Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.10 Pretoria Out. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i10.950
RESEARCH
Ocular manifestations of HIV infection at Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
I IsmailI; M VenterII; S IsmailIII; N AllyIV, V
IMB BCh; Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IICert ID Phys (SA); Division of Infectious Diseases, Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIFC Opth (SA); Consultant ophthalmologist, Johannesburg, South Africa
IVFC Opth (SA); Manchester Royal Eye Hospital, Manchester, UK
VFC Opth (SA); Division of Ophthalmology, Department of Neurosciences, St John Eye Hospital, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: The pattern of HIV-associated eye disease has changed with ongoing advancements in highly active antiretroviral therapy (HAART). HIV-infected individuals now live longer, enabling us to observe the long-term effects of HIV and HAART on the eye. There are few recent studies on HIV-related ocular disease in sub-Saharan Africa
OBJECTIVES: To describe the ocular manifestations of HIV in patients attending the Nthabiseng HIV clinic at Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
METHODS: A cross-sectional study was conducted in 2021 and 2022 using convenience sampling of patients at the HIV clinic. The participants' clinical history was taken, their files were reviewed, and they underwent ocular examination. Correlation between eyes was managed by taking disease in one eye as the presence of disease in the participant. Descriptive statistics were used to summarise participant characteristics. Univariate and multivariate logistic regression models were used to assess the odds ratio (OR) of developing HIV-associated ocular diseases, and a p-value of <0.05 was used to define statistical significance
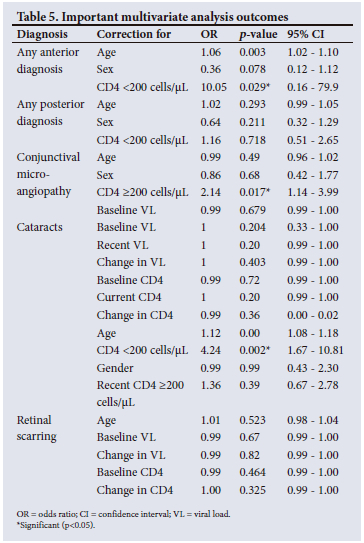
RESULTS: There were 182 participants (139 females and 43 males), with a mean (standard deviation) age of 48.9 (10.6) years. The most common anterior segment diagnoses were conjunctival microangiopathy (34.6%), pinguecula (31.3%) and cataracts (30.2%), while the most common posterior segment finding was peripheral retinal scarring with features in keeping of previous cytomegalovirus retinitis (24.2%). Notably, only 1.1% of patients had HIV retinopathy. A CD4 count <200 cells/(iL showed an increased OR for cataracts (OR 4.24; p=0.003) and any anterior segment diagnoses (OR 10.05; p=0.029), while a CD4 count >200 cells/(iL showed an increased risk of conjunctival microangiopathy (OR 2.14; p=0.017
CONCLUSION: With the advent of HAART, ocular manifestations of HIV are changing and the incidence of severe ocular opportunistic infections and HIV retinopathy has decreased precipitously. Although this study has shown that patients with a CD4 count <200 cells/(µL are at increased risk of developing anterior ocular manifestations of HIV, including cataracts, these diseases are relatively innocuous or easily treatable. Routine ocular screening of HIV patients seems to be substantially less important now than it was in the pre-HAART era
Since the initial description of ophthalmic disease in HIV-positive individuals more than 38 years ago, there have been many studies describing the spectrum of HIV-associated eye pathology. In the pre-highly active antiretroviral therapy (HAART) era, HIV-related ocular disease was extremely common and estimated to affect 70 - 80% of HIV-infected patients at some point during their illness.[1] The ocular manifestations of HIV are protean and may involve the adnexa, as well as the anterior and posterior segments of the eye. Anterior segment involvement includes tumours and external infections, while posterior segment involvement manifests as HIV retinopathy and opportunistic infections of the retina and the choroid.[2] Posterior segment manifestations are a common finding in HIV/AIDS, with significant morbidity associated with these illnesses. Posterior segment manifestations can be classified as those related to opportunistic infections (cytomegalovirus (CMV) retinitis, toxoplasma retinochoroiditis, retinal necrosis (both acute and progressive outer), and bacterial and fungal retinitis); those directly related to HIV infection (HIV retinopathy, cotton wool spots, telangiectatic vessels and retinal haemorrhages), and those caused by the toxicity secondary to drug therapy.[3] The most common and important conditions are HIV retinopathy and CMV retinitis, both of which have been reported in 30 - 40% of HIV-infected patients.[4] CMV retinitis is a clinical diagnosis that can be confirmed with polymerase chain reaction testing for CMV DNA. CMV retinitis is a common AIDS-defining illness, most notably in patients with CD4 counts <50 cells/µL.[5] In the Longitudinal Study of the Ocular Complications of AIDS (LSOCA),[5] the incidence of CMV retinitis was 7% compared with the pre-HAART era, when Hoover et al.[6] reported an incidence of 25% in 1996. CMV retinitis was responsible for 40% of severe visual loss in AIDS patients.
With ongoing advancements in HAART, a global shift from infectious-based to non-infectious-based ocular diseases is occurring. The introduction of HAART has changed the spectrum of ocular disease, with a significant reduction in infectious ocular conditions among HIV-infected individuals.[7] HIV-infected individuals also live longer, and we can therefore observe the long-term effects on patients taking HAART. Many patients are on lifelong HAART, but the long-term effects of HAART on the occurrence and severity of ocular diseases are largely unknown.[2,7,8] However, patients in South Africa (SA) continue to present to point-of-care services at advanced stages of disease,[9] so this pattern may not be applicable locally. This study aimed to document and describe the ocular manifestations of HIV in patients attending the HIV clinic at Chris Hani Baragwanath Academic Hospital (CHBAH), Johannesburg, SA. Quantifying HIV-associated ocular disease in a periurban SA setting is important, as it assists medical practitioners to screen, recognise, and appropriately refer patients to ophthalmological services timeously.
Methods
This was a cross-sectional study conducted at CHBAH and St John Eye Hospital in Soweto, Johannesburg. The hospital is a tertiary institution that services a large catchment area in a periurban area of Gauteng Province, but also receives referrals from secondary hospitals, private practitioners and primary healthcare clinics, as well as from hospitals in other provinces. Patients are referred to the Nthabiseng HIV/infectious diseases clinic at CHBAH for further monitoring and management of their HIV illness. Potential participants in the study were conveniently sampled from the clinic and invited to participate in the study. Patients were included in the study if they were >18 years old, attended the Nthabiseng HIV clinic, and consented to take part in the study. Patients were excluded if they were known to have congenital ocular disease, a history of ocular or orbital trauma, or documented clinically significant ocular disease from non-HIV-related comorbidity such as diabetes mellitus or hypertension, and if they would be driving a motor vehicle immediately after the eye examination.
Study approval was obtained from the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (ref. no. M201079). The study adhered to the tenets of the Declaration of Helsinki.
Informed consent was obtained from the participants, with a translator where necessary. The clinical history included demographics (sex determined using biological factors), comorbidities including treatment and length of therapy, and previous opportunistic infections, including the year/s of diagnosis and therapy. The year of HIV diagnosis, current HAART regimen and a history of previous HAART regimens, including defaulting periods and length of time on each regimen, were recorded, together with the baseline or first documented CD4 count and the most recent documented immunological markers (CD4 count and viral load). Participants were examined at St John Eye Hospital (the ophthalmology department of CHBAH) by an ophthalmologist (authors NA or SI). The ophthalmic assessment consisted of unaided distance visual acuity and pinhole acuity using the ETDRS chart, slit-lamp biomicroscopy and fundoscopy, and anterior segment and fundus photography using a Canon CR-2 AF digital non-mydriatic retinal camera (Canon, USA). These were conducted on all participants.
Statistical analysis
Stata version 16.1 (StataCorp., USA) was used to conduct all statistical analyses. Descriptive statistics were used to summarise participant characteristics. The frequencies and percentages of categorical variables were calculated and tabulated. Means and standard deviations (SDs) and medians and interquartile ranges of continuous variables such as CD4, HIV viral load and age were calculated and tabulated.
Univariate and multivariate logistic regressions were used to assess the odds ratios (ORs) of developing HIV-associated ocular diseases. Performance of the models was assessed using receiver operating characteristic curves and the area under the curve. Correlation between eyes was managed by taking disease in one eye as the presence of disease in the participant. A p-value <0.05 was used to define statistical significance.
Results
A total of 182 participants were recruited for the study, of whom 139 (76.4%) were female. The mean (SD) age was 48.9 (10.6) years (95% confidence interval 47.3 - 50.5). Table 1 summarises the baseline and most recent immunological results.
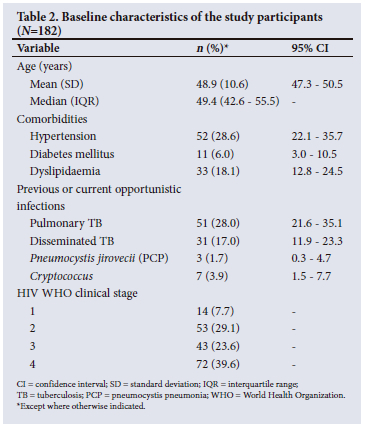
The most common comorbidity noted in the cohort was hypertension, followed by dyslipidaemia and diabetes mellitus (Table 2). Table 3 describes the participants' current antiretroviral therapy as well as the total number of ART regimens that they had used during their HIV journey.
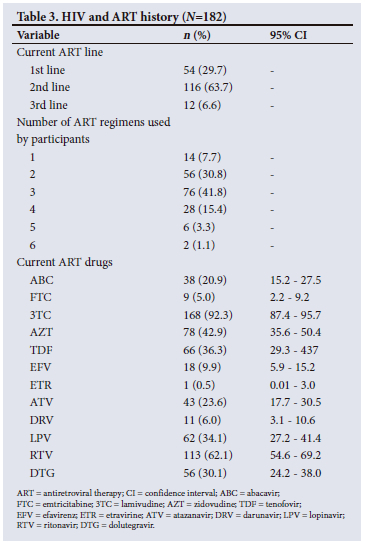
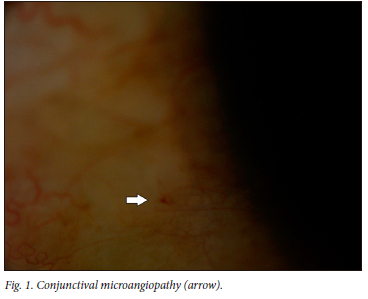
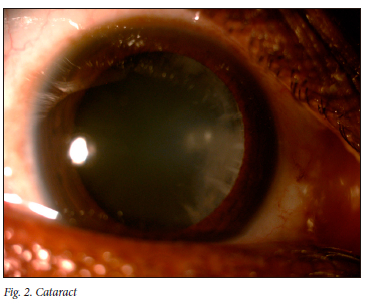
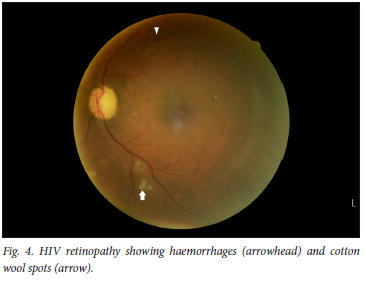
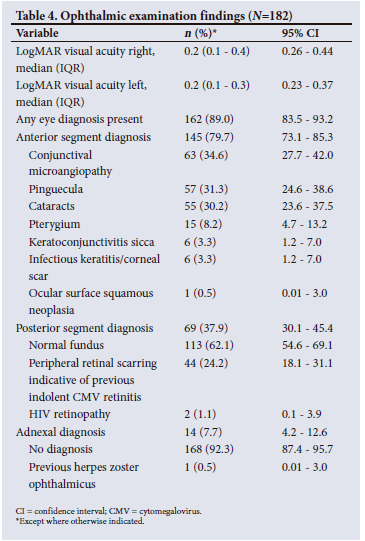
Eighty-nine percent of participants had a positive eye diagnosis, with the most common anterior segment diagnosis being conjunctival microangiopathy (34.6%) (Fig. 1), followed by pinguecula (31.3%) and cataracts (30.2%) (Fig. 2). The most common posterior segment finding was peripheral retinal scarring (Fig. 3), with features in keeping with previous CMV retinitis (24.2%). HIV-associated retinopathy (Fig. 4) was noted in only 1.1% of the study population. Table 4 summarises the ophthalmic examination findings.

After accounting for age and gender, a current CD4 count <200 cells/µL was shown to place an individual at a 10 times increased risk of an anterior segment diagnosis and a 4 times increased risk of cataracts, while those with a current CD4 count >200 cells/µL were twice as likely to have conjunctival microangiopathy (Table 5).
A variety of multivariate analyses were conducted for time on HAART, number of HAART regimens, number of treatment interruptions and previous opportunistic infections. None of these were significantly associated with the presence of any ocular disease.
Discussion
This study attempted to document ocular disease among people living with HIV (PLWH).
Ocular disease was found in up to 70 - 80% of HIV patients in the pre-HAART era, with figures between 19% and 70% reported for the HAART era.[1,10-12] In our study, 89% of the cohort of HIV-infected individuals had some form of ocular disease. Participants on a second-line regimen made up 63.7% of the cohort, which can be explained by the fact that it was a conveniently sampled cohort in a centre receiving referrals from nearby clinics and hospitals that had treated these patients using a first-line regimen. The cohort also comprised a majority female population, which supports the view that females are more likely to seek medical care for counselling, reproductive health and general health.[13]
The multivariate analysis using the dichotomous variable of a CD4 count <200 cells/µL v. >200 cells/µL showed an increased OR for cataracts and any anterior segment diagnosis. This finding may be due to the accelerated ageing process seen in PLWH, or a consequence of opportunistic infections with immune recovery inflammation, both of which cause accelerated cataracts.[14] A higher recent viral load was not associated with the increased risk of cataract or any other eye disease that had been seen in the pre-HAART era.[15] Reasons for this include earlier antiretroviral therapy (ART) initiation and more efficacious ART, especially with recent integrase inhibitor roll-out.[16]
Our findings are comparable to a 2013 Cape Town study by Pathai et al.,[14] who found that CD4 counts <200 cells/µL placed individuals at a statistically increased risk for cataract in comparison with non-infected individuals. A Danish study that assessed the risk of cataract surgery in HIV-infected individuals compared with age- and sex-matched counterparts also showed cataracts to be associated with CD4 counts <200 cells/µL.[17]
The most common ocular diseases in the pre-HAART era as described by Cunningham and Margolis[1] in 1998 were conjunctival microangiopathy (70 - 80%), CMV retinitis (40%), HIV retinopathy (50 - 70%) and Kaposi's sarcoma (25%). In the HAART era, multiple studies describing ocular disease have been conducted globally, with keratoconjunctivitis sicca and HIV retinopathy the most common findings.[12,18] There are few SA studies on ocular manifestations of HIV. In the present study, HIV retinopathy accounted for only 1% of our cohort. Schaftenaar et al[11] assessed 342 patients between August 2014 and March 2015 at three hospitals and three primary healthcare centres in SA, evaluating ocular manifestations in patients on long-term and short-term HAART and in those who were HAART naive. Eighteen percent of HIV-infected individuals had eye disease, with the most common condition overall being blepharitis. The main conditions affecting the anterior segment were clinically detectable cataracts, keratoconjunctivitis sicca and pterygium.[11]
In the present study, the most common anterior segment diagnosis was conjunctival microangiopathy (34.6%). A CD4 count >200 cells/µL showed an increased OR for conjunctival microangiopathy. Cunningham and Margolis[1] postulated that the cause of conjunctival microangiopathy was increased plasma viscosity, immune complex deposition and direct vascular endothelial infection by HIV.
Schaftenaar et al.[11] also showed that posterior eye conditions were more common among patients who had been on HAART for a longer period, including the diagnosis of HIV retinopathy. The most common posterior eye condition was HIV retinopathy (10% in patients on long-term HAART v. 9% in short-term patients).[11] However, a study by Acharya et al.[19] found that 25 - 50% of patients had HIV retinopathy. As mentioned previously, the above was not the case in our study (1.1%). Our study also showed that time on HAART did not predict ocular disease. This finding could be explained by current guidelines that advise test and treat, whereas older protocols focused on reduced CD4 levels prior to HAART initiation, as well as the advent of more robust HAART with quicker times to complete virological suppression. Earlier viral suppression would also result in a lower ocular viral load, resulting in less retinal inflammation, as shown by Hsu et al.[20] in a case series published in 2004. They studied plasma and ocular fluid HIV viral loads and showed that a higher HIV viral load correlated with an increased risk of ocular opportunistic infections and HIV retinopathy. Initiation of HAART and subsequent reduction in plasma viral load resulted in a reduction in ocular fluid viral load.[20]
Peripheral retinal scarring was assessed as most likely to result from previous indolent CMV infection. Of our cohort, 24.2% had features in keeping with indolent CMV infection; however, on multivariate analysis, age, gender, baseline CD4 and baseline viral load were not found to be statistically significant (Table 5). This finding could be explained by various reasons: more efficacious HAART, early introduction of HAART, and closer monitoring of PLWH. It is also not in keeping with findings from the LSOCA, which showed that up to 30% of PLWH will have CMV retinitis.[21] Sugar et al.[5] also showed that a higher viral load (>10 000 copies/mL, 4 on the log10 scale) was associated with a significant increase in the risk of retinitis (hazard ratio 37; p<0001), which was not the case in our study.
The results of the present study show that the number of ocular complications of HIV has remained unchanged, while the type of complications has changed. Cataracts have increased while HIV retinopathy has decreased in the HAART era in comparison with findings from the pre-HAART era.
Study limitations
The primarily female study participants represent a conveniently sampled group that may not be fully representative of the population. Many patients in our cohort were on second-line therapy (63.7%), representing patients who have had previous treatment failure. The clinic manages patients who are already on HAART and receives referrals from primary and secondary centres to assist in complicated cases, which also explains why one-third of the cohort were on a dolutegravir-based regimen and 60% were on a ritonavir regimen combined with lopinavir or atazanavir. This referral pattern to the clinic could therefore bias the type of participants that were seen and the HIV complications that were found. Ideally, a longitudinal study with a larger cohort at multiple sites could assist with reducing the above limitations. A study of this type would allow for better follow-up of patients to assess the effects of HIV and HAART on the eye.
Conclusion
The use of HAART is of paramount importance in reducing severe ocular disease. With the advent of HAART, the incidence of ocular manifestations of HIV is changing, with a decrease in the incidence of severe ocular opportunistic infections. In addition, our study showed that HIV retinopathy is not a significant finding in the HAART era. The study has shown that patients with a CD4 count <200 cells/µL are at high risk for developing any anterior ocular manifestation of HIV, including cataracts. Fortunately, early HAART seems to have almost entirely eliminated dangerous or permanently sight-threatening eye diseases, and the other eye pathology that is increasing is relatively innocuous or easily treatable. Routine ocular screening of HIV patients seems to be substantially less important than it was in the pre-HAART era.
Declaration. The research for this study was done in partial fulfilment of the requirements for II's MMed (Int Med) degree at the University of the Witwatersrand.
Acknowledgements. We thank the patients, colleagues, staff and support staff of CHBAH, Nthabiseng Clinic and St John Eye Hospital for enabling us to further research in this field. We are most grateful for research assistant Nazreen Chopdat's time and expertise.
Author contributions. II formulated the study, conducted the study (recruitment, consent, data collection) and wrote the manuscript. NA and SI conducted the ophthalmic examinations on the consenting participants. NA assisted with the design of the study, statistical analysis, and review of the manuscript. MV and SI assisted with the design and review of the manuscript.
Funding. None.
Conflicts of interest. None.
Disclaimer. The opinions expressed in this article are the authors' own and do not reflect the views of the University or the Department of Health.
References
1. Cunningham ET, Margolis TP. Ocular manifestations of HIV infection. N Engl J Med 1998;339(4):236-244. https://doi.org/10.1056/NEJM199807233390406 [ Links ]
2. Govender P, Hansraj R, Naidoo KS, Visser L. Ocular manifestations of HIV/AIDS: A literature review* (Part 1). S Afr Optom 2010;69(4):193-199. https://doi.org/10.4102/aveh.v69i4.141 [ Links ]
3. Govender P, Hansraj R, Naidoo KS, Visser L. Ocular manifestations of HIV/AIDS: A literature review (Part 2)*. S Afr Optom 2011;70(2):81-88. https://doi.org/10.4102/aveh.v70i2.97 [ Links ]
4. Moraes HV. Ocular manifestations of HIV/AIDS. Curr Opin Ophthalmol 2002;13(6):397-403. https://doi.org/10.1097/00055735-200212000-00010 [ Links ]
5. Sugar EA, Jabs DA, Ahuja A, Thorne JE, Danis RP, Meinert CL. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol 2012;153(6):1016-1024.e5. https://doi.org/10.1016/j.ajo.2011.11.014 [ Links ]
6. Hoover DR, Peng Y, Saah A, et al. Occurrence of cytomegalovirus retinitis after human immunodeficiency virus immunosuppression. Arch Ophthalmol 1996;114(7):821-827. https://doi.org/10.1001/archopht.1996.01100140035004 [ Links ]
7. Accorinti M, Pirraglia MP, Corradi R, Corsi C, Fabiani C, Pivetti-Pezzi P. Changing patterns of ocular manifestations in HIV seropositive patients treated with HAART. Eur J Ophthalmol 2006;16(5):728-732. https://doi.org/10.1177/112067210601600511 [ Links ]
8. Sudharshan S, Nair N, Curi A, Banker A, Kempen JH. Human immunodeficiency virus and intraocular inflammation in the era of highly active antiretroviral therapy - an update. Indian J Ophthalmol 2020;68(9):1787-1798. https://doi.org/10.4103/ijo.IJO_1248_20 [ Links ]
9. Osler M, Hilderbrand K, Goemaere E, et al. The continuing burden of advanced HIV disease over 10 years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis 2018;66(Suppl 2):S118-S125. https://doi.org/10.1093/cid/cix1140 [ Links ]
10. Schaftenaar E, van Gorp ECM, Meenken C, et al. Ocular infections in sub-Saharan Africa in the context of high HIV prevalence. Trop Med Int Health 2014;19(9):1003-1014. https://doi.org/10.1111/tmi.12350 [ Links ]
11. Schaftenaar E, Khosa NS, Baarsma GS, et al. HIV-infected individuals on long-term antiretroviral therapy are at higher risk for ocular disease. Epidemiol Infect 2017;145(12):2520-2529. https://doi.org/10.1017/S0950268817000978 [ Links ]
12. Saini N, Hasija S, Kaur P, Kaur M, Pathania V, Singh A. Study of prevalence of ocular manifestations in HIV positive patients. Nepal J Ophthalmol 2019;11(21):11-18. https://doi.org/10.3126/nepjoph.v11i1.25411 [ Links ]
13. Otwombe K, Dietrich J, Laher F, et al. Health-seeking behaviours by gender among adolescents in Soweto, South Africa. Glob Health Action 2015;8:25670. https://doi.org/10.3402/gha.v8.25670 [ Links ]
14. Pathai S, Lawn SD, Weiss HA, Cook C, Bekker LG, Gilbert CE. Increased ocular lens density in HIV-infected individuals with low nadir CD4 counts in South Africa: Evidence of accelerated aging. J Acquir Immune Defic Syndr 2013;63(3):307-314. https://doi.org/10.1097/QAI.0b013e31828ad759 [ Links ]
15. Jabs DA, van Natta ML, Holbrook JT, et al Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology 2007;114(4):780-786. https://doi.org/10.1016/j.ophtha.2006.11.008 [ Links ]
16. Rahangdale L, Cates M, Potter J, et al. Integrase inhibitors in late pregnancy and rapid HIV viral load reduction. Am J Obstet Gynecol 2016;214(3):385.e1-7. https://doi.org/10.1016/j.ajog.2015.12.052 [ Links ]
17. Rasmussen LD, Kessel L, Molander LD, et al. Risk of cataract surgery in HIV-infected individuals: A Danish nationwide population-based cohort study. Clin Infect Dis 2011;53(11):1156-1163. https://doi.org/10.1093/cid/cir675 [ Links ]
18. Saadouli D, Ammari L, Ben Mansour K, et al Ocular manifestations of people living with HIV in Tunisia. South Afr J HIV Med 2021;22(1):1193. https://doi.org/10.4102/sajhivmed.v22i1.1193 [ Links ]
19. Acharya PK, Venugopal KC, Karimsab DP, Balasubramanya S. Ocular manifestations in patients with HIV infection/AIDS who were referred from the ART Centre, Hassan, Karnataka, India. J Clin Diagn Res 2012;6(10):1756-1760. https://doi.org/10.7860/JCDR/2012/4738.2637 [ Links ]
20. Hsu W-M, Chiou S-H, Chen SS-L, et al. The HIV RNA levels of plasma and ocular fluids in AIDS patients with ophthalmic infections. Ophthalmologica 2004;218(5):328-332. https://doi.org/10.1159/000079475 [ Links ]
21. Jabs DA, van Natta ML, Holland GN, Danis R. Cytomegalovirus retinitis in patients with AIDS after initiating antiretroviral therapy. Am J Ophthalmol 2017;174:23-32. https://doi.org/10.1016/j.ajo.2016.10.011 [ Links ]
 Correspondence:
Correspondence:
I Ismail
ibismiles@ymail.com
Accepted 20 July 2023














