Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.8 Pretoria Ago. 2023
http://dx.doi.org/10.7196/samj.2023.v113i8.710
RESEARCH
The association between cytology and histopathology in thyroid nodules over a 6-year period in an urban hospital in South Africa
M ChettyI; B MbathaII; P FruIII
IMB ChB; Department of Surgery, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB BCH; FCS (SA) Wits; Department of Endocrine and Bariatrics, Helen Joseph Hospital, Johannesburg, South Africa
IIIPhD; Department of Surgery, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Thyroid cancer is prevalent both internationally and locally, and is the most common cause of endocrine malignancies in Africa. The prognosis of thyroid cancer in general is quite good, but to achieve good outcomes, appropriate methods for diagnosis are important. A cytology result obtained from a fine needle aspiration and biopsy (FNAB) is one such method, and is less invasive and has less risk compared with obtaining a histological result via open surgery. However, there are accuracy differences that have been reported in different literature findings
OBJECTIVES: To determine the diagnostic accuracy rate of thyroid FNAB compared with histopathological samples at an urban hospital in South Africa (SA), and compare this with international standards
METHODS: A retrospective observational study was conducted of patients who had undergone both FNAB and thyroidectomies at Helen Joseph Hospital, Gauteng Province, SA, a public tertiary hospital, between 1 January 2016 and 31 December 2021. Various analytical methods were used, including Poisson generalised linear model, binomial generalised linear model, a two-proportion z-test, McNemar's test and the F1 score
RESULTS: There were 164 participants in this study who were between the ages of 21 and 82 years old. Thirty-six records were excluded for use as a comparison between cytology and histology, as they were in Bethesda categories 1, 3 and 4. Out of the 128 records that were compared, there was statistically significant agreement v. non-agreement between cytology and histology findings for thyroid nodules (109 v. 19, p<0.001, respectively). A comparison between our sample population and internationally published standards in terms of rate of malignancy noted that our rate of malignancy was slightly higher in Bethesda 2 patients (5.88%) v. internationally published standards (0 - 3%), and slightly lower in Bethesda 4 (23.52%) and 6 (77.77%) v. internationally published standards (25 - 40% and 97 - 99%, respectively
CONCLUSION: There was good correlation between cytology and histology for thyroid nodules. Differences were noted between the percentage of malignant cases in the different Bethesda categories compared with international standards as noted above. We recommend that further studies are conducted locally to improve knowledge on this topic
Thyroid cancer is prevalent both internationally and locally in South Africa (SA),[1] and is the most common cause of endocrine malignancy in Africa.[1] Rahbari et al.[2] found that it is 2.9 times more common in females than males, and is currently on an increasing trend worldwide.[1] This increase may be due to various factors, including improvements in diagnostic methods and changes in iodination of salt in our diets.[3] Internationally, according to the National Cancer Database in the USA, the incidence of thyroid cancer has increased over the last decade from an incidence of 7.1 per 100 000 in 2000 to 17.6 per 100 000 in 2013.[4] This increase was noted predominantly in papillary carcinoma.[4] The 2019 SA National Cancer Registry Guidelines noted that thyroid cancer was the 15th most common cancer among females, compared with the 32nd most common in males, with incidences of 1.28% v. 0.4%, respectively[5]
The prognosis of thyroid cancer in general is quite good, with an up to 98% cure rate in papillary and follicular carcinomas.[6] In order to achieve this rate of curative intervention, appropriate methods of diagnosis of the type of carcinoma are important, as this affects subsequent treatment. Papillary carcinomas can be diagnosed on cytology, and management is subsequently a surgical resection and/or postoperative radio-ablative therapy[7] Cytology alone may not always be adequate in distinguishing benign v. malignant follicular and Hurthle cell carcinoma, and may require further histological sampling.[7] A cytology result is obtained from a fine needle aspiration and biopsy (FNAB).[7] It is less invasive and poses fewer risks compared with obtaining a histological result via open surgery. A diagnostic lobectomy is the alternative to thyroid FNAB.
Risks of surgical intervention include bleeding, recurrent laryngeal nerve and external branch of the superior laryngeal nerve injury infection, tracheal injury, hypothyroidism, hypoparathyroidism and thyroid storm.[8] Thus, considering the extent of risks possible during an open histological sampling, it would be ideal to avoid unnecessary thyroidectomies unless necessary. Hence, an appropriate system or classification was necessary to ensure that unnecessary surgery is minimised without missing indications where surgical intervention is warranted.
In 2009, the Bethesda system of grading cytology based on risk of malignancy was developed,[9] and revised in 2017.[10] It graded thyroid nodules into six categories, allowing surgeons to aim intervention regarding the nodule appropriately.[10] Table 1 illustrates the various grades used with the Bethesda system and how they have been used to help manage thyroid nodules.[10] The Bethesda system of categorising thyroid nodules based on the risk of malignancy provides a means of avoiding unnecessary surgery and its complications in the process.
Various studies addressed the accuracy of this cytological assessment in diagnosing those at risk of malignancy. A 2018 Jordanian study by Abdullah et al.[11] found that cytology compared well with histopathology, with a sensitivity and specificity of 95.6% v. 54.8%, respectively. The positive predictive value of FNAB was 75.4%, and the negative predictive value was 89.5%.[1l] A 2018 Brazilian study[12] concluded that the corresponding malignancy rates for each grade of the Bethesda classification were within the expected ranges of malignancy. Furthermore, a Turkish study in 2020[9] found that cytology corresponded well to the expected ranges of malignancy on histology. Meta-analysis studies have been done internationally, with one such study involving 25 445 cases showing a sensitivity rate of 97%, a specificity rate of 50.7% and a diagnostic accuracy of 68.8% when comparing cytology v. histopathology.[13] However, all these studies supporting this cytological classification system involve patient samples outside of Africa.[9,11,12] Hence, to the best of our knowledge, there are no local SA studies that support or refute the accuracy of the use of the Bethesda classification as a modality in the management of thyroid nodules. This is concerning as there is evidence to suggest that there are differences in the incidence and epidemiology of thyroid nodules in SA v. international settings owing to differences in iodine availability in the diet.[3] The main aim of this study was to determine the diagnostic accuracy rate of thyroid FNAB in relation to histopathological samples taken at a SA setting from 2016 to 2021, and compare the findings to international standards.
Methods
This was a retrospective observational study of patients who had undergone both FNAB and thyroidectomies at Helen Joseph Hospital, Johannesburg, SA, between 1 January 2016 and 31 December 2021. This is a public tertiary hospital affiliated with the University of the Witwatersrand's medical school. Permission to conduct this study was granted by the University of the Witwatersrand Human Research Ethics Committee (ref. no. M220502). Patient confidentiality was maintained by keeping all identification parameters anonymous and only accessible to the principal investigator. The sample population consisted of all adult patients for whom both cytology and histopathological analysis had been done and who were aged >18 years. Patients younger than this were excluded due to paediatric patients not attending this institution, as well as investigators' choice for adolescent patients.
Patients were identified initially using the general surgery theatre record book from the hospital. The patients' corresponding files were then obtained from the records office to confirm that a cytological sample had been taken and to capture additional study details. De-identified details from the database were transcribed onto an Excel (Microsoft Corp., USA) database. Each entry was re-checked twice to ensure data accuracy.
The relevant data obtained for each patient included a histology result and cytology result, which were obtained from the file, as well as online from the National Health Laboratory Service (NHLS) system, patient demographics including age, sex, the number of cytological samples taken for each patient and the grading of each sample in terms of the Bethesda classification.
Statistical analysis
A statistical analysis was done using R and RStudio software using various statistical models, including Poisson generalised linear model (to calculate the frequency of each thyroid nodule subtype), binomial generalised linear model (to calculate the age and gender association with thyroid nodules), a two-proportion z-test (to calculate both the proportion of malignancy per Bethesda category and its comparison to internationally published rates), McNemar's test (to calculate the degree of agreement between cytology and histology) and the Fl score (to calculate the performance parameters for cytology). Statistical significance was present where p<0.05. The sensitivity, specificity, positive and negative predictive values and diagnostic analysis were also determined
From the patients included in the study, there were none who presented with Bethesda classification 1, 3 and 4, i.e. they had either no definitive diagnosis of benign or malignant pathology on cytology, or had inadequate samples on cytology. This therefore prevented comparisons between their cytological result and their histopathological result. These patients had a comparison of their histological results done as to whether they were benign or malignant.
For patients who presented with Bethesda 2, 5 or 6, there was a definitive diagnosis of either benign or malignancy on cytology, and therefore these were analysed in terms of the full spectrum of objectives for this study in order to determine the accuracy of cytology v. histopathology.
Results
Demographics
In total, there were 176 patients with thyroid disease in the study period. Twelve of these were excluded as they did not meet the study inclusion criteria, hence the sample size for the study was 164 participants. Of the 164 participants, 36 were either Bethesda 1, 3 or 4, resulting in them being included in the study for analysis but excluded from cytology-histology comparability. The other 128 patients were Bethesda 2, 5 or 6, and therefore a comparison of histology and cytology was done. The age range of the patients was between 21 and 82 years. The average age of patients with thyroid nodules (both benign and malignant) was 48.07 years, while that for patients with malignancy was 45.24 years. The median age of malignancy was higher in males than females (59 v. 41 years, respectively; p=0.841), as shown in Fig. 1.
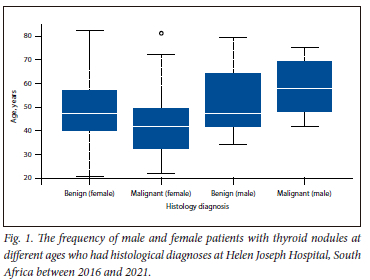
Of the eligible participants, there were significantly more females (92.68%) than males (7.32%) with thyroid nodules (152 v. 12, p<0.001). A total of 29 patients were diagnosed with malignancy on both cytology and histology, and also showed significantly more females than males (24 v. 5, p=0.004) owing to the larger sample size of females. However, a percentage comparison showed that males had a higher malignancy rate compared with females (41.67% v. 15.79%; p=0.0006).
Cytology v. histology findings
There was statistically significant agreement (v. non-agreement) between cytology and histology for thyroid nodules (109 v. 19; p<0.001).
The cytology and histology results were compared for all 128 patients in our cohort with Bethesda scores of 2, 5 and 6 (78.04% of sample). Of these 128 patients, there was agreement between in the cytology and histology results for 109 patients (85.15%). The remaining 36 patients in the included sample were either Bethesda 1, 3 or 4, and therefore could not be compared in terms of histology and cytology. The confusion matrix and associated metrics that highlight the high level of agreement between the two different diagnoses are provided (Fig. 2). An agreement of 109 patients is noted on the confusion matrix, 29 being malignant on both cytology and histology and a further 80 benign on both cytology and histology. There was non-agreement between cytology and histology of 19 patients, 14 presenting as malignant on cytology but benign on histology, and 5 who were benign on cytology and malignant on histology.
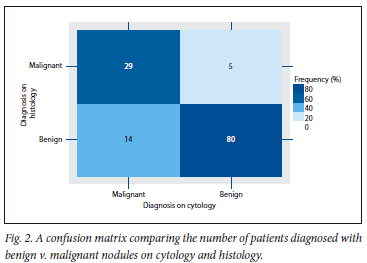
Use of cytology in the probability of malignancy
The use of cytology in diagnosing the probability of malignancy showed 85.29% sensitivity, a specificity of 85.11%, a positive predictive value (PPV) of 67.44% and a negative predictive value (NPV) of 94.12%. There was a false negative rate of 14.71% and a false positive rate of 14.89%. Cytology in this sample showed 85.15% diagnostic accuracy in predicting malignancy.
Proportion of benign v. malignant cases based on the Bethesda system
Of the 6 patients who had a Bethesda 1 in the sample, all 6 (100%) were noted to have a benign result on histopathology. Of the 13 patients who had a Bethesda 3 on cytology, 10 (76.92%) had a benign result on histopathology v. the 3 (23.07%) who had a malignant result on histopathology. Of the 17 patients who had a Bethesda 4 on cytology, 13 (76.47%) showed a benign result on histology v. the 4 patients (23.52%) who had a malignant result (Fig. 3).
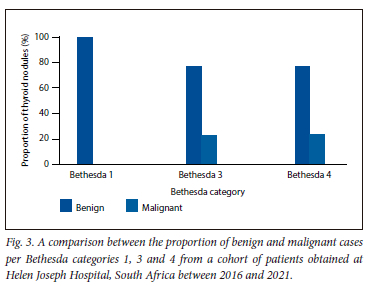
When comparing our study s rate of malignancy in each category with those of international standards,it was noted that our rate of malignancy was lower than that published internationally for Bethesda 4 and 6 (23.5% v. 25 - 40% and 77.77% v. 97 - 99%, respectively). However, our rate of malignancy was higher in Bethesda 2 (5.88% v. 0 - 3%) compared with international rates (Table 2).
Subtypes of thyroid disease
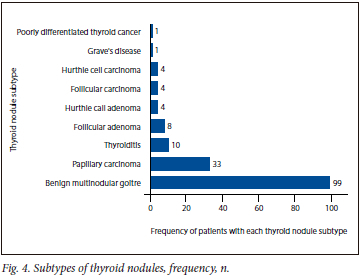
The majority of patients had a benign multinodular goitre (60.36%), followed by papillary carcinoma (20.12%) and thyroiditis (6.09%) (Fig. 4).
Discussion
Thyroid disease usually occurs more often in females than males, often owing to it being autoimmune in nature.[13] The average sex distribution of thyroid nodules in both endemic and non-endemic regions is 3:1 in female:male ratio.[14] In addition to this, a metaanalysis by le Clair et al.[15] suggested that females have a higher risk of thyroid malignancy than males. Our study concurred that thyroid nodules are 4.8 times higher in females than males; however, it was notable that the percentage of males with malignancies was higher than that of females. We postulate that this could have been due to the small sample size in our study.
Further, a study done in Brazil by Girardi[16] focused only on thyroid malignancies and noted a mean age of 48.14 years of incidence of thyroid cancer. Similar findings were reported in this study (around 45.24 years), in agreement with these international findings. Cancer Research UK noted that the highest number of women diagnosed with thyroid cancer are between the ages of 44 and 49 years, with men being more likely to develop thyroid cancer at an older age.[17] These findings are similar to those reported here, with the median age of malignancy in males v. females being 59 years v. 41 years, respectively. Although the reason for this difference in incidence of age is not clear, there are various theories as to why the onset of thyroid nodules occur earlier in females than males.[17] These theories revolve around hormones and include pregnancy, the use of oral contraceptives, hormone replacement therapy, the start age of menstruation and the age of menopause.[17]
A good correlation between cytology and histology was present in this study, with 85.16% of our patients' samples correlating. A recent 2018 study by Abdullah et al.[11] found that a comparison between cytology and histology revealed test sensitivity v. specificity of 95.6% v. 54.8%, respectively. The PPV was 75.4% while the NPV was 89.5%.[11] Our study found the sensitivity and specificity to be around the 85% mark, while the PPV and NPV were 67.44% v. 94.12%, respectively. This may suggest that the NPV may offer valuable information when analysing results, i.e. showing true negatives. This is relevant as it affects further management of patients, avoiding unnecessary surgical intervention and subsequent possible complications. On the other hand, our PPV was significantly low at 67.44%, and also noted to be much lower than that found in international studies. Therefore, the reliability of obtaining a Bethesda score of 5 and 6 comes into question. The false positive rate in our study is calculated to be 14.89%. In general, the management principles of obtaining a Bethesda 5 and 6 result are to proceed with surgery. However, due to the low PPV, quite a few of these surgical interventions maybe unnecessary, owing to the likelihood of benign disease. We postulate that this could be due to a small sample size, and therefore studies with a larger sample size should be conducted to correlate this finding.
In addition, the local malignancy rates for each grade of Bethesda was compared against the 2017 Bethesda System for Reporting Thyroid Cytopathology by Cibas and Ali.[10] The results showed that the local rates for malignancy for Bethesda 1, 4 and 6 were lower than expected, while Bethesda 2 was higher than the norm. Bethesda 3 and 5 were in keeping with international standards. Although most other studies align with the international Bethesda ranges, our results were not always in keeping with these ranges. We postulate that this could be due to ethnic differences among a predominantly African population, or possibly due to HIV infection, which is not uncommon in our local setting. However, there is limited evidence in the literature to support or refute this. It is also important to note that the sample size only involves a single population cohort, which may have limited representation of the population as a whole. This may be a contributing factor to the difference in rates between international and local standards.
The present study also noted that based on histology results, the majority of patients had a multinodular goiter (MNG) (60.36%), followed by papillary carcinoma (20.12%), thyroiditis (6.09%) and follicular carcinoma (2.4%). Various factors have been implicated in the development of MNG, the most common factor being iodine deficiency[18] MNG incidence is directly proportional to the degree of iodine deficiency[19] Studies have shown that MNG has a prevalence of between 0.4 and 7.2% in iodine-deficient areas, and around 4% in iodine-sufficient areas.[18] Evidence has suggested that iodine or the deficiency thereof plays a large role in influencing the prevalence and morphology of thyroid nodules.[3] In iodine-replete areas, it has been noted that the less aggressive papillary thyroid carcinomas are more common, whereas in iodine-depleted areas, follicular thyroid carcinoma is more common.[3] In general, iodine deficiency increases the rate of thyroid carcinoma.[3] Studies have shown that improvements in iodine in the diet have influenced the morphology of thyroid nodules.[3] Thus, the frequency of papillary carcinoma v. follicular carcinoma can indicate whether or not an iodine-depleted or repleted population is involved. In our case, we had an incidence of papillary carcinoma of 20.12%, which was much higher than the incidence of follicular carcinoma of 2.4%. Although this does suggest the presence of iodine in our population sample's diet, the extensively high rate of MNG of 60.36% suggests that we are still in a very iodine-deficient setting.
Limitations
This study was limited by various factors. Firstly, it was a single-centre study, and so limited in representation. Only the information related to thyroid disease and demographics was recorded, and no comorbidities or ethnicity were recorded to look at other factors affecting the incidence of thyroid disease. The poor handwriting in theatre record books led to difficulty in getting correct patient identification details, thus decreasing the sample size. Furthermore, owing to the method of identification of the patients from the surgical theatre record books, there was a significant selection bias as only patients involved in a surgical intervention from the thyroid nodule were identified, and thus it is highly likely that patients who presented within the relevant period with thyroid nodes as outpatients but did not proceed to surgery were left out of the study, which could possibly skew the study results.
Conclusion
There was good correlation between cytology and histology for thyroid nodules. Although a higher number of females had thyroid cancers, the percentage of males who presented with a thyroid nodule was higher than that of females. Differences were noted between the percentage of malignant cases in the different Bethesda categories compared with international standards. We recommend that further studies are conducted locally to improve knowledge on this topic.
Declaration. This study was done as MC's MMed for an FCS (SA).
Acknowledgements. Uthamaganthan Chetty Savithree Chetty & Rushern Chetty - moral support and guidance. Dhesan Swaminathan - continued support and guidance.
Author contributions. MC was the primary researcher, author and writer of the study. The study was further supervised and facilitated by MB and PF in terms of both the clinical and research aspects of the study.
Funding. Self-funded by principal investigator (MC).
Conflicts of interest. None.
References
1. Chagi N, Bombil I, Manneli A. The profile of thyroid cancer in patients undergoing thyroidectomy at Chris Hani Baragwanath AcademicHospital. S Afr J Surg 2019;57(3):55. https://doi.org/10.17159/2078-5151/2019/v57n3a2928 [ Links ]
2. Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity Future Oncol Lond Engl 2010;6(11):1771-1779. https://doi.org/10.2217%2Ffon.10.127 [ Links ]
3. Kalk WJ, Sitas F, Patterson AC. Thyroid cancer in South Africa - an indicator of regional iodine deficiency. S Afr Med J 1997,87(6).731-733. [ Links ]
4. Olson E, Wintheiser G, Wolfe KM, Droessler J, Siiberstein PT. Epidemiology of thyroid cancer. A review of the National Cancer Database, 2000-2013. Cureus 2019;11(2):e4127. https://doi.org/10.7759/cureus.4127 [ Links ]
5. Cancer Association of South Africa. Cancer Statistics. CANSA, 2012. https://cansa.org.za/south-african-cancer-statistics/ (accessed 29 March 2022). [ Links ]
6. Endocrine Web. Thyroid cancer. Symptoms, diagnosis, and treatment - causes, symptoms, diagnosis, treatments, and support, https://www.endocrineweb.com/conditions/thyroid-cancer/thyroid-cancer (accessed 20 October 2021). [ Links ]
7. Singh P, Moodley M. Lecture notes in general surgery. A students guide, UKZN. 1st ed. Durban? University of KwaZulu-Natal, 2012. [ Links ]
8. Sharma PK. Complications of thyroid surgery. https://emedicine.medscape.com/article/852184-overview (accessed 20 October 2021). [ Links ]
9. Bayrak BY, Eruyar AT. Malignancy rates for Bethesda III and IV thyroid nodules. A retrospective study of the correlation between fine-needle aspiration cytology and histopathology. BMC Endocr Dis 2020;20:48. https://doi.org/10.1186/sl2902-020-0530-9 [ Links ]
10. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathoJogy. J Am Thyroid Assoc 2017;27(11):1341-1346. https://doi.org/10.1016/j.jasc.2017.09.002 [ Links ]
11. Abdullah N, Hajeer M, Abudalu L, Sughayer M. Correlation study of thyroid nodule cytopathology and histopathology at two institutions in Jordan. Cyto J 2018;15(15):24. https://doi.org/10.4103/cytojournal.cytojournal_53_ 17 [ Links ]
12. Brites CA, Baisimelli LBS, Coelho KMPA, Fronza-Júnior H, Stall J, França PHC. Investigation of correlation between cytologicai and histological findings in suspected carcinoma of thyroid. J Bras Patol E Med Lab 2018;54:407-411. https://doi.org/10.5935/1676-2444.20180061 [ Links ]
13. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda system for reporting thyroid cytopathology. A meta-analysis. Acta Cytol 2012;56(4):333-339. https://doi.org/10.1159/000339959 [ Links ]
14. Mulder JE. Thyroid disease in women. Med Clin North Am 1998;82(1):103-125. https://doi.org/10.1016/s0025-7125(05)70596-4 [ Links ]
15. Le Clair K, Bell KJL, Furuya-Kanamori L, Doi SA, Francis DO, Davies L. Evaluation of gender inequity in thyroid cancer diagnosis. Differences by sex in US thyroid cancer incidence compared with a metaanalysis of subclinical thyroid cancer rates at autopsy. JAMA Intern Med 2021;181(10):1351-1358. https://doi.org/10.1001/jamainternmed.2021.4804 [ Links ]
16. Girardi F. Thyroid carcinoma pattern presentation according to age. Int Arch Otorhinol 2016;21(01):38-41. https://doi.org/10.1055/s-0036-1585095 [ Links ]
17. Cancer Research UK. Risks and causes of thyroid cancer. Cancer Research UK, 2021. https://www.cancerresearchuk.org/about-cancer/thyroid-cancer/causes-risks (accessed 5 December 2022). [ Links ]
18. Khatawkar AV, Awati SM. Multi-nodular goiter. Epidemiology, etiology, pathogenesis and pathology IAIM 2015;2(9):152-156. [ Links ]
19. Pinchera A, Aghini-Lombardi F, Antonangeli L, Vitti P. Gozzo multinodulare. Epidemiologia e prevenzione [Multinodular goiter. Epidemiology and prevention]. Ann Ital Chir 1996;67(3):317-325. [ Links ]
 Correspondence:
Correspondence:
M Chetty
chetmerish@gmail.com
Accepted 25 April 2023














