Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 n.10 Pretoria Oct. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i10.968
RESEARCH
Prevalence and outcome of acute kidney injury in burn victims at a tertiary centre in Cape Town, South Africa
L VaziI; W KleintjesII; M Y ChothiaIII
IMB ChB; Division of Nephrology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIMMed (Plast), PhD; Burns Unit, Department of Surgery, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIIMMed (Int Med), PhD; Division of Nephrology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND: Burn victims commonly experience acute kidney injury (AKI), which can lead to significant morbidity and mortality
OBJECTIVE: To investigate the prevalence of AKI in burn patients, the causes of AKI and the rate of in-hospital mortality
METHODS: A retrospective cohort study was conducted on patients admitted to the Tygerberg Hospital Burn Unit between 1 April 2018, and 31 March 2019. The study included all burn patients >18 years old, except for those with end-stage kidney disease or cold burn wounds, skin donors or readmissions. AKI was defined using the Kidney Disease Improving Global Outcomes criteria, and multivariable logistic regression was used to identify predictors of AKI and death, along with Kaplan-Meier survival analysis
RESULTS: The prevalence of AKI was 27% (58/215). The most common causes of burns were open fires (37%) and shack fires (17%). Patients with AKI had higher scores on the abbreviated burn severity index (ABSI) (7 v. 5, p<0.01), required more mechanical ventilation (69% v. 33%, p<0.01) and experienced more sepsis (35% v. 12%, p<0.01). Predictors of AKI included ABSI score (adjusted odds ratio (aOR) 1.48, 95% confidence interval (CI) 1.21 - 1.80, p<0.01), mechanical ventilation (aOR 7.75, 95% CI 1.23 - 48.65, p=0.03) and high admission lactate (aOR 1.57, 95% CI 1.04 - 2.39, p=0.03). Mortality was higher in patients with AKI (34% v. 6%, p<0.01). ABSI score (aOR 2.16, 95% CI 1.56 - 2.99, p<0.01) and vasopressor use (aOR 7.71, 95% CI 2.15 - 27.60, p<0.01) were identified as predictors of death. The survival analysis revealed that AKI was associated with higher mortality (log rank, p<0.01
CONCLUSION: The study highlights the high prevalence of AKI among burn victims requiring tertiary care and its association with high mortality rates. Improving living conditions in informal settlements could help prevent burns and their complications
Burn injuries are common worldwide, totalling 7.1 million injuries annually, with an estimate death toll of 250 000 patients worldwide. Low- to middle-income countries (LMICs) account for 90% of this total, according to the latest epidemiological data.[1]
In these LMICs, burn injuries are usually associated with poor socioeconomic status, with flame burns accounting for the majority of burns in these populations.[2] It is well documented that the rapidly rising expansion of informal settlements in urban areas, coupled with the use of kerosene (paraffin) stoves and open flames for cooking and heating, has resulted in an increase in total burn injuries.[3-5]
Over the years, burn injuries have become less frequently fatal owing to the innovative interventions developed in treating burned patients. However, there is a paucity of research on acute kidney injury (AKI) in burns, particularly in Africa. Furthermore, there is no clear consensus offered as to how to effectively treat these patients when they develop AKI, in addition to the supportive and preventative measures currently advocated.[6]
AKI is a common complication, with high morbidity and mortality.[6-8] The prevalence of AKI in burns has been reported to be 30% in all major burns, with the severity of AKI being directly related to the percentage of total body surface area (TBSA) of the burn.[9] The latest meta-analysis from Folkestad et al.[10] revealed a higher pooled prevalence of 38%.
AKI in burns can be divided into acute phase AKI (<72 hours post burn) and late phase AKI (4 days - 4 weeks post burn).[7,9] Acute phase AKI (<72 hours) is generally related to hypovolaemia, burn shock, release of denatured proteins, inflammatory mediators and cardiac dysfunction.[7,9] The causes for late phase AKI (4 days - 4 weeks) are usually multifactorial. These include sepsis, multi-organ dysfunction, nephrotoxic agents, fluid overload or the so-called 'fluid creep'.[7,9,11,12] The last refers to overzealous fluid resuscitation predicted by the Parkland formula. Fluid overload following overestimation during early resuscitation can cause life-threatening complications such as pulmonary oedema and intra-abdominal hypertension leading to abdominal compartment syndrome.[12] To reduce fluid overload, colloids and other starch-containing intravenous fluids have been used when high fluid resuscitation is needed.[11-13]
The development of AKI in burn patients can be categorised into pre-renal and intra-renal factors. Pre-renal factors encompass several causes, including reduced intravascular volume due to fluid loss from the burn wound, bleeding, fluid redistribution into extracellular spaces and diminished cardiac output resulting from burn shock. The resulting decrease in renal perfusion causes ischaemia and subsequent reduced glomerular filtration.[12] Burn shock is a state of hypovolaemia due to a massive acute inflammatory response resulting in microvascular and macrovascular dysfunction. Microvascular dysfunction is characterised by heightened capillary permeability, leading to decreased intravascular volume and leakage of proteins and intracellular electrolytes, while macrovascular dysfunction is manifested by reduced cardiac output and increased vascular tone.[12] Intra-renal causes are related to the type of burn or mechanism of burn. As a result, most of these injuries are associated with rhabdomyolysis.[12]
The significant risk factors associated with an increased risk for AKI are older age, degree of TBSA affected, burn type (flame and inhalational burn being the major risk), abbreviated burn severity index (ABSI), baseline kidney function, sequential organ failure assessment (SOFA) score on admission, and sepsis.[8-10] In addition, the degree of AKI is directly associated with an increased risk of mortality. Mortality rates of up to 80% have been reported in patients requiring kidney replacement therapy (KRT).[7,9,10] The high mortality in this latter group is attributed to severe nosocomial sepsis due to drug-resistant organisms. Therefore, there is a growing trend to withhold KRT when it is required in this population of patients.[7]
There is a paucity of epidemiological data on the outcome of patients with burns complicated by AKI from the African continent. Therefore, we aim to identify the period prevalence, causes and in-hospital mortality of burns patients complicated by AKI.
Methodology
A retrospective cohort study was conducted on patients admitted to Tygerberg Hospital Burn Unit from 1 April 2018 to 31 March 2019. All burn patients >18 years old were included. Patients with end-stage kidney disease, cold burn wounds, skin donors or patients who required readmission were excluded. The Kidney Disease Improving Global Outcomes (KDIGO) AKI 2012 criteria were used. Only serum creatinine was used in this study to define AKI owing to inconsistent documenting of urine output data. The measurements of serum creatinine were taken on admission, after 48 hours of hospital stay and at discharge.
Extracted data included demographic including age, sex, comorbidities, and clinical and biochemical data during admission. The clinical information collected included TBSA and ABSI score, mechanism of burn injury (shack fire, boiling water, petrol, electrical and open fire), the burn circumstance (accidental v. non-accidental, with the latter subcategorised into deliberate self-harm (suicide) and homicide or assault), presence or absence of inhalation injury, development of sepsis, outcome (AKI and in-hospital mortality) and length of hospital stay. Alcohol intoxication was not reported.
Biochemical data collected included serum creatinine, creatine phosphokinase levels and septic markers (white cell count, blood cultures and C-reactive protein). Urine output monitoring data, when available, were documented.
Data on therapies received included oral and intravenous fluids (type, volume, duration and Parkland formula calculated for each patient), need for blood transfusions, and all medications administered during hospitalisation, including the type of antibiotics and need for vasopressor support. The need for mechanical ventilation and kidney replacement therapy were also recorded.
Ethical considerations
Ethical approval was be obtained from the Human Research Ethics Committee (HREC) of Stellenbosch University (project ID: 18691, ref. no: S20/09/248). This included a waiver of consent due to the retrospective nature of the research study design. The research was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
All descriptive data were reported as mean (standard deviation (SD)) where data had a normal distribution, and median and interquartile range (IQR) where data were not normally distributed. Chi-squared or Fisher's exact tests were used to compare categorical variables. Student's f-test was used to compare means of continuous variables that were normally distributed, whereas the Mann-Whitney U test was used to compare median values. The Kruskal-Wallis test was used to compare continuous variables for the three AKI group. Univariable and stepwise backward multivariable logistic regression analysis were used to identify predictors of AKI and in-hospital death. Predictor variables with p<0.1 were included in the multivariable model. Kaplan-Meier survival analysis and associated log-rank p-values were also performed. P<0.05 was regarded as statistically significant, and 95% confidence intervals (CIs) were reported. Data were analysed using Stata version 16.1 (StataCorp, USA).
Results
We screened a total of 253 patients admitted to the burn unit for inclusion. Thirty-eight were excluded due to age <18 years (n=12), readmissions (n=10) and incorrect or missing data (n=16). Therefore, a total of 215 patients were included in the final analysis Fig 1).
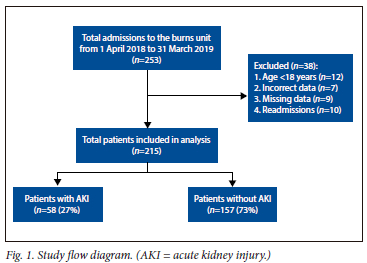
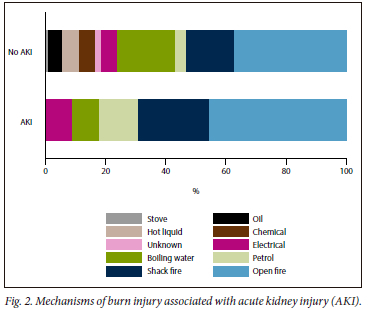
The period prevalence of AKI was 27% (58/215). There were no significant differences regarding age or sex in patients with or without AKI; however, most were male (72%), with few overall having comorbid conditions. Patients with AKI had higher TBSA burns (30% v. 11%, p<0.01) and ABSI score (7 v. 5, p<0.01). Most burn injuries associated with AKI were accidental (74%). The most frequent mechanisms of burn injury associated with AKI were open fires (43%), shack fires (22%) and petrol (12%) (Fig. 2). Mechanisms of burn injury in non-accidental circumstances (homicide/assault and deliberate self-harm) included open fires (n=12), boiling water (n=9), petrol (n=7), paraffin (n=4), chemicals (n=3) and shack fires (n=2). Patients with AKI required more mechanical ventilation (69% v. 33%, p<0.01), blood transfusions (66% v. 34%, p <0.01) and developed more sepsis (35% v. 12%, p<0.01), requiring more antibiotic therapy (66% v. 45%, p<0.01) as well as vasopressor support (38% v. 10%, p<0.01). The volume of intravenous (IV) fluid administered within the first 24 hours calculated using the Parkland formula was higher in patients with AKI (6 187 mL v. 4 350 mL, p=0.03), as was the combined daily average oral and IV fluids administered during hospitalisation (2 749 mL v. 2 500 mL, p<0.01). There were no differences in length of stay in hospital between groups (22 days v. 16 days, p=0.18) (Table 1).
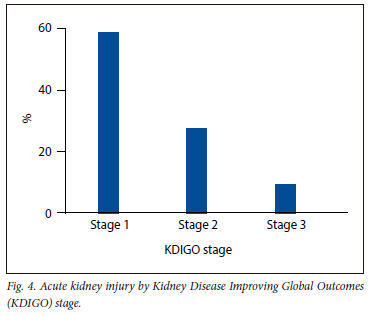
The distribution of AKI by KDIGO stages 1, 2 and 3 were 59%, 28% and 14%, respectively (Fig. 4). There were no differences regarding ABSI score, TBSA burns, volume of IV fluid administered within the first 24 hours calculated using the Parkland formula, or the average daily oral and IV fluids administered during hospitalisation by KDIGO AKI stage (Table 2).
The median serum creatinine concentration at admission was higher in patients with AKI (83 umol/L v. 67 umol/L, p<0.01). Patients with AKI had higher white cell counts (15.2 x 109/L v. 10.6 x 109/L, p<0.01), haemoglobin concentrations (15.2 g/dL v. 10.6 g/dL, p<0.01) and creatine phosphokinase (CPK) concentrations (2 643 IU/L v. 263 IU/L, p<0.01) compared with patients without AKI.
On univariable logistic regression, ABSI score (crude odds ratio (cOR) 1.57, 95% CI 1.34 - 1.84, p<0.01), inhalation injury (cOR 3.07, 95% CI 1.65 - 5.73, p<0.01), mechanical ventilation (cOR 4.49, 95% CI 2.35 - 8.58, p<0.01), blood transfusion (cOR 3.55, 95% CI 1.88 -6.70, p<0.01), vasopressor support (cOR 5.79, 95% CI 2.73 - 12.26, p<0.01), treatment with antibiotics (cOR 2.36, 95% CI 1.26 - 4.42, p<0.01) and serum lactate concentration (cOR 2,28, 95% CI 1.57 -3.30, p<0.01) were associated with AKI; however, only ABSI score (adjusted OR (aOR) 1.48, 95% CI 1.21 - 1.80, p<0.01) and mechanical ventilation (aOR 7.75, 95% CI 1.23 - 48.65, p=0.03) and serum lactate concentration (aOR 1.57, 95% CI 1.04 - 2.39, p=0.03) were associated on multivariable analysis (Table 3).
In-hospital death was higher in patients with AKI (34% v. 6%, p<0.01). ABSI score (cOR 1.91 - 3.38, 95% CI 1.91 - 38, p<0.01), AKI (cOR 7.74, 95% CI 3.34 - 17.90, p<0.01), average volume of daily oral and IV fluids during hospitalisation (cOR 1.00, 95% CI 1.000026 -1.000263, p=0.02), mechanical ventilation (cOR 8.81, 95% CI 3.22 - 24.08, p<0.01), inhalation injury (cOR 7.75, 95% CI 3.01 - 19.93, p<0.01), blood transfusion (cOR 3.68, 95% CI 1.60 - 8.49, p<0.01), vasopressor support (cOR 31.17, 95% CI 11.86 - 81.90, p<0.01) and serum lactate concentration (cOR 1.54, 95% CI 1.16 - 2.04, p<0.01) were associated with in-hospital death. However, only ABSI score (aOR 2.16, 95% CI 1.56 - 2.99, p<0.01) and vasopressor support (aOR 7.71, 95% CI 2.15 - 27.60, p<0.01) were associated with mortality on a multivariable model (Table 3).
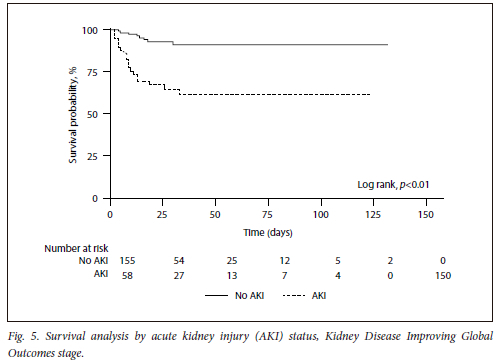
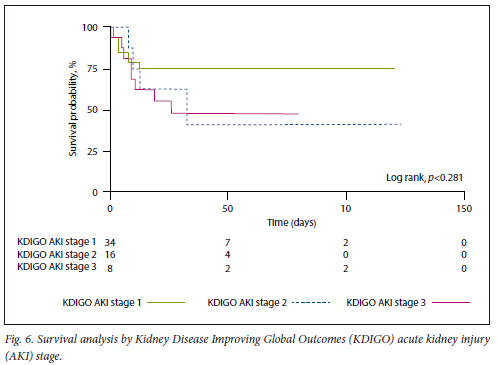
On Kaplan-Meier survival analysis, AKI was associated with higher mortality (log rank, p<0.01) (Fig. 5), with no statistically significant difference regarding survival between the KDIGO AKI stages (log rank, p=0.28) (Fig. 6). Only one patient was offered KRT due to refractory hyperkalaemia.
Discussion
To the best of our knowledge, this is the first study to investigate the outcomes of AKI in burn victims in SA. The period prevalence of AKI in our study was slightly lower than others.[8-10] The reasons for this could be related to lack of blood sampling at admission, leading to patients with potential AKI being missed. Also, AKI may have been at a recovery phase by the time of the admission, leading to an underestimation.[14] Other considerations may be related to the pre-admission intravenous fluid resuscitation protocols by first responders. The younger age of our cohort along with the low frequency of comorbid diseases may have reduced the risk of burn-related AKI. Others have consistently reported older age as a risk factor for the development of AKI.[8-10,14,15]
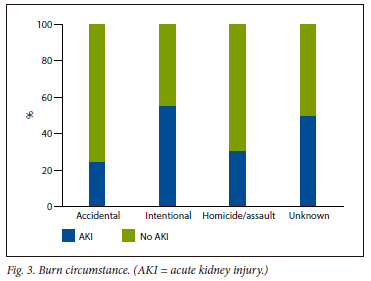
Informal settlements and poor socioeconomic circumstances remain a leading cause of burn injuries in LMICs.[16] The materials used to build homes in informal settlements are highly combustible and include plastic and wood. Also, kerosene is used as a fuel for lanterns, and fires are used for heating and cooking. This, along with the proximity of homes to each other, creates a perfect storm for the high burden of fires in these communities. The type of accelerant used in the building material is also a reason for the high prevalence of inhalation injury due to the toxic smoke released from the burning material. In our study, most shack fires occurred over weekends during the early hours of the morning. Reported causes of the fires were due to patients falling asleep with lit cigarettes in hand or mouth, candles left alight and kerosene light sources that were not extinguished. An interesting finding was that males were mostly affected. This was speculated to be related to alcohol intoxication, with more risky behaviour leading to homicide/assault.[17] According to a recent report by the World Bank, SA has the highest Gini index in the world, which indicates the income disparity between the wealthy and the poor.[18] If the poor continue to rely on combustible sources of energy, the threat of fires in informal settlements is likely to persist.[16] Non-accidental injuries had a higher prevalence of AKI. This may be due to a lack of employing protective reflexes such as the 'drop-and-roll' manoeuvre and removing burning clothing items, resulting in longer burn contact time, with subsequent higher TBSA burns and ABSI scores. Petrol bomb attacks were a common mechanism for non-accidental burns. The reasons were not documented, but were thought to be related to homicide/assault and deliberate self-harm. These patients required more mechanical ventilation due to inhalation injuries, likely related to inhalation from burning clothing items and petrol fumes.
The causes of AKI in burn victims were multifactorial and included hypovolaemia, sepsis and nephrotoxicity related to rhabdomyolysis, and use of nephrotoxic antibiotics. One third of our patients developed sepsis, which was similar to results reported by others.[14] Common causes were related to burn wound sepsis and ventilator-associated pneumonia. In addition, the use of nephrotoxic antibiotics was more frequent in patients with AKI, especially colistin and aminoglycosides in combination with piperacillin-tazobactam. These findings were similar to those reported by Witkowski et al.[9] and Clark et al. '121 Since many patients developed multidrug-resistant nosocomial sepsis, the prescription of these antibiotics was unavoidable. A recent study by van Langeveld et al.[19] also reported that Pseudomonas aeruginosa, Staphylococcus aureus and Acinetobacter baumannii were frequently identified multidrug-resistant organisms in severe burn victims. Sepsis is particularly difficult to prevent and control in burn patients due to impaired immune function and the loss of the skin as a protective barrier to pathogens.[19]
Most patients in this study developed KDIGO AKI stage 1, with only a few progressing to higher stages of AKI. This could be due to the higher volume of fluid administered within the first 24 hours and the higher daily volumes administered during hospitalisation. Patients with AKI had more severe rhabdomyolysis, as reflected by the CPK concentrations that were 10 times higher than those without AKI. The administration of large volumes of isotonic fluids within the first 24 hours may have prevented progression to rhabdomyolysis-associated AKI and pre-renal AKI.
However, excessive fluid resuscitation leading to fluid overload could also result in AKI and oedema-related complications such as abdominal compartment syndrome. Therefore, accurate assessment of the TBSA involved is crucial to avoid overor underdosing of IV fluids. It should be noted that excessive fluid administration may not always prevent AKI, and a more individualised approach may be necessary.
The predictors of AKI in our study were a high ABSI score, mechanical ventilation and raised serum lactate concentration, which align with findings from other studies.[8,10,14,15] However, our cohort consisted of a younger population with fewer comorbidities. We believe that the reason for our younger cohort is due to the demographics of SA, where 85% of the population is between the ages of 15 and 59 years.[20] Additionally, the high rates of intentional burns (suicide) and homicide/assault among the young could also contribute to the age distribution in our study.
Patients who developed AKI had a six-fold higher mortality rate than those without AKI. High ABSI score and vasopressor requirements were identified as predictors of in-hospital death, which is consistent with findings from other studies.[11,14,15] ABSI score is a measure of burn severity that considers age, sex, inhalation injury, presence of full thickness burns and TBSA. It was specifically designed to estimate survival following burn injuries. The need for vasopressor requirements likely indicates the higher frequency of septic shock, as more patients with AKI received treatment with combination antibiotics frequently used for multidrug-resistant bacteria.
This is the first study in SA to report on the outcomes of AKI in burn victims and its associated complications in patients admitted to a tertiary level facility. However, the study had some limitations. It was retrospective, which means that missing data could have introduced bias. Additionally, the use of serum creatinine alone may have underestimated the prevalence of AKI due to variations in sampling time. Finally, the study was conducted at a single tertiary center that primarily treats severe cases, which limits its generalisability due to potential selection bias.
Conclusion
We found a high prevalence of AKI in burn victims requiring tertiary care, and this was associated with high mortality. Educating the population living in informal settlements on safe practices to prevent burns, and more importantly, improving living conditions in the long term, will reduce the frequency of burns and associated complications.
Declaration. The research for this study was done in partial fulfilment of the requirements for LV's MMed (Int Med) degree at Stellenbosch University.
Acknowledgments. None.
Authors contributions. LV, WK and MYC conceptualised the study and developed the protocol. LV performed data collection. MYC performed the data analysis. LV and MYC wrote the first manuscript draft and interpreted the data. All authors contributed to and approved the final manuscript.
Funding. None.
Conflict of interest. None.
References
1. Rybarczyk MM, Schafer JM, Elm CM, et al. A systematic review of burn injuries in low- and middle-income countries: Epidemiology in the WHO-defined African Region. Afr J Emerg Med 2017;7(1):30-37. https://doi.org/10.1016/j.afjem.2017.01.006 [ Links ]
2. Den Hollander D, Albert M, Strand A, Hardcastle TC. Epidemiology and referral patterns of burns admitted to the Burns Centre at Inkosi Albert Luthuli Central Hospital, Durban. Burns 2014;40(6):1201-1208. https://doi.org/10.1016/j.burns.2013.12.018 [ Links ]
3. Godwin Y, Wood S. Major burns in Cape Town: A modified burns score for patient triage. Burns 1998;24(1):58-63. https://doi.org/10.1016/s0305-4179(97)00053-3 [ Links ]
4. Maritz D, Wallis L, Van Der Merwe E, Nel D. The aetiology of adult burns in the Western Cape, South Africa. Burns 2012;38 (1):120-127. https://doi.org/10.1016/j.burns.2010.12.007 [ Links ]
5. Blom L, Van Niekerk A, Laflamme L. Epidemiology of fatal burns in rural South Africa: A mortuary register-based study from Mpumalanga Province. Burns 2011;37(8):1394-1402. https://doi.org/10.1016/j.burns.2011.07.014 [ Links ]
6. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8(4):1-9. https://doi.org/10.1186/cc2872 [ Links ]
7. Ibrahim A, Sarhane K, Fagan S, Goverman J. Renal dysfunction in burns: A review. Ann Burn Fire Disasters 2013;26(1):16-25. [ Links ]
8. Wu G, Xiao Y, Wang C, et al. Risk factors for acute kidney injury in patients with burn injury: A meta-analysis and systematic review. J Burn Care Res 2017;38(5):271-282. https://doi.org/10.1097/bcr.0000000000000438 [ Links ]
9. Witkowski W, Kawecki M, Surowiecka-Pastewka A, Klimm W, Szamotulska K, Niemczyk S. Early and late acute kidney injury in severely burned patients. Med Sci Monit 2016;22:3755. https://doi.org/10.12659%2FMSM.895875 [ Links ]
10. Folkestad T, Brurberg KG, Nordhuus KM, et al. Acute kidney injury in burn patients admitted to the intensive care unit: A systematic review and meta-analysis. Crit Care 2020;24(1):1-11. https://doi.org/10.1186/s13054-019-2710-4 [ Links ]
11. Tejiram S, Romanowski KS, Palmieri TL. Initial management of severe burn injury. Curr Opin Crit Care 2019;25(6):647-652. https://doi.org/10.1097/mcc.0000000000000662 [ Links ]
12. Clark A, Neyra JA, Madni T, et al Acute kidney injury after burn. Burns 2017;43(5):898-908. https://doi.org/10.1016/j.burns.2017.01.023 [ Links ]
13. Béchir M, Puhan MA, Fasshauer M, Schuepbach RA, Stocker R, Neff TA. Early fluid resuscitation with hydroxyethyl starch 130/0.4 (6%) in severe burn injury: A randomised, controlled, double-blindclinical trial. Crit Care 2013;17(6):1-8. https://doi.org/10.1186/cc13168 [ Links ]
14. Odutayo A, Adhikari NKJ, Barton J, et al. Epidemiology of acute kidney injury in Canadian critical care units: A prospective cohort study. Can J Anaesth 2012;59(10):934-942. https://doi.org/10.1007/s12630-012-9761-1 [ Links ]
15. Queiroz LFT, Anami EH, Zampar EF, Tanita MT, Cardoso LT, Grion CMC. Epidemiology and outcome analysis of burn patients admitted to an intensive care unit in a university hospital. Burns 2016;42(3):655-662. https://doi.org/10.1016/j.burns.2015.08.002 [ Links ]
16. Kimemia D, Van Niekerk A. Energy poverty, shack fires and childhood burns. S Afr Med J 2017;107(4):289-291. https://doi.org/10.7196%2FSAMJ.2017.v107i4.12436 [ Links ]
17. Osuafor GN, Okoli CE. Alcohol consumption as a factor in gun or knife crimes in South Africa. Afr J Drug Alcohol Stud 2019;18(2):85-96. [ Links ]
18. Sulla V, Zikhali P, Cuevas PF. Inequality in southern Africa: An assessment of the Southern African Customs Union, 2022. https://documents.worldbank.org/en/publication/documents-reports/documentdetail/099125303072236903/p1649270c02a1f06b0a3ae02e57eadd7a82 (accessed 11 April 2023). [ Links ]
19. Van Langeveld I, Gagnon RC, Conrad PF, et al. Multiple-drug resistance in burn patients: A retrospective study on the impact of antibiotic resistance on survival and length of stay. Burn Care Res 2017;38(2):99-105. https://doi.org/10.1097/bcr.0000000000000479 [ Links ]
20. Statistics South Africa. Statistical release: Mid-year population estimates. Pretoria: Stats SA, 2019. https://www.statssa.gov.za/publications/P0302/P03022019.pdf (accessed 11 April 2023). [ Links ]
 Correspondence:
Correspondence:
M Y Chothia
yaziedc@sun.ac.za
Accepted 3 August 2023














