May%202020%20final%20EN.pdf (accessed 15 July 2021). [ Links ]
57. Khoza C, du Plessis R. Behavioural and health impacts of the COVID-19 pandemic in South Africa. Pretoria: Statistics South Africa, 2021. [ Links ]
58. Spaull N, Ardington C, Bassier H, et al. Overview and findings: NIDS-CRAM Synthesis Report Wave 1. NIDS-CRAM Technical Document, 30 September 2020. 2020. https://cramsurvey.org/wp-content/uploads/2020/07/Spaull-et-al.-NIDS-CRAM-Wave-1-Synthesis-Report-Overview-and-Findings-1.pdf (accessed 15 June 2022). [ Links ]
59. De Onis M, Branca F. Childhood stunting: A global perspective. Matern Child Nutr 2016;12(Suppl 1):12-26. https://doi.org/10.1111/mcn.12231 [ Links ]
60. Torche F, Echevarria G. The effect of birthweight on childhood cognitive development in a middle-income country. J Epidemiol 2011;40(4):1008-1010. https://doi.org/10.1093/ije/dyr030 [ Links ]
61. Victoria CG, Adair L, Fall C, et al. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008;371(9609):340-357. https://doi.org/10.1016/s0140-6736(07)61692-4 [ Links ]
62. Walker SP, Chang SM, Younger N, Grantham-McGregor SM. The effect of psychosocial stimulation on cognition and behaviour at 6 years in a cohort of term low-birthweight Jamaican children. Dev Med Child Neurol 2010;52(7):148-154. https://doi.org/10.1111/j.1469-8749.2010.03637.x [ Links ]
63. Sabet F, Richter LM, Ramchandani PG, et al. Low birthweight and subsequent emotional and behavioural outcomes in 12-year-old children in Soweto, South Africa: Findings from Birth to Twenty. J Epidemiol 2009;38(4):944-954. https://doi.org/10.1093/ije/dyp204 [ Links ]
64. Gupta R, de Wit ML, McKeown D. The impact of poverty on the current and future health status of children. Paediatr Child Health 2007;12(8):667-672. https://doi.org/10.1093/pch/12.8.667 [ Links ]
65. Emond AM, Lira PIC, Lima MC, Grantham-McGregor SM, Ashworth A. Development and behaviour of low-birthweight term infants at 8 years in northeast Brazil: A longitudinal study. Acta Paediatrica 2007;95(10):1249-1257. https://doi.org/10.1080/08035250600615127 [ Links ]
 Correspondence:
Correspondence:
R E Drysdale
rdrysdale@wrhi.ac.za
Accepted 2 May 2023














