Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.8 Pretoria ago. 2023
http://dx.doi.org/10.7196/samj.2023.v113i8.348
RESEARCH
Signal of harm in morphine use in adults with acute pulmonary oedema: A rapid systematic review
C HendrikseI, II; V NgahIII; I I KallonIV; G ThornV, VI; T D LeongVII, VIII; K CohenIX, X; M McCaulXI, XII, XIII
IMB ChB, MMed (EM); Division of Emergency Medicine, Department of Family, Community and Emergency Care, Faculty of Health Sciences, University of Cape Town South Africa
IIMB ChB, MMed (EM); Ministerially appointed PHC/Adult Hospital Level Expert Review Committee of the National Essential Medicines List Committee, National Department of Health (2019 - 2023), Pretoria, South Africa
IIIBSc (Nursing), MSc (Clin Epi); Centre for Evidence-based Health Care, Division of Epidemiology and Biostatistics, Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IVMTech (Envr Health), PhD; Centre for Evidence-based Health Care, Division of Epidemiology and Biostatistics, Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
VMB ChB, M Prax Med; Ministerially appointed PHC/Adult Hospital Level Expert Review Committee of the National Essential Medicines List Committee, National Department of Health (2019 - 2023), Pretoria, South Africa
VIMB ChB, M Prax Med; Amajuba District Clinical Specialist Team, KwaZulu-Natal Department of Health, Pretoria, South Africa
VIIBPharm, MSc (Clin Epi); Secretariat to the PHC/Adult Hospital Level Expert Review Committee (2020 - 2023); Secretariat to the National Essential Medicines List Committee, National Department of Health (2021 - 2022), Durban, South Africa
VIIIBPharm, MSc (Clin Epi); Cochrane South Africa, South African Medical Research Council, Cape Town, South Africa
IXMB ChB, MMed (Clin Pharm); Ministerially appointed PHC/Adult Hospital Level Expert Review Committee of the National Essential Medicines List Committee, National Department of Health (2019 - 2023), Pretoria, South Africa
XMB ChB, MMed (Clin Pharm); Division of Clinical Pharmacology, Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
XIMSc (Clin Epi), PhD; Ministerially appointed PHC/Adult Hospital Level Expert Review Committee of the National Essential Medicines List Committee, National Department of Health (2019 - 2023), Pretoria, South Africa
XIIMSc (Clin Epi), PhD; Centre for Evidence-based Health Care, Division of Epidemiology and Biostatistics, Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
XIIIMSc (Clin Epi), PhD; South African GRADE Network, Centre for Evidence-based Health Care, Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND: Heart failure affects nearly 65 million people globally, resulting in recurrent hospital admissions and substantial healthcare expenditure. The use of morphine in the management of acute pulmonary oedema remains controversial, with conflicting guidance and significant variation in practice. Synthesised evidence is needed to inform standard treatment guidelines and clinical practice.
OBJECTIVE: To determine whether morphine should be used in the treatment of acute pulmonary oedema (APE) in adults.
METHODS: A rapid review of systematic reviews of randomised controlled trials or observational studies, and then randomised controlled trials, was conducted searching three electronic databases (PubMed, Embase, Cochrane Library) and one clinical trial registry on 12 February 2022. We used a prespecified protocol following Cochrane rapid review methods and aligned to the National Standard Treatment Guidelines and Essential Medicines List methodology. We first considered relevant high-quality systematic reviews of randomised controlled trials or observational studies, then (if required) randomised controlled trials to inform time-sensitive or urgent evidence requests, clinical practice, policy, or standard treatment guidelines.
RESULTS: We identified four systematic reviews of observational studies. The two most relevant, up-to-date, and highest-quality reviews were used to inform evidence for critical outcomes. Morphine may increase in-hospital mortality (odds ratio (OR) 1.78; 95% confidence interval (CI) 1.01 - 3.13; low certainty of evidence; six observational studies, n=151 735 participants), resulting in 15 more per 1 000 hospital deaths, ranging from 0 to 40 more hospital deaths. Morphine may result in a large increase in invasive mechanical ventilation (OR 2.72; 95% CI 1.09 - 6.80; low certainty of evidence; four observational studies, n=167 847 participants), resulting in 45 more per 1 000 ventilations, ranging from 2 more to 136 more. Adverse events and hospital length of stay were not measured across reviews or trials.
CONCLUSION: Based on the most recent, relevant and best-available quality evidence, morphine use in adults with APE may increase in-hospital and all-cause mortality and may result in a large increase in the need for invasive mechanical ventilation compared to not using morphine. Recommending against the use of morphine in pulmonary oedema may improve patient outcomes. Disinvesting in morphine for this indication may result in cost savings, noting the possible accrued benefits of fewer patients requiring invasive ventilation and management of morphine-related side-effects.
Heart failure is a significant public health challenge, with nearly 65 million people affected globally[13] It is a heterogenous clinical syndrome with a global prevalence estimated at 2% in the general adult population in high-income countries, with an increase of >10% in patients >70 years of age.[3-5] Population-based estimates in sub-Saharan Africa are lacking, but the combination of poorer outcomes and an anticipated higher prevalence results in a significant burden on the health system and substantial healthcare expenditure.[6] In contrast to high-income countries, where heart failure is mainly considered a disease of the older population, it affects younger people in sub-Saharan Africa, with predominantly non-ischaemic aetiologies.[5] Acute pulmonary oedema (APE) is a well-defined manifestation of acute decompensated heart failure, with an in-hospital mortality of 4 - 7% globally, increasing to 11 % 2-3 months post discharge.[7,8] It is a life-threatening emergency that progresses to cardiorespiratory collapse in minutes to hours, if not treated promptly[9]
The use of opioids in the management of APE is controversial, mainly because of their side-effect profile. The rationale behind their use is based on the potential beneficial effects on physiological parameters by reducing the preload and afterload, as well as on the nervous system by decreasing anxiety, dyspnoea and chest pain.[2,10,11] Variations on its haemodynamic effects have been reported, however, such as a paradoxical increase in the afterload secondary to catecholamine release, as well as evidence of coronary vasospasm associated with morphine administration.[12,13] The use of morphine may also result in serious side-effects, including hypotension (especially in those with existing volume depletion), a reduction in respiratory drive and nausea and vomiting.[12,13] Despite the theoretical benefits, lack of consensus and evidence on serious adverse events, it is still being used for APE, especially in patients with agitation and anxiety[14,15]
There is significant variation in practice with regard to clinical practice guidelines for APE. The American Heart Society and the American College of Cardiology advise against the routine administration of morphine, and only recommend morphine in patients receiving palliative care.[16] The use of morphine in terminally ill and end-of-life patients with symptoms of chronic heart failure is an accepted practice, and widely advocated.[16,17] The Acute Decompensated HEart Failure National REgistry (ADHERE) of > 175 000 patients in >250 hospitals across the USA reports that 14% of patients received morphine during their initial hospital visit.'151 The European Society of Cardiology advises against the routine use of morphine in APE, and recommends prescribing it with caution in those with severe dyspnoea in APE, due to the side-effect profile and the risk of mortality.[17,18] Guidelines in Australia recommend against the routine use of morphine in APE, but state that low doses of morphine may be used to facilitate the tolerance of non-invasive ventilation, as well as in patients with APE with associated ischaemic chest pain.[19] In South Africa (SA), the 2019 adult hospital level Standard Treatment Guidelines (STGs) and Essential Medicines List (EML) recommend morphine as standard practice for patients with APE with anxiety and severe dyspnoea.[15,20]
The SA National Essential Medicines List Committee (NEMLC) is a ministerially appointed, non-statutory advisory committee that is responsible for the development and maintenance of the National EML and the STGs.[21,22] An essential medicines list is defined by the World Health Organization as a list of medicines that satisfy the priority healthcare needs of a population, and includes medicines that people should have access to at all times and in sufficient amounts.[23] The process of conducting rapid reviews for the NEMLC has been previously described for COVID-19 therapeutic interventions.[24] The rapid review methodology has also been adopted for essential medicines list evidence reviews more generally in SA, supported by the SA Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Network.[25] Rapid reviews are a form of knowledge synthesis that accelerate the process of conducting a traditional systematic review through streamlining specific methods to produce evidence for stakeholders in a resource-efficient and timely manner.[26] The response to COVID-19 highlighted the need for timely evidence review to inform decision-making and advanced rapid review methods, specifically in response to urgent or emergency evidence requests from decisionmakers.[24] One rapid review method is to use a tiered approach whereby reviewers first consider high-quality, relevant and up-to-date clinical practice guidelines, then systematic reviews, randomised controlled trials and other designs if the review question is still not answered.[27] To settle the uncertainty and inform the adult hospital level STGs and EML for Emergency and Injuries, we conducted a rapid review to determine whether intravenous morphine should be used in the management of adults with APE.
Methods
We used a prespecified protocol following the Cochrane methodology and SA National EML Health Technology Assessment guidelines for rapid systematic reviews (SRs).[26] The methodology aims to balance rigour with speed, and reduce research waste and duplication of effort by first considering relevant high-quality SRs of randomised controlled trials (RCTs) or observational studies, then (if required) RCTs to inform time-sensitive or urgent evidence requests, clinical practice, policy or standard treatment guidelines.[28-30]
We searched for SRs of RCTs, then, if needed, RCTs or observational studies, comparing morphine with standard of care[20] (i.e. intravenous (IV) and sublingual nitrates, and IV and per os furosemide) in adult patients with APE. Prioritised clinically relevant patient important outcomes included mortality, adverse events ( AEs), serious adverse events (SAEs), intensive care unit (ICU) length of stay and hospital length of stay. We systematically searched three databases (Ovid MEDLINE, Embase and the Cochrane Library) and one trial registry for ongoing studies (Pan African Clinical Trial Registry). The search strategy was developed and conducted by an experienced information specialist with no language or publication restrictions on 12 February 2022 (appendix: https://www.samedical.org/file/2046).
Screening of title and abstracts, full-text screening, selection of studies and data extraction were conducted independently and in duplicate by two reviewers (IK and VN). Screening was done using the Covidence (Covidence, USA) software. AMSTAR-2 (A Measurement Tool to Assess systematic Reviews (AMSTAR, USA)) was used to appraise all the systematic reviews included by a single reviewer (VN) and checked by a second reviewer (IK). Any disagreements were resolved through discussion or in consultation with a third reviewer (MM orCH).
Where multiple eligible SRs were included, we reported evidence from the most relevant, recent and high-quality review or reviews in order to provide evidence across all a priori outcomes. If any eligible RCTs were not included by the SRs authors, these were included in the pooled synthesis if appropriate, and assessed using the Cochrane Risk of Bias 2.0 tool (Cochrane, UK) or ROBINS-I tool for non-randomised studies of interventions.[31] Where possible, only multivariate adjusted measures of effect were pooled from observational studies.[32] We conducted a GRADE assessment to establish the certainty of the evidence across each outcome, considering risk of bias, directness, consistency, precision and other considerations such as publication bias to determine whether the confidence in the overall results was high, moderate, low or very low.[33] Pooled effects across outcomes and certainty of evidence are reported in summary of findings tables using GRADEPro (McMaster University, Canada).
Results
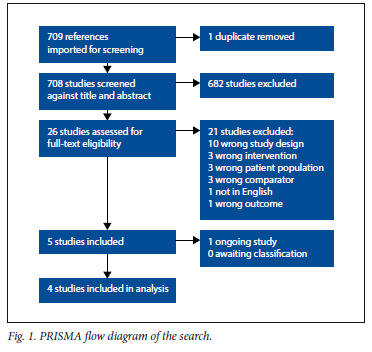
The search produced 709 records, and included 26 reports for full-text screening, and 4 SRs in the final review (Fig. 1). We found no SRs of RCTs or RCTs addressing this question. Of the four reviews, Gao et al. [7] and Zhang et al[34] were assessed to be of moderate quality (AMSTAR 2) and were considered most relevant and up to date. Relevant pooled outcomes from Gao and Zhang were re-GRADED (Table 1).
Description of included studies
The four included studies were SRs of observational studies, with three using meta-analyses to aggregate results. The effect estimates in the meta-analysis were adjusted.
Gao et al.[7] investigated the risk of mortality associated with opioid use in acute heart failure. They included six observational retrospective studies, with 151 735 participants in total. Treatment given to the control groups was not described. The authors report extracting adjusted measures of effect from primary studies for meta-analysis. However, they do not report which factors were adjusted. Gil et al[2] assessed morphine use in the treatment of acute cardiogenic pulmonary oedema. They included seven studies (one RCT, one non-randomised control trial and five observational studies), and 150 639 participants. Lin etal[14] studied intravenous morphine in heart failure and reviewed five studies (three propensity-matched cohorts and two retrospective analyses (one unpublished) with 14 9967 participants. Zhang et al[34] investigated the safety of morphine in patients with acute heart failure, and included seven retrospective case-control studies and 172 226 participants, including adjusted measures of effect similar to Gao et al.[7] The treatment given to control groups in included studies was not described (appendix: https://www.samedical.org/file/2059 shows tables of characteristics of included and excluded studies).
Internal validity of the systematic reviews, GRADE and absolute effects
In order to reduce duplication of efforts in synthesis, we used the most relevant, recent and highest-quality SRs (based on the PICO).
We prioritised reviews using GRADE to complement the downstream evidence to decision framework when developing recommendations for the NEMLC and STGs.[33] If a selected review did not report on all relevant outcomes, the next best review with relevant reported outcomes was used. Where needed, outcomes were re-GRADED accounting for differences in contextual/clinical interpretation such as indirectness and imprecision. Gao et al.[7] included one secondary analysis of a previously conducted RCT, which was excluded from our list of included studies to avoid double counting.
Gao et al.[7] and Zhang et al.[34] had the highest AMSTAR 2 scores overall (moderate quality review). However, Gao et al. was considered overall to be the most relevant, up-to-date and internally valid review. Gao et al. did not report their reasons for selecting the studies included in the review; neither did they report on the funding sources for each study included in the review, and hence the review was scored as moderate quality. The Lin et al.[14] and Gil et al. [2]reviews were of critically low quality.
Absolute effects were calculated from pooled-effect data where possible. In the absence of baseline event data (control event rates for pooled effects), absolute effects were calculated using the baseline events (where available) either from pooled control event data from included reviews or large prognostic observational studies for that outcome to determine baseline prevalence. This was done for mortality and SAEs. Refer to the GRADE summary of findings table reported in Table 1.
Effect of interventions
Mortality (in-hospital mortality and 30-day mortality)
Morphine may increase in-hospital mortality (odds ratio (OR) 1.78; 95% confidence interval (CI) 1.01 - 3.13; low certainty of evidence; 6 observational studies, ?i=151 735 participants), resulting in 15 more per 1 000, from 0 fewer to 40 more in-hospital deaths, and may increase 30-day mortality (Fig. 2[7,15,33-39]). Zhang et al[34] found no association between morphine and in-hospital mortality (OR 1.94; 95% CI 0.93 - 4.03; p=0.08)). However, the direction of effect is still in line with that of Gao et al.[7] Gao et al. did not report any baseline event rates for standard of care or intervention arms. Therefore, to calculate absolute effects we assumed a baseline control event rate of 2% for overall mortality based on Lin et al.[14]
Zhang et al.[34] found that morphine treatment was associated with an increased 7- and 30-day all-cause mortality (OR 1.59; 95% CI 1.16 - 2.17) from three studies (n=9 904; Fig. 3). [37-39] Gao et al.[7] reported a similar association between morphine use and 30-day mortality (OR 1.56; CI 1.14 - 2.15) from two studies (n=986, Fig. 2).[7,15,35-39]
Serious adverse events (need for invasive mechanical ventilation)
Morphine may result in a large increase in invasive mechanical ventilation (OR 2.72; 95% CI 1.09 - 6.80; low certainty of evidence, four observational studies; n=167 847 participants, Fig. 4),[15,35,39,40] resulting in 45 more per 1 000, ranging from 2 more to 136 more.[34] Baseline event rate not reported in reviews was calculated from estimates of mechanical ventilation baseline event rate and was based on that of Gray et al.[41]
Adverse events and ICU or hospital length of stay outcomes were not reported or measured in the included reviews.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Discussion
In this rapid systematic review, we found that administration of morphine in adult patients with APE was associated with an increase in the in-hospital and 30-day all-cause mortality rate. Considering a baseline mortality rate of 2% in adults with APE, 15 more patients per 1 000 population who received morphine died.[14] This signal of harm was consistent throughout the included reviews. [2,7,14,34] The review also found an increased risk of requiring invasive ventilation (45 more per 1 000 population) and the occurrence of serious adverse events when morphine is prescribed in patients with APE.
This review was conducted to inform NEMLC decision-making by providing GRADE evidence profiles for use in the GRADE evidence-to-decision framework.[33] Additionally, we anticipate that cost savings would be generated if the recommendation not to use morphine for pulmonary oedema is implemented in clinical practice, noting the possible accrued benefits of fewer patients requiring invasive ventilation and management of morphine-related side-effects.
The removal of morphine from the SA standard treatment guidelines may have implications for the current practice of clinicians in emergency centres, paramedics, intensivists and cardiologists. The ongoing use of morphine in APE despite the signal orfharm indicates either a lack of a suitable substitute, uncertainty with regard to the current evidence or a challenge with knowledge translation. [15] With regard to the former, patients with APE who are severely anxious/distressed or dyspnoeic, and those who require sedation to help facilitate the application of non-invasive ventilation, may require a therapeutic alternative. Alternatives to morphine for sedation in patients requiring non-invasive ventilation are being used, and several options have been assessed.[42,43] A RCT found that midazolam and dexmedetomidine are both effective and safe
agents to facilitatenon-invasive ventilation, although not assessed in patients with APE.[42] The Midazolam versus Morphine (MIMO) in APE trial,[44] the results of which were published after this search was conducted, compared midazolam v. morphine for patients with APE. This multicentre, open-label, blinded endpoint clinical trial randomised 111 patients from several Spanish emergency departments.[44] It found no difference of in-hospital mortality between the two groups, but the trial was stopped early after a planned interim analysis because of a significantly lower rate of serious adverse events in the midazolam arm (18.2% v. 42.9%; risk ratio 0.42; 95%; CI 0.22 - 0.8; p=0.007).[44] The early introduction of non-invasive ventilation and/or nitrate infusion in patients with severe APE with hypoxia and anxiety/distress may even mitigate the need for a sedative in the severely distressed and has been included in some international guidelines - this will, however, need to be explored further in the SA and low- and middle-income setting.[16-19] The removal of morphine also raises the debate as to whether the signal of harm extends to all opioids equally, as the synthetic opioids have a more favourable cardiovascular risk profile with less histamine release. Cardiovascular complications of opioids are complex, and vary between the natural and synthetic options. Effects on systemic vascular resistance and cardiovascular output are more common with the natural opioids, while effects on action potentials and impulse conduction have been reported with the synthetic options.'45,461 Further research should attempt to delineate these effects. For example, an assessment of the safety of morphine in acute coronary syndromes has also highlighted an association with an increased risk of in-hospital mortality and major cardiac adverse events by increasing platelet reactivation by decreasing the antiplatelet effect of P2Y12 inhibitors.[47] Evidence suggests that the effect on platelet aggregation could be due to a drug class effect instead of just morphine, as similar outcomes were found when morphine was substituted with fentanyl.[48]
This review provides no clear signal of any anticipated beneficial effect of morphine in APE, despite the theorised physiological benefits and the rationale behind its traditional inclusion in treatment guidelines.[2,7,14,34] The argument to consider its use in certain scenarios by assessing the risk/benefit ratio of individual patients therefore becomes difficult to sustain, as there is a clear signal of anticipated harmful effects.
Although the overall certainty of evidence across outcomes ranges from very low to low, indicating further evidence is likely to change our confidence in the treatment effect, future trials in this setting are unlikely to be acceptable or ethically possible due to signal of harm associated with morphine in APE. Future research is possible at evidence synthesis level, considering methods such as individual patient data meta-analysis and meta-regression to further analyse effect modification, dose response effects and plausible confounding to strengthen causal claims and confidence in treatment effects. Further primary research would unlikely provide estimates substantially different from the pooled synthesis that was conducted.
Conclusion
This rapid review of the use of intravenous morphine for the treatment of APE included four SRs of observational studies. This review focuses on adjusted pooled evidence from two high-quality relevant and recent reviews pooling more than 150 000 participants, with direction and magnitude of effects consistent across other included SRs. Based on this most recent, relevant and best-available quality reviews, morphine may increase in-hospital and all-cause mortality and may result in a large increase in the need for invasive mechanical ventilation compared with not using morphine. There is a paucity of data on whether morphine increases non-fatal adverse events, ICU or hospital length of stay. Morphine use in pulmonary oedema may result in an important net harm for patients, and disinvesting in morphine for this indication may result in significant cost savings.
Declaration. None.
Acknowledgements. This research was supported by Vittoria Lutje, information specialist for Cochrane Infectious Diseases Group, for supporting the searches and Rephaim Mpofu, who assisted with the costing analysis. We thank the members of the Primary Healthcare/Adult Hospital Level Expert Review Committee and the NEMLC, who provided insight and expertise that supported the research. We also thank members of the SA-GRADE Network for insights and support (Solange Durao, Tamara Kredo). However, the views expressed in this article are those of the authors and do not necessarily reflect the views or policies of Cochrane SA, the SA Medical Research Council or Stellenbosch University.
Author contributions. Study conception and design: CH, MM, GT; methodology: CH, MM, GT, TL, KC; analysis and interpretation of results: MM, VN, IK; writing (original draft): CH, MM, IK, VN; writing (review and editing): CH, MM, IK, VN, GT, TL, KC. All authors approved the final version of the manuscript.
Funding. MM, CH and IK are partly supported by the Research, Evidence and Development Initiative (READ-It). READ-It (project number 300342-104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK governments official policies. Research reported in this publication is the sole responsibility of the researchers and does not reflect the official views or position of the SA MRC or Stellenbosch University.
Conflicts of interest. None.
References
1. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020;22(8):1342-1356. https://doi.org/10.1002/ejhf.l858 [ Links ]
2. Gil V, Domínguez-Rodríguez A, Masip J, Peacock WF, Miro Ò. Morphine use in the treatment of acute cardiogenic pulmonary edema and its effects on patient outcome. A systematic review. Curr Heart Fail Rep 2019;16(4):81-88. https://doi.org/10.1007/s11897-019-00427-0 [ Links ]
3. Bleumink G, Knetsch A, Sturkenboom M, et al. Quantifying the heart failure epidemic Prevalence, incidence rate, lifetime risk and prognosis of heart failure. The Rotterdam Study Eur Heart J 2004;25(18):1614-1619. https://doi.org/10.1016/j.ehj.2004.06.038 [ Links ]
4. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93(9):1137-1146. https://doi.org/10.1136/hrt.2003.025270 [ Links ]
5. Kraus S, Ogunbanjo G, Sliwa K, Ntusi NAB. Heart failure in sub-Saharan Africa. A clinical approach. S Afr Med J 2015;106(1):23. https://doi.org/10.7196/samj.2016.v106i1.10325 [ Links ]
6. McMurray JJV, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33(14):1787-1847. superscript 2 [ Links ]
7. Gao D, David C, Rosa MM, Costa J, Pinto FJ, Caldeira D. The risk of mortality associated with opioid use in patients with acute heart failure. Systematic review and meta-analysis. J Cardiovasc Pharmacol 2021;77(2):123-129. https://doi.org/10.1097/fjc.0000000000000954 [ Links ]
8. Adams KF, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States. Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149(2):209-216. https://doi.org/10.1016/j.ahj.2004.08.005 [ Links ]
9. Masip J, Peacock WF, Price S, et al. Indications and practical approach to non-invasive ventilation in acute heart failure. Eur Heart J 2018;39(1):17-25. https://doi.org/10.1093/eurheartj/ehx580 [ Links ]
10. Sosnowski MA. Lack of effect of opiates in the treatment of acute cardiogenic pulmonary oedema. Emerg Med Australasia 2008;20(5):384-390. https://doi.org/10.1111/j.1742-6723.2008.01113.x [ Links ]
11. Allison RC. initial treatment of pulmonary edema. A physiological approach. Am J Med Sci 1991;302(6):385-391. https://doi.org/10.1097/00000441-199112000-00013 [ Links ]
12. Agewall S. Morphine in acute heartfailure. J Thorac Dis 2017;9(7):1851-1854. https://doi.org/10.21037/jtd.2017.06.129 [ Links ]
13. Vatner SF, Marsh JD, Swain JA. Effects of morphine on coronary and left ventricular dynamics in conscious dogs. J Clin invest 1975;55(2):207-217. https://doi.org/10.1172/JCI107923 [ Links ]
14. Lin Y, Chen Y, Yuan J, et al. Intravenous morphine use in acute heart failure increases adverse outcomes. A meta-analysis. Rev Cardiovasc Med 2021;22(3):865. https://doi.org/10.31083/j.rcm2203092 [ Links ]
15. Peacock WF, Hollander JE, Diercks DB, Lopatin M, Fonarow G, Emerman CL. Morphine and outcomes in acute decompensated heart failure. An ADHERE analysis. Emerg Med J 2008;25(4):205-209. https://doi.org/10.1136/emj.2007.050419 [ Links ]
16. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heartfailure. An update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016;134(13). https://doi.org/10.1161/cir.0000000000000435 [ Links ]
17. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37(27):2129-2200. https://doi.org/10.1093/eurheartj/ehwl28 [ Links ]
18. Hitzeroth J, Mpe M, Klug E, et al. 2020 Heart Failure Society of South Africa perspective on the 2016 European Society of Cardiology Chronic Heart Failure Guidelines. S Afr Med J 2020;110(9b):935-951. https://doi.org/10.7196/SAMJ.2020.v110i8.14681 [ Links ]
19. Purvey M, Allen G. Managing acute pulmonary oedema. Aust Prescr 2017;40(2):59-63. https://doi.org/10.18773/austprescr.2017.012 [ Links ]
20. National Department of Health, South Africa. Hospital Level (Adults) Standard Treatment Guidelines and Essential Medicines List. 2nd ed. https://knowledgehub.health.gov.za/elibrary/hospital-level-adults-standard-treatment-guidelines-and-essential-medicines-list-2nd (accessed 20 June 2023). [ Links ]
21. Gray A, Suleman F, Pharasi B. South Africa's national drug policy. 20 years and still going? S Afr Health Rev 2017;2017(1):49-58. https://doi.org/10.10520/EJC-c80c69129 [ Links ]
22. National Department of Health, South Africa. National Drug Policy for South Africa Pretoria. NDoH, 1996. https://www.gov.za/documents/national-drugs-policy (accessed 22 June 2023). [ Links ]
23. World Health Organization. Essential medicines, health product policy and standards, medicines selection, IP and affordability. WHO model list of essential medicines, Geneva. WHO, 2019. https://www.who.int/publications-detail-redirect/WHOMVPEMPIAU2019.06 (accessed 22 June 2023). [ Links ]
24. Leong TD, McGee SM, Gray AL, et al. Essential medicine selection during the COVID-19 pandemic Enabling access in uncharted territory. S Afr Med J 2020;110(11):1077. https://doi.org/10.7196/samj.2020.v110il1.15271 [ Links ]
25. South Africa GRADE Network The Centre for Evidence-based Health Care (CEBHC). https://www.cebhc.co.za/research-what-we-do/south-africa-grade-network/ (accessed 20 June 2023). [ Links ]
26. Garritty C, Gartlehner G, Nussbaumer-Streit B, et al. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol 2021;130:13-22. https://doi.org/10.1016/j.jclinepi.2020.10.007 [ Links ]
27. Cochrane. Collaborating in response to COVID-19. Editorial and methods initiatives across Cochrane. Cochrane Database Syst Rev 2020,12(Suppl 1):CD202002. https://doi.org/10.1002/14651858.CD202002 [ Links ]
28. Tri ceo AC, Garritty CM, Boulos L, et al. Rapid review methods more challenging during COVID-19. Commentary with a focus on 8 knowledge synthesis steps. J Clin Epidemiol 2020;126:177-183. https://doi.org/10.1016/j.jclinepi.2020.06.029 [ Links ]
29. Akl EA, Morgan RL, Rooney AA, et al. Developing trustworthy recommendations as part of an urgent response (1-2 weeks). A GRADE concept paper. J Clin Epidemiol 2021;129:1-11. https://doi.org/10.1016/j.jclinepi.2020.09.037 [ Links ]
30. McCaul M, Tovey D, Young T, et al. Resources supporting trustworthy, rapid and equitable evidence synthesis and guideline development. Results from the COVID-19 evidence network to support decision-making (COVID-END). J Clin Epidemiol 2022,151.88-95. https://doi.org/10.1016/j.jclinepi.2022.07.008 [ Links ]
31. Sterne J A, He man MA, Reeves BC, et al. ROBINS-I. A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355d4919. https://doi.org/10.1136/bmj.i4915 [ Links ]
32. Sterne JAC, Savovic J, Page MJ, et al. RoB 2. A revised tool for assessing risk of bias in randomised trials. BMJ 2019:14898. https://doi.org/10.1136/bmj.14898 [ Links ]
33. Guyatt GH, Oxman AD, Vist GE, et al. GRADE. An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-926. https://doi.org/10.1136/bmj.39489.470347.AD [ Links ]
34. Zhang D, Lai W, Liu X, Shen Y, Hong K. The safety of morphine in patients with acute heart failure. A systematic review and meta-analysis. Clin Cardiol 2021;44(9):1216-1224. https://doi.org/10.1002/clc.23691 [ Links ]
35. Caspi O, Naami R, Halfin E, Aronson D. Adverse do se-dep end ent effects of morphine therapy in acute heart failure. Int J Cardiol 2019;293:131-136. https://doi.org/10.1016/j.ijcard.2019.06.015 [ Links ]
36. D ominguez-Rodriguez A, Avanzas R Burilio-Putze G, A breu-Gonzalez P. Influence of morphine treatment on in-hospital mortality among patients with acute heart failure. Med Intensiva 2017;41(6):382-384. https://doi.org/10.1016/j.medin.2016.05.007 [ Links ]
37. Gray A, Goodacre S, Seah M, Tilley S. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema. Analysis from the 3CPO trial QJM 2010;103(8):573-581. https://doi.org/10.1093/q|med/hcq077 [ Links ]
38. Iakobishvili Z, Cohen E, Garty M, et al. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care 2011;13(2):76-80. https://doi.org/10.3109/17482941.2011.575165 [ Links ]
39. Miro Ò, Gil V, Martin-Sanchez FJ, et al. Morphine use in the ED and outcomes of patients with acute heart failure. A propensity s core-matching analysis based on the EAHFE registry. Chest 2017;152(4):321-832. https://doi.org/10.1016/j.chest.2017.03.037 [ Links ]
40. Sacchetti A, Ramoska E, Moakes ME, McDermott R Moyer V. Effect of ED management on ICU use in acute pulmonary edema. Am J Emerg Med 1999;17(6):571-574. https://doi.org/10.1016/s0735-6757(99)90198-5 [ Links ]
41. Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J. Noninvasive ventilation in acute cardiogenic pulmonary edema. New Engl J Med 200810;359(2):142-151. https://doi.org/10.1056/nejmoa0707992 [ Links ]
42. Senoglu N, Oksuz H, Dogan Z, Yildiz H, Demirkiran H, Ekerbicer H. Sedation during noninvasive mechanical ventilation with dexmedetomidine or midazolam. A randomized, double-blind, prospective study. Curr Ther Res Clin Exp 2010;71(3):141-153. https://doi.org/10.1016/j.curtheres.2010.06.003 [ Links ]
43. Longrois D, Conti G, Mantz J, Faltlhauser A, Aantaa R, Tonner P. Sedation in non-invasive ventilation. Do we know what to do (and why)? Multidiscip Respir Med 2014;9(1):56. https://doi.org/10.1186/2049-6958-9-56 [ Links ]
44. D ominguez-Rodriguez A, Suero-Mendez C, Burillo-Putze G, et al. Midazolam versus morphine in acute cardiogenic pulmonary oedema. Results of a multicentre, open-label, randomized controlled trial. Eur J Heart Failure 2022;24(10):1953-1962. https://doi.org/10.1002/ejhf.2602 [ Links ]
45. Krantz MJ, Palmer RB, Haigney MCR Cardiovascular complications of opioid use. J Am Coll Cardiol 2021;77(2):205-223. https://doi.org/10.1016/j.jacc2020.11.002 [ Links ]
46. Behzadi M, Joukar S, Beik A. Opioids and cardiac arrhythmia. A literature review. Med Princ Pract 2018;27(5):401-414. https://doi.org/10.1159/000492616 [ Links ]
47. Molina PE. Opioids and opiates. Analgesia with cardiovascular, haemodynamic and immune implications in critical illness. J Int Med 2006;259(2):138-154. https://doi.org/10.1111/j.l365-2796.2005.01569.x [ Links ]
48. Duarte GS, Nunes-Ferrei ra A, Rodrigues FB, et al. Morphine in acute coronary syndrome. Systematic review and meta-analysis. BMJ Open 2019;9(3):e025232. https://doi.org/10.1136/bmjopen-2018-025232 [ Links ]
 Correspondence:
Correspondence:
M McCaul
mmccaul@sun.ac.za
Accepted 20 April 2023














