Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 n.10 Pretoria Oct. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i10.861
RESEARCH
Prevention of vertical transmission of HIV in Khayelitsha, South Africa: A contemporary review of services after 20 years
F M PhelanyaneI, II; A HeekesIII, IV; M SmithV, VI; K JenningsVII, VIII; V MudalyIX; P PietersX; J ArendseXI; S KariemXII; D CoetzeeXIII, XIV; A BoulleXV, XVI; E KalkXVII
IBSc, MPH; Centre for Infectious Disease Epidemiology and Research, School of Public Health, Faculty of Health Sciences, University of Cape Town, South Africa
IIBSc, MPH; Health Intelligence Directorate, Western Cape Government: Health and Wellness, Cape Town, South Africa
IIIBMedSci Hons, PhD; Centre for Infectious Disease Epidemiology and Research, School of Public Health, Faculty of Health Sciences, University of Cape Town, South Africa
IVBMedSci Hons, PhD; Health Intelligence Directorate, Western Cape Government: Health and Wellness, Cape Town, South Africa
VBSc Hons, MSc; Centre for Infectious Disease Epidemiology and Research, School of Public Health, Faculty of Health Sciences, University of Cape Town, South Africa
VIBSc Hons, MSc; Health Intelligence Directorate, Western Cape Government: Health and Wellness, Cape Town, South Africa
VIIMB ChB, DCH; HIV/AIDS/STI/TB Unit, City Health, Cape Town, South Africa
VIIIMB ChB, DCH; Western Cape Government: Health and Wellness, Cape Town, South Africa
IXMB ChB, MPH; Services Priorities Coordination Directorate, Western Cape Government: Health and Wellness, Cape Town, South Africa
XRN, RNAdmin; Western Cape Government: Health and Wellness, Cape Town, South Africa
XIRN, MPH; Emergency and Clinical Services Support, Western Cape Government: Health and Wellness, Cape Town, South Africa
XIIMB ChB, EMBA; Western Cape Government: Health and Wellness, Cape Town, South Africa
XIIIMB ChB, MS; Centre for Infectious Disease Epidemiology and Research, School of Public Health, Faculty of Health Sciences, University of Cape Town, South Africa
XIVMB ChB, MS; Health Intelligence Directorate, Western Cape Government: Health and Wellness, Cape Town, South Africa
XVMB ChB, PhD; Centre for Infectious Disease Epidemiology and Research, School of Public Health, Faculty of Health Sciences, University of Cape Town, South Africa
XVIMB ChB, PhD; Health Intelligence Directorate, Western Cape Government: Health and Wellness, Cape Town, South Africa
XVIIMB BCh, PhD; Centre for Infectious Disease Epidemiology and Research, School of Public Health, Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: The first vertical transmission of HIV prevention (VTP) programme in South Africa was launched in 1999 in Khayelitsha, Western Cape Province (WC). Since then, VTP guidelines have expanded in complexity and scope
OBJECTIVES: To describe contemporary VTP uptake in Khayelitsha and quantify vertical transmission (VT) risk factors based on linked routine electronic health data
METHODS: In the WC, all patients at public health facilities have a unique identifier allowing linkage across electronic health platforms through a health information exchange hosted within the WC Department of Health. We conducted a cohort analysis of mother-infant pairs where the mother was living with HIV and attended any obstetric care in Khayelitsha in 2017. Descriptive statistics assessed VTP coverage along the care cascade, including maternal viral load (VL) testing and early infant diagnosis (EID). Logistic regression analysis quantified a priori-defined risk factors associated with VT
RESULTS: Antenatal HIV prevalence in the cohort was 31.3%, and VT was 1.8% by 12 months. Of women living with HIV, 88.3% knew of their positive status at the first antenatal visit and 77.9% were already receiving antiretroviral therapy (ART). Most women diagnosed prior to delivery (94.5%) were initiated on ART; 85.0% received an antenatal VL test, of whom 88.0% were virologically suppressed. Women who were not virally suppressed had a five-fold (adjusted odds ratio (aOR) 5.3; 95% confidence interval (CI) 2.5 - 12.3) increased VT risk compared with those who were suppressed. Women who attended no antenatal care were at higher risk of VT (aOR 1.6; 95% CI 0.7 - 3.6) than those who did attend. EID coverage was suboptimal: a birth HIV polymerase chain reaction (PCR) test was available for 79.2% of infants, and a low proportion with a negative birth test had a repeat test around 10 weeks (57.9%). Data linkage identified an additional 15 infants living with HIV who were not detected by HIV-PCR testing alone
CONCLUSION: Although most women presented to care already knowing their HIV status, ART initiation was suboptimal prior to the first antenatal visit but improved over the course of pregnancy. The VT rate based on laboratory HIV-PCR testing alone underestimated HIV transmission: linked data from multiple sources suggested higher VT than programme-reported rates based on HIV-PCR testing alone
It has been 20 years since Western Cape Province (WC), South Africa (SA), launched its first vertical transmission of HIV prevention (VTP) pilot programme in Khayelitsha, an urban township 50 km from Cape Town city centre.[1] The programme, supported by Médecins sans Frontières, provided antenatal voluntary counselling and testing in primary care obstetric facilities with short-course zidovudine (AZT) from 36 weeks' gestation. Initially, only ante and perinatal prophylaxis was provided; continuity of care beyond delivery was poor, and postnatal services were fragmented.[1] At the time, national support for VTP was politically contentious and there was no infrastructure to facilitate testing. Since then, the WC VTP programme has expanded in range and efficiency, mirroring the World Health Organization (WHO) guidelines, and in 2013 the VTP programme adopted WHO Option B+: the provision of lifelong antiretroviral therapy (ART) to pregnant women irrespective of CD4 count, antenatal viral load (VL) testing in women already on ART, and support for exclusive breastfeeding for up to 6 months.[2]
Since the inception of the first programme in 1999, VTP services and outcomes in Khayelitsha have been reviewed regularly.[1,3] These studies looked at single elements of the care cascade, e.g. maternal testing or maternal prophylaxis. More recently, early infant diagnosis (EID) and infant treatment have been investigated. Throughout this time, Khayelitsha has remained the WC subdistrict with the highest antenatal HIV seroprevalence. Here we present an updated analysis of the entire VTP cascade in Khayelitsha from maternal HIV diagnosis to ART, VL monitoring and vertical HIV transmission (VT) in an era of universal ART in pregnancy with the advantage of a novel data linkage system. The WC Provincial Health Data Centre (PHDC) is a health information exchange that harmonises all electronic health data linked via a unique patient identifier issued to users of WC public health services. Routine electronic programme data entered in real time into healthcare administrative, pharmacy and laboratory information systems are consolidated in the PHDC, generating a longitudinal individual-level population database.[4] Using this resource, we defined a cohort of pregnant and postpartum women living with HIV (WLHIV) linked to their infants that allowed for assessment of exposure (maternal HIV, ART, VL) and outcome (VT of HIV), and likely timing of transmission.
We aimed to describe VT up to 12 months post partum and the associated risk factors in infants born to WLHIV who presented for antenatal care (ANC) or, in the absence of ANC, delivered in a public healthcare facility in the Khayelitsha subdistrict during 2017, in a setting with a well-established VTP presence and universal ART eligibility in pregnancy.
Methods
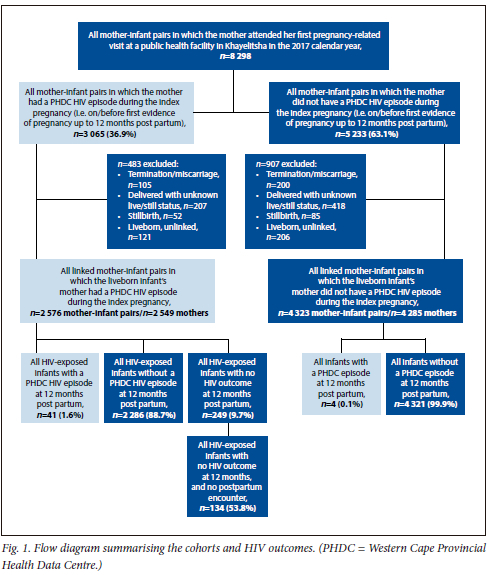
Using the PHDC, a cohort of WLHIV attending Khayelitsha obstetric services between 1 January and 31 December 2017 was defined, and linked mother-infant pairs were followed up until the infants reached 12 months of age. The cohort included women who attended ANC at any health facility in Khayelitsha (primary care or hospital), regardless of where they delivered, and those who delivered at any facility in Khayelitsha. Women who were diagnosed as living with HIV between delivery and 12 months post partum were included as a separate group (Fig. 1). A woman could enter the cohort at any date from her first electronic evidence of pregnancy up to 12 months post partum. This was defined as the index pregnancy period. Women and infants living with HIV were defined in the PHDC using multiple data sources including but not limited to laboratory test results, visits to HIV clinics, electronically captured ART start dates, and ART dispensing events.[4]
Prevention of VT of HIV in the WC
WHO Option B+ was implemented in the WC in 2013. This was superseded in 2016 by the introduction of a universal test-and-treat policy, the provision of lifelong ART upon HIV diagnosis to people living with HIV, regardless of clinical or immunological stage.[4] In addition, the VTP guidelines recommended routine HIV screening for negative women at presentation to ANC and regularly thereafter until the end of breastfeeding.[2] From December 2015, EID guidelines recommended that all HIV-exposed infants receive an HIV polymerase chain reaction (PCR) test at birth (within 1 week of birth), and a repeat HIV-PCR test at 10 weeks if the first result was negative or inconclusive; an HIV-PCR test was also indicated at any point up to 10 weeks of age if the infant did not receive one at birth.[5] Post-exposure prophylaxis (PEP) is recommended for all HIV-exposed infants, the regimen and duration differing according to the mother's HIV transmission risk profile. HIV-exposed infants born to low-risk mothers (documented VL <1 000 copies/mL <12 weeks before delivery) received nevirapine (NVP) for 6 weeks, regardless of feeding choice. HIV-exposed infants born to high-risk women are given NVP daily and AZT twice daily for 6 weeks, if the mother is not breastfeeding; breast-fed infants at high risk of VT receive NVP daily for at least 12 weeks and AZT twice daily for 6 weeks (NVP only stops when the maternal VL has fallen to <1 000 copies/mL).[5]
Data analysis
Analysis was performed using R statistical software (v 3.6.2) (R Foundation for Statistical Computing, Austria). Continuous variables were categorised according to pre-set thresholds (i.e. low birthweight (<2 500 g), viral suppression (VL <1 000 copies/mL), maternal age in 10-year bands, and first/subsequent pregnancy). Categorical variables were described using proportions, and frequency tables were used for comparison.
Logistic regression analysis was used to estimate associations with VT. Risk factors (maternal age, number of pregnancies, ANC attendance, timing of ART initiation and virological suppression) were selected a priori. Mother-infant pairs in which the infant HIV status was unknown at the end of the index period, as well as pairs where the mother was diagnosed post partum, were excluded from the logistic regression analyses.
Ethical considerations
The study was approved by the University of Cape Town's Human Research Ethics Committee (ref. nos HREC 817/2019 and HREC 541/2015) and the Provincial Government of the WC Department of Health Research Committee. There was no recruitment process, as we used data collected and processed by the WC Department of Health as part of its service provision. As the study was based on anonymised routine data, a waiver of informed consent was granted.
Results
The PHDC identified 8 298 mother-infant pairs in which the mother had her first electronic evidence of pregnancy at a public sector health facility in Khayelitsha during the 2017 calendar year. In this group, 3 065 fetuses/infants (36.9%) were exposed to HIV during the index period (i.e. antenatally or up to 12 months postpartum). Of the WLHIV, 483 were excluded antenatally because the pregnancy ended in termination of pregnancy/miscarriage/stillbirth or the woman could not be linked to an infant (Fig. 1 and Table 1). The final cohort consisted of 2 576 mother-infant pairs (including multiple births) in which the infant's mother was living with HIV during the index period. Of 2 576 infants, 41 (1.6%) had evidence of HIV infection, 2 286 (88.7%) had a negative HIV test result (HIV-PCR and/or serology) at some point over 12 months, and 249 (9.7%) had no electronic evidence of an HIV test result. Of these infants with an unknown HIV outcome, 53.8% (n=134) had no electronic evidence of a post-birth encounter with the health system, e.g. no evidence of well-baby or vaccination visits (Fig. 1). This figure represents 5.2% of the entire HIV-exposed cohort. The rate of antenatal VT was 1.4% (n=37/2 576 infants exposed to HIV antenatally). There were an additional 4 infants with a PHDC HIV episode where there was no electronic evidence of maternal HIV.

VT in the observation cohort was 45 infants (41 infants from mothers with an HIV episode in the PHDC and 4 infants whose mothers' HIV status was unknown in the PHDC).
The median (interquartile range) age of the pregnant WLHIV was 30 (26 - 34) years. This was the first pregnancy recorded in the dataset (i.e. primigravida) in 46.9%, and most women had electronic evidence of ANC (81.8%), defined as first evidence of attendance at a healthcare facility at least 7 days before the date of delivery.
Eighty-eight percent (n=2 251) of WLHIV knew their HIV-positive status at presentation to ANC, and 10.1% (n=257) were diagnosed during the index pregnancy. Seventy-eight percent (n=1 753) of those who knew their positive HIV status at the first ANC visit had started ART prior to this visit, and 94.5% (n=2 369) of the 2 508 women diagnosed prior to delivery started ART before delivery. Of the entire cohort (n=2 548), 80.6% received a VL test at some point during the index period (i.e. first evidence of pregnancy to 12 months post partum). Approximately 41% (n=922) of the 2 251 women diagnosed prior to the first ANC visit received a VL test prior to the first ANC visit, and virological suppression was 92%. Among the 2 508 women diagnosed prior to delivery, 85.0% (n=2 131) received an antenatal VL test and 88.0% were virologically suppressed. Seventy-three out of 139 (52.5%) with no digitally recorded ART, who received a VL test antenatally, had a VL <1 000 copies/mL (Table 1).
There was little electronically documented infant mortality over the study period, with 0.58% and 0.19% early and late neonatal deaths, respectively. Most infants (84.4%) had a normal birthweight (Table 1).
First HIV-PCR at birth
Most (79.3%) of the 2 537 in utero HIV-exposed infants received a birth HIV-PCR test, of which 15 (0.7%) were positive (Table 2). Fifty-eight percent (n=1156) of those who tested negative or indeterminate at birth (n=1 998) were re-tested around 10 weeks, when 2 infants (0.2%) were positive. Of the 42.1% (n=842) who did not receive a repeat test at 10 weeks, 12.2% were tested beyond 10 weeks and 1 (1.0%) was positive (Table 2).
First HIV-PCR at 10 weeks
Five hundred and twenty-four eligible infants (20.7%) did not receive an HIV-PCR test at birth, of whom 71.2% received their first test around 10 weeks of age: 4 (1.1%) tested positive. One hundred and fifty-one infants with antenatal HIV exposure had no evidence of a laboratory-based test within 12 months.
In the PHDC, infant HIV status was determined using multiple sources which identified an additional 15 infants living with HIV who would have been missed if evidence was limited to laboratory assays alone.
Risk factors for VT
The final cohort for logistic regression excluded infants with a missing HIV outcome (n=151) and those born to women who tested HIV positive post partum (n=40). Table 3 shows the results of the logistic regression analysis with VT outcome (HIV positive/negative) as the dependent variable. Maternal age category >34 years and a 10-year increase in maternal age both decreased the odds of VT by 20% in univariable analysis (odds ratio (OR) 0.8; 95% confidence interval (CI) 1.0 - 4.0 and OR 0.8; 95% CI 0.3 - 2.3, respectively).
However, this effect did not persist in the multivariable models (Table 3). WLHIV who did not attend any antenatal services but presented to the health facility for the first time for delivery had double the risk of VT in the univariable model (OR 2.1; 95% CI 1.0 - 4.0), although the effect was attenuated after adjustment for potential confounders (adjusted OR (aOR) 1.6; 95% CI 0.7 - 3.6).
Women who were not virologically suppressed antenatally had more than five times higher VT risk compared with those who were suppressed antenatally in both the univariable (OR 5.5; 95% CI 2.4 - 12.6) and multivariable (aOR 5.3; 95% CI 2.5 - 12.3) models, whereas women with no virological assessment antenatally had a sixfold increased VT risk (aOR 6.1; 95% CI 2.7 -12.3). There was no evidence of an increased risk of VT in the small proportion of women with no definitive evidence of antenatal ART. As the dataset may overestimate the proportion of women who did not attend any ANC, we performed an analysis excluding this variable (model 2). This increased the adjusted odds of VT in women who were not virologically suppressed, indicating that non-attendance of ANC is a confounder in the association between VT and maternal viral suppression.
Discussion
We present an updated analysis of the VTP programme in Khayelitsha, more than 20 years after its launch as the first such initiative in SA in 1999. At that time, antenatal HIV prevalence was between 16.0% (January 1999 - May 2000) and 20.3% (June - December 2000), and VT was estimated to be 20 - 30% without intervention^ and 12.5% with short-course AZT.[7] WHO Option B+ had been implemented in 2013 and was updated to include birth HIV-PCR for all HIV-exposed infants by 2015.[5] While antenatal maternal HIV prevalence had increased from 16% in 1999 to 31.3%, this prevalence was similar to the national prevalence (30.7%) reported in the 2017 National Antenatal Sentinel HIV Survey report (ANSUR).[7] The VTP programme had matured significantly, reaching more women by 2017. More WLHIV were aware of their status (88.9%) before their first ANC visit, slightly higher than the provincial and national estimates (70.0% and 60.8%, respectively) reported in the ANSUR. Ninety-five percent of those diagnosed before delivery had initiated ART prior to delivery and most (85.0%) received a VL test antenatally, 88.0% being virologically suppressed. VTP decreased from 20 - 30% in 1999 to 1.8% in 2017. While not yet meeting the 95-95-95 UNAIDS targets for
Antenatal ART uptake and effectiveness
The proportion of women on ART at presentation to ANC (77.9%) was slightly lower than the provincial and national estimates (81.4% and 91.1%, respectively) reported in the 2017 ANSUR.[7] This could be a result of selection bias of individuals who chose to participate in the survey (i.e. patients who attend clinic) and may have better ART-seeking practices, or the effect of pooled samples where better-performing regions increased the overall percentage.[7] However, rates of ART at delivery were similar to the survey national estimate (98.2%).[7] The 2017 ANSUR did not report on virological suppression; however, we reported both higher coverage and suppression rates than a study on maternal HIV VL testing (around the time of delivery) from four obstetric units in Gauteng Province, which reported a 34.0% testing coverage, and 77.5% with a VL <1 000 copies/mL.[9]
Early infant diagnosis
The present study showed relatively good coverage of birth HIV-PCR testing (79.3%), but poor uptake of the repeat test around 10 weeks of age. The birth HIV-PCR coverage was lower than the provincial estimates for the WC reported by the National Health Laboratory Service (NHLS) between April 2016 to March 2017 (87.9%) using laboratory data only.[10] This difference could be attributed to de-duplication and disaggregation of HIV-PCR data by the PHDC, compared with duplicated and aggregated NHLS data,[11] which allowed for better ascertainment of the timing of HIV-PCR uptake and prevented double-counting in our cohort. Overall coverage of any HIV-PCR by 10 weeks was 92.7%, similar to that reported by Kalk et al.[12] in nearby Mitchell's Plain and to the 93% national EID coverage reported by Moyo et al.[13] in their review of the 2015 routine HIV birth testing in the South African National Consolidated Guidelines.
VT of HIV
VT at 12 months post partum was 1.8%, which is similar to the 1.6% reported in the 'Closing the gaps' study conducted at Khayelitsha's site B community health centre in 2014.[14] The slightly higher rates in the present study may reflect our use of the multiple linked data sources used to identify infants and WLHIV, i.e. limiting evidence of HIV to laboratory parameters alone underestimated the rate of VT. Dependence on laboratory tests alone (HIV-PCR) would have missed an additional 15 infants living with HIV (who were identified by ART initiation in an HIV register, VL testing, and HIV rapid assays). In addition, we included WLHIV who were diagnosed antenatally and up to 12 months post partum (which includes 6 infants whose mothers were first identified during pregnancy and 4 infants born to women with no evidence of HIV before the infant's diagnosis).
We included diagnoses of 4 infants whose mothers were not identified as HIV positive by the PHDC (i.e. no maternal HIV episode) during the index period, suggestive of postpartum seroconversion or missed antenatal testing.[151 Results from mathematical modelling developed to simulate changes in VT over time in SA suggest that mothers who seroconvert during pregnancy and breastfeeding contribute substantially to VT. Model simulations suggest that postnatal transmission contributes to VT more than perinatal transmission, and the significance of VT from maternal seroconversion is therefore likely to depend on duration of breastfeeding.[15]
Consistent with the literature,[16] virological suppression had the biggest impact on VT. Compared with WLHIV who were virally suppressed antenatally, women who were not suppressed antenatally and those with no virological assessment had a five- and six-fold increase in VT risk, respectively. The increased risk indicates missed opportunities to optimise HIV treatment antenatally.
Study strengths and limitations
The consolidated data environment in the WC (PHDC) enabled high levels of ascertainment of exposures and outcomes. Previous assessments of the VTP programme often relied solely on laboratory data[10,17] and acknowledged difficulties in linking infants with maternal exposures, and in the de-duplication of results.[11] Within the PHDC, we were able to use additional data sources to identify infants living with HIV.[6] The cohort has the advantage over previous studies that maternal exposures (such as the timing of HIV diagnosis and ART initiation, as well as maternal VL test outcomes) could be linked to the infant's outcome.
One limitation, however, is that we were limited to electronically captured data by the various source information systems. We therefore had no control over the quality of data entered and could not account for missing data (i.e. missing electronic laboratory tests and/or electronic drug dispensing events, or missing or incorrect linkage of mother-infant pairs). However, missingness of infant HIV outcome data was very low. We also lacked information on women and infants who 'drop out' of the WC population and receive care in other provinces in SA (134 of the infants lost to follow-up (5.2% of the HIV-exposed cohort) had no electronic evidence of post-birth encounters with the health system). Infants living with HIV, particularly those not initiated on ART, have relatively high mortality and are more likely to migrate out of the province.[18] Missing data on ANC attendance undermined our ability to fully assess the impact of this confounder on the association between VT and maternal antenatal VL.
In addition, the PHDC consolidates data from the public sector only; ~25% of the WC population are members of private sector medical schemes,[19] so these results are only representative of WLHIV who access the public sector health facilities, approximately two-thirds of the population in the WC.
Data on infant PEP and breastfeeding are poorly documented electronically and we were unable to determine adherence to and the impact of infant PEP on VT, and in particular the risks associated with breastfeeding. Similarly, gestational age data are not robust and were not included. Postpartum transmission was not possible to determine as a proportion, as not all women who were HIV negative antenatally were assessed for HIV postnatally, those with positive children being more likely to be tested.
Conclusion
We present an updated analysis using routine programme data of the implementation and outcomes of the WC VTP guidelines in Khayelitsha, an area with high antenatal HIV prevalence and a history of promotion of and engagement with HIV care. While the VTP uptake indicators remained below the UNAIDS 95-95-95 targets in 2017, there is evidence of an established HIV care programme effective for the majority of pregnant WLHIV, and VT has fallen dramatically since the first interventions in 1999. However, there remains a vulnerable group of women who are not supported by the VTP programme and may require more targeted interventions. VTP assessed through aggregate data from VTP registers, or solely from centralised laboratory data, may be under-estimated in the absence of unique identifiers and availability of other clinical data sources.
Declaration. The research for this study was done in partial fulfilment of the requirements for FMP's MPH degree at the University of Cape Town.
Acknowledgements. None.
Author contributions. AB and EK designed the study. FMP, AH and MS curated and managed the data. FMP conducted the data analyses. FMP wrote the original manuscript draft. KJ, VM, PP, JA, SK and DC provided subject matter expertise essential to the interpretation of the research. All authors read and approved the final manuscript.
Funding. Research reported in this publication was supported by the US National Institutes of Health (R01HD080465, U01AI069924), Bill and Melinda Gates Foundation (OPP1191327, OPP1164272) and United States Agency for International Development (72067418CA00023). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest. AB and EK have received funding from ViiV Healthcare since this analysis was performed.
References
1. Stinson K, Giddy J, Cox V, et al. Reflections on a decade of delivering PMTCT in Khayelitsha, South Africa. South Afr J HIV Med 2014;15(1):30-32. https://doi.org/10.4102/sajhivmed.v15i1.41 [ Links ]
2. Poolman M, van der Walt N, Luwaca B. Western Cape Provincial Aids Council Annual Progress Report 2015/16. 2017. https://sanac.org.za/wp-content/uploads/2018/08/Western-Cape.pdf (accessed 31 May 2022). [ Links ]
3. Coetzee D, Hilderbrand K, Boulle A, Draper B, Abdullah F, Goemaere E. Effectiveness of the first district-wide programme for the prevention of mother-to-child transmission of HIV in South Africa. Bull World Health Organ 2005;83(7):489-494. [ Links ]
4. National Department of Health, South Africa. Implementation of the universal test and treat strategy for HIV positive patients and differentiated care for stable patients. Pretoria: NDoH, 26 August 2016. https://sahivsoc.org/Files/22%208%2016%20Circular%20UTT%20%20%20Decongestion%20CCMT%20Directorate%20(2).pdf (accessed 31 May 2022). [ Links ]
5. Provincial Government of the Western Cape Department of Health. The Western Cape Consolidated Guidelines for HIV Treatment: Prevention of Mother-to-Child Transmission of HIV (PMTCT), Children, Adolescents and Adults, amended. October 2016. Western Cape Department of Health, Cape Town. https://www.westerncape.gov.za/sites/www.westerncape.gov.za/files/the-western-cape-consolidated-guidelines-for-hiv-treatment-2015-v26012016.pdf (accessed 31 May 2022) [ Links ]
6. Boulle A, Heekes A, Tiffin N, et al. Data centre profile: The Provincial Health Data Centre of the Western Cape Province, South Africa. Int J Popul Data Sci 2019;4(2):1143. https://doi.org/10.23889/ijpds.v4i2.1143 [ Links ]
7. Woldesenbet SA, Kufa T, Lombard C, et al. The 2017 National Antenatal Sentinel HIV Survey, South Africa. Pretoria: National Department of Health, 2019. https://doi.org/10.13140/RG.2.2.25252.01928 [ Links ]
8. Joint United Nations Programme on HIV/AIDS (UNAIDS). Understanding fast-track: Accelerating action to end the AIDS epidemic by 2030. Geneva: UNAIDS, 2015. https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pd (accessed 31 May 2022). [ Links ]
9. Moyo F, Haeri Mazanderani A, Murray T, et al. Characterizing viral load burden among HIV-infected women around the time of delivery. J Acquir Immune Defic Syndr 2020;83(4):390-396. https://doi.org/10J097/QAL0000000000002267 [ Links ]
10. Goga A, Chirinda W, Ngandu NK, et al. Closing the gaps to eliminate mother-to-child transmission of HIV (MTCT) in South Africa: Understanding MTCT case rates, factors that hinder the monitoring and attainment of targets, and potential game changers. S Afr Med J 2018;108(3a):s17-s24. https://doi.org/10.7196/SAMJ.2017.v108i3b.12817 [ Links ]
11. Radebe L, Haeri Mazanderani A, Sherman GG. Evaluating patient data quality in South Africa's National Health Laboratory Service Data Warehouse, 2017 - 2020: Implications for monitoring child health programmes. BMC Public Health 2022;22:1266. https://doi.org/10.1186/s12889-022-13508-y [ Links ]
12. Kalk E, Kroon M, Boulle A, et al. Neonatal and infant diagnostic HIV-PCR uptake and associations during three sequential policy periods in Cape Town, South Africa: A longitudinal analysis. J Int AIDS Soc 2018;21(11):e25212. https://doi.org/10.1002/jia2.25212 [ Links ]
13. Moyo F, Haeri Mazanderani A, Barron P, et al. Introduction of routine HIV birth testing in the South African National Consolidated Guidelines. Pediatr Infect Dis J 2018;37(6):559-563. https://doi.org/10.1097/INF.0000000000001840 [ Links ]
14. Ibeto M, Giddy J, Cox V. Closing the gaps: Steps towards elimination of mother-to-child transmission of HIV. South Afr J HIV Med 2014;15(3):108-109. https://doi.org/10.7196/SAJHIVMED.1047 [ Links ]
15. Johnson LF, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr 2012;59(4):417-425. https://doi.org/10.1097/QAI.0b013e3182432f27 [ Links ]
16. Myer L, Phillips TK, Hsiao N-Y, et al. Plasma viraemia in HIV-positive pregnant women entering antenatal care in South Africa. J Int AIDS Soc 2015;18(1):20045. https://doi.org/10.7448/IAS.18.L20045 [ Links ]
17. Sherman GG, Haeri Mazanderani A, Barron P, et al. Toward elimination of mother-to-child transmission of HIV in South Africa: How best to monitor early infant infections within the Prevention of Mother-to-Child Transmission Program. J Glob Health 2017;7(1):010701. https://doi.org/10.7189/jogh.07.010701 [ Links ]
18. Anglewicz P, VanLandingham M, Manda-Taylor L, Kohler HP. Migration and HIV infection in Malawi. AIDS 2016;30(13):2099-2105. https://doi.org/10.1097/QAD.0000000000001150 [ Links ]
19. Statistics South Africa. General Household Survey 2017. Statistical release P0318. Pretoria: Stats SA, 2018. http://www.statssa.gov.za/publications/P0318/P03182017.pdf (accessed 15 March 2020). [ Links ]
 Correspondence:
Correspondence:
E Kalk
emma.kalk@uct.ac.za
Accepted 20 July 2023














