Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.10 Pretoria oct. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i10.1364
CORRESPONDENCE
Specimen rejection in a high-throughput TB laboratory: A descriptive study
To the Editor: In 2015, the South African (SA) National Health Laboratory Service (NHLS) reported an 8.3% (220 000/2.65 million) national tuberculosis (TB) sputum rejection rate.[1] Patients with rejected specimens will likely have a delayed diagnosis or remain undiagnosed. Regardless, local studies have yet to be performed to evaluate the reasons and magnitude for specimen rejection related to TB investigations. Areas with high rejection rates need to be identified in order to implement pragmatic quality improvement training interventions.
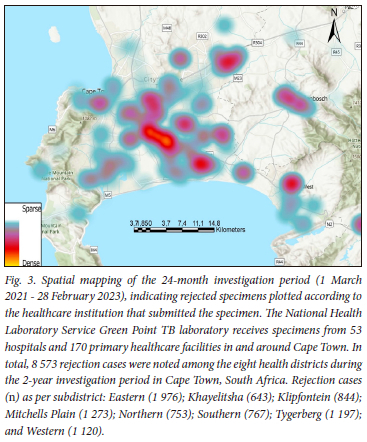
This descriptive study evaluated retrospective data spanning 2 years (1 March 2021 - 28 February 2023) on the reasons for rejecting specimens submitted to the NHLS Green Point complex TB laboratory. Monthly rejection rates were determined by dividing the number of tests rejected by the total performed. Reasons for rejecting a specimen, among others, included: information on the container does not match that on the specimen, no request form received with the specimen, specimen unsuitable (for example, saliva), container empty on arrival, volume insufficient, container not labelled, leaking, or specimen not received with the laboratory request form.[2] Duplicate specimen rejections for different TB-related tests from the same container were excluded from the analysis. Rejection cases were plotted according to the healthcare institution where the specimen was submitted, using ArcGISPro version 2.0 (Environmental Systems Research Institute, USA).
Ethical approval with a waiver for informed consent was obtained from the Human Research Ethics Committee of Cape Town University (ref. no. 270/2023).
The 24-month evaluation showed a rejection total of 13 396 in the Western Cape Province, SA, among 588 116 TB-related tests. The rejection rate varied from 3.1% (612/19 511) in March 2021 to 1.0% (252/25 282) in August 2022 (Fig. 1), with an average of 2.3% (558 cases/month) over the 2 years. Leaking specimen containers were the leading cause of TB specimen rejection, at 60.9% (8 157/13 396), followed by the specimen container being empty at 14.2% (1 903/13 396) and insufficient specimen volume at 7.9% (1 052/13 396), respectively (Fig. 2). Geographical maps showed a potential high rejection area among the Cape Metropole's Mitchells Plain, Tygerberg and Eastern sub-health districts (Fig. 3). Further studies will focus on ascertaining the number of specimens processed in these specific areas with possible enhanced training of TB healthcare workers for appropriate specimen collection to reduce rejection rates.
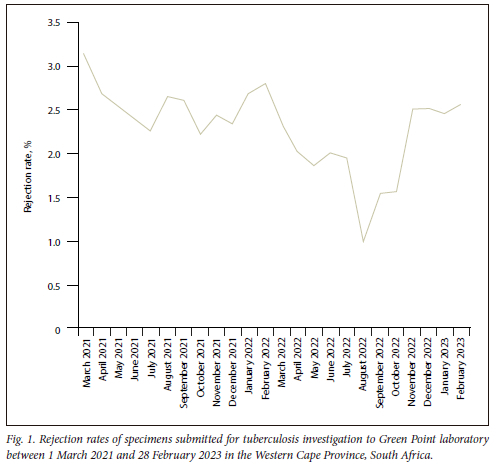
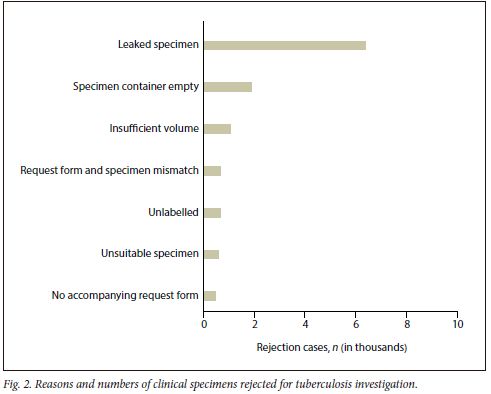
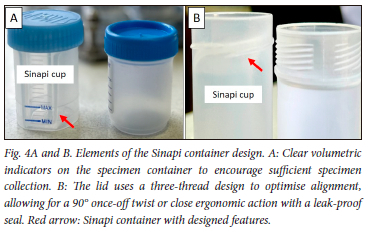
The rejected specimens for TB investigation are not only a financial burden on the healthcare system but a potential missed opportunity to diagnose Mycobacterium tuberculosis complex in a patient. Although the average rejection rate of 2.3% over the 2 years was below the accepted 3% standard for specimen rejection,[3] submitting an empty container (14.2%) or not labelling specimens (4.7%) is avoidable. In this regard, collaboration with local laboratories can be vital in supporting healthcare institutions with appropriate specimen collection training and surveillance of rejection rates to ensure timely interventions. In addition, the current study revealed that TB specimen leakage (60.9%) remains a significant impediment, and can be addressed with container design improvement (Fig. 4A and B). All specimens were processed and cultured at the National Health Laboratory Service, Green Point laboratory, Cape Town, South Africa.
Acknowledgements. We would like to acknowledge the frontline TB healthcare workers for their continuous efforts in our communities. We would also like to recognise the staff of the Green Point laboratory for their high-quality TB diagnostic service.
Author contributions. MZ analysed the data. CJO conceptualised the investigation. All authors drafted, edited and approved the final letter.
Funding. CJO receives funding from the NHLS Research Trust Development Grant (ref. no. PR2232714) and Harry Crossley Foundation.
Conflict of interest. Dr Magda Botha is employed by Sinapi Biomedical, but received no financial incentive to collaborate in the research.
Data sharing. The data supporting this study's findings are available from the corresponding author, CJO, upon reasonable request upon NHLS institutional approval from a controlled access repository.
Maria Stella Zerbini
American School of Cape Town, South Africa
Sarishna Singh
National Health Laboratory Service, Green Point TB Laboratory, and SAMRC Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Stellenbosch University, Cape Town, South Africa
Magda Botha
Sinapi Biomedical, Stellenbosch, South Africa
Yonas Ghebrekristos
National Health Laboratory Service, Green Point TB Laboratory, and SAMRC Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Stellenbosch University, Cape Town, South Africa
Christoffel Johannes Opperman
Division of Medical Microbiology, Faculty of Health Sciences, University of Cape Town, South Africa; National Health Laboratory Service, Green Point TB Laboratory, Cape Town, South Africa; SAMRC Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Stellenbosch University, Cape Town, South Africa. Christoffel.opperman@nhls.ac.za
References
1. Marokane P, Ndlovu S, Mahlatsi O, Ntsimane S, da Silva P, Berrie L. Anaylsis of Xpert MTB/ RIF rejection rates using laboratory data 2011 - 2015, South Africa. National priority program. Poster: African Society for Laboratory Medicine Annual Conference, 3 - 8 December 2016. Cape Town, South Africa [ Links ]
2. National Health Laboratory Service. Scope of tests offered by the NHLS handbook. Section 11.5: Specimen rejection criteria (GPQ0064 version 2). https://www.nhls.ac.za/diagnostic-services/type-of-tests (accessed 2 August 2023). [ Links ]
3. National Health Laboratory Service. Standard Operating Procedure. Selection and monitoring of quality indicators for process improvement of the quality management system (GPQ0043 version 4) (accessed 2 August 2023). [ Links ]














