Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.7 Pretoria jul. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i7.560
RESEARCH
Profile of human papillomavirus genotypes in breast and oesophageal cancer patients in Pretoria, South Africa
N MarogaI; T MokoenaII; N LedibaneIII; A MusekiwaIV; M BidaV; M KgomoVI; R LebeloVII
IMSc; Department of Surgery, School of Medicine, Faculty of Health Sciences, University of Pretoria and Steve Biko Academic Hospital, Pretoria, South Africa
IIPRCS, DPhil; Department of Surgery, School of Medicine, Faculty of Health Sciences, University of Pretoria and Steve Biko Academic Hospital, Pretoria, South Africa
IIIMB ChB, MPH; School of Health Systems and Public Health, Faculty of Health Sciences, University of Pretoria, South Africa
IVPhD; School of Health Systems and Public Health, Faculty of Health Sciences, University of Pretoria, South Africa
VMSc, MMed (Path); Department of Anatomical Pathology, School of Medicine, Faculty of Health Sciences, University of Pretoria and National Health Laboratory Service, Pretoria, South Africa
VIFCP (SA), PhD; Gastroenterology Unit, Department of Internal Medicine, School of Medicine, Faculty of Health Sciences, University of Pretoria and Steve Biko Academic Hospital, Pretoria, South Africa
VIIPhD; Department of Virology, National Health Laboratory Service, Sefako Makgatho Health Sciences University, Pretoria, South Africa
ABSTRACT
BACKGROUND: The association between human papillomavirus (HPV) and cervical cancer is well established, and cervical cancer can be prevented through HPV vaccination. Little has been reported on the association between HPV and breast carcinoma (BC) or oesophageal squamous cell carcinoma (OSCC) in Africa. It is possible that use of appropriate HPV vaccines against genotypes responsible for these cancers may also prevent their development
OBJECTIVES: To investigate HPV genotype prevalence in BC and OSCC patients in Pretoria, South Africa (SA
METHODS: A retrospective cross-sectional study of BC and OSCC patients managed at Steve Biko Academic Hospital from 2015 to 2019 was undertaken. Patient medical records were analysed, and DNA was extracted from their archived pathology material and amplified by polymerase chain reaction before hybridisation for HPV genotypes
RESULTS: There were 101 patients with BC and 50 with OSCC. The prevalence of HPV infection in BC patients was 77.2%, with 35.6% high-risk (HR) genotypes, and that in OSCC patients 90.0%, with 56.0% HR genotypes. The most prevalent HPV genotypes (>20% each) were HPV 16, 70 and 51 for BC and HPV 51, 70, 16 and 82 for OSCC, with 31.7% and 60.0% of patients, respectively, having co-infection with > 2 genotypes
CONCLUSION: The high prevalence of infection with multiple HPV genotypes in BC and OSCC patients, with HPV 16, 51, 70, 35 and 82 the most common genotypes in these cancers, warrants expansion of the current SA bivalent HPV 16/18 vaccine for girls to include boys, and inclusion of HPV 51, 70, 35 and 82, in order to prevent BC and OSCC as well as cervical cancer
According to the World Health Organization, global estimates of the burden of cancer reached 18.1 million new cases and 9.1 million deaths in 2018.[1] A meta-analysis of the global burden of disease for the period 1990 - 2017 indicated that the burden of cancer quantified as disability-adjusted life-years had risen from sixth place in 1990 to second in 2017[2]
Breast carcinoma (BC) is the most prevalent cancer among women worldwide.[1] The association of human papillomavirus (HPV) with cervical and other genital squamous cell carcinomas is well established.[3,4] A number of studies from around the world have identified the presence of HPV infection in patients with BC,[57] although a few failed to demonstrate this association in some regions.[8-10]
Oesophageal squamous cell carcinoma (OSCC) is a significant public health problem in many countries, including South Africa (SA). Persistent HPV infection has been implicated in the causation of OSCC.[11,12] HPV infection has been demonstrated in patients with OSCC in many geographical areas, especially those with a high OSCC prevalence.[13-18]
HPV oncogene products can inhibit tumour suppressor genes such as p53[19,20] and retinoblastoma protein (pRb),[21,22] inhibit antigen presentation and effective local immune response,[19,23] and induce carcinogenic host cell somatic mutations and genomic instability[24]
These changes have also been observed in BC.[25]
There are no scientific reports on the association between HPV infection and BC in sub-Saharan Africa or SA, and there is a paucity of reports on the association of HPV with OSCC in the region.[26,27] A cross-sectional study was therefore undertaken to investigate the presence and genotypes of HPV infections in patients with BC or OSCC, the leading causes of cancer deaths among women and men, respectively, in southern Africa.[28] HPV infection affects >60% of young women in SA.[29]
It is hoped that the demonstration of HPV infection in patients with these cancers, especially BC, in SA would motivate for expansion of HPV vaccination, which is currently limited to girls for the prevention of cervical cancer, [30] to include prevention of BC and OSCC and therefore to be extended to boys as well.
Methods
Study population and data collection
A retrospective cross-sectional analysis of patients with confirmed BC or OSCC treated at Steve Biko Academic Hospital, Pretoria, SA from 2015 to 2019 was conducted. Medical records were accessed and patient demographic details were recorded. The patients' archived formalin-fixed paraffin-embedded (FFPE) pathology blocks were retrieved from the Department of Anatomical Pathology, National Health Laboratory Service (NHLS), for DNA extraction and amplification and HPV genotyping. Patients who had undergone prior neoadjuvant chemotherapy or radiotherapy were excluded as were patients whose medical records were incomplete and those whose archived pathology samples were inadequate.
Tissue preparation
All selected pathology blocks were histologically re-examined to confirm the pathological diagnosis. FFPE tissue blocks were first placed on ice for 1 hour to harden the wax to facilitate sectioning. Three 20 μm sections were trimmed from the blocks before the test sections were cut. Ten sections of 8 μm each were cut from the selected blocks using a Leica 2245 microtome (Leica Biosystems, Germany) and placed in sterile 2 ml Eppendorf tubes (Merck KGa, Germany) using disposable toothpicks. The microtome and blades were cleaned with xylene followed by 70% ethanol after cutting each block, and a fresh toothpick was used for each sample. The tubes were labelled and stored at room temperature until DNA extraction.
DNA extraction
DNA extraction was performed according to the QIAamp DNA Mini and Blood Mini Handbook (Qiagen, Germany).[31] This involved cell lysis using physicochemical methods. Lipids were removed by the addition of detergent (Qiagen, USA), followed by the removal of proteins with Proteinase Κ solution (Qiagen, USA). RNA was removed with RNase (Qiagen, USA), and the remaining DNA was quantified.
Polymerase chain reaction
HPV genotyping was performed using the Roche Linear Array HPV genotyping test (Roche Diagnostics, Germany), according to the manufacturer's recommendation. This method uses biotinylated primers to define a sequence of nucleotides within the polymorphic L1 region of the HPV genome that is approximately 450 base pairs long (supplementary file, available online at https://www.samedical.org/file/2040).
Ethical considerations
Study patients' information was kept confidential, and their identity was protected by assigning each with a unique study number.
The study was approved by the Faculty of Health Sciences Research Ethics Committee, University of Pretoria (ref. no. 343/2020). The study was conducted according to the Helsinki Declaration ethical principles for research on human subjects.
The hospital and NHLS management, as custodians of patient records and materials, gave permission to access patients' medical records and their archived pathological material, respectively, and to publish the research findings.
Statistical analysis
Both descriptive and inferential statistics were used. To determine the prevalence of HPV infection in patients with histologically confirmed BC or OSCC, the number of cases with HPV infection was divided by the total number tested and expressed as a percentage. Descriptive statistics were used to describe demographic characteristics and HPV genotypes. Numerical characteristics such as age in years were summarised using means, standard deviations (SDs) and ranges. Categorical characteristics such as genotypes were tabulated, showing counts and percentages for each category. Bar charts were used to visualise categorical data. For inferential statistics, Pearson's χ2 test was used to compare high-risk (HR) HPV infection between BC and OSCC patients. All statistical analyses were performed using Stata version 15 (StataCorp, USA) software, and a p-value <0.05 was considered statistically significant.
Results
One hundred and fifty-one patients (101 BC and 50 OSCC) had complete medical records and pathological samples for analysis. All 101 BC patients were female, with a mean (SD) age of 56.2 (15.7) years (range 19 - 96). Seventy-eight BC patients (77.2%) tested positive for HPV infection (Table 1). The highest HPV infection rate was in the middle age range (50 - 65 years).
There were 26 male and 16 female OSCC patients (sex was not stated in 8 patient records and could not be deduced from the patients' names), with a mean (SD) age of 51.9 (25.7) years (range 18 - 88). Forty-five OSCC patients (90.0%) tested positive for HPV infection (Table 1). The highest HPV infection rate was also in the middle age range (50 - 65 years).
HPV genotypes in patients with BC
Fig. 1 shows the number of BC patients with HR-HPV or low-risk (LR) HPV genotypes. It was noted that the most common HPV genotypes were HPV 16 and HPV 70 at -37% each, followed by HPV 51 at 23%, with HPV 35 and HPV 82 trailing at 16% each.
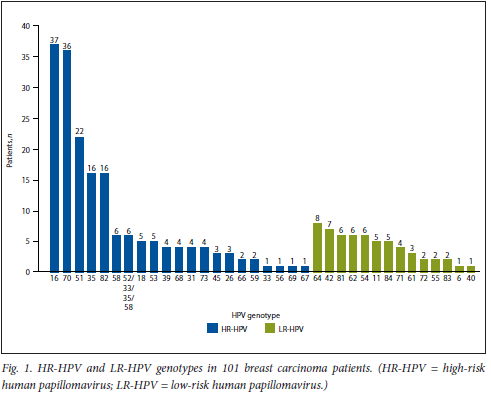
HPV genotypes in patients with OSCC
Fig. 2 shows the number of OSCC patients with HR-HPV or LR-HPV genotypes. The most common genotypes were HPV 51 (62.0%), HPV 70 (48.0%), HPV 16 (44.0%) and HPV 82 (34.0%).
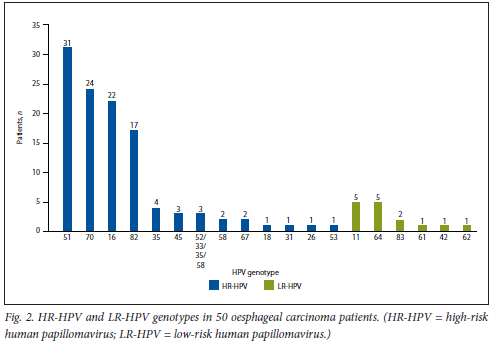
Overall, 35 genotypes were identified, of which 21 were HR and 14 LR.
Comparison of HR-HPV infection between BC and OSCC patients
A comparison between BC and OSCC patients in respect of selected HPV genotypes is shown in Table 1. It was noted that OSCC patients had a higher prevalence of HPV infection overall (90.0% v. 77.2%; p=0.057) and had significantly more HR-HPV genotypes (56.0% v. 35.6%; p<0.017). The profile of HR-HPV genotypes was similar but not identical in the two cancer types. While HPV genotypes 16, 70 and 51 were the most prevalent in both, OSCC patients had a higher proportion of HPV 51 than BC patients (62.0% v. 21.8%; p<0.001). Interestingly, HPV 82 was significantly more prevalent in OSCC patients than in BC patients (34.0% v. 15.8%; p=0.011). In contrast, BC patients had a higher proportion of HPV 35 (15.8% v. 8.0%; p=0.181). It was noted that the prevalence of HPV 18 was relatively low in both cancer types (5.0% in BC and 2.0% in OSCC).
Multiple HR-HPV co-infection in BC and OSCC patients
A number of patients in both cancer groups were co-infected with different HR-HPV genotypes (Tables 2 and 3). It was noted that 60.0% of OSCC patients had >2 HR-HPV genotypes (Table 2), compared with 31.7% of BC patients (Table 3). However, these differences were not statistically significant. Interestingly, 19 OSCC patients (38.0%) had co-infection with 3 or 4 HR-HPVs, namely 16, 51, 70 or 82, compared with only 14 BC patients (13.9%).
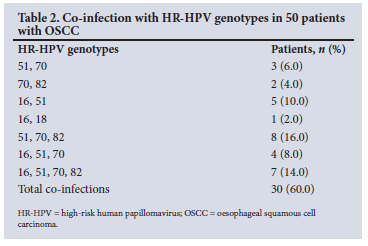
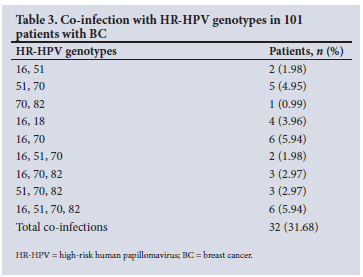
A qualitative comparison of the degree of HPV co-infection between BC and OSCC patients is shown in Table 4. The median number of HPV co-infections in OSCC was 3 genotypes, compared with 2 in BC.
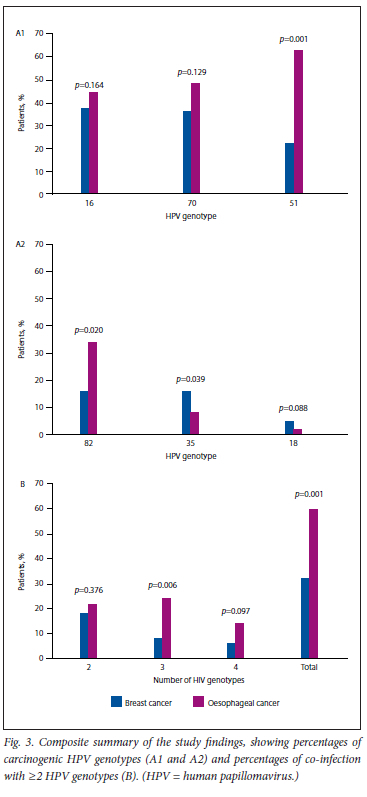
Fig. 3 shows a composite summary of the key findings of the study.
Discussion
The study identified 35 HPV genotypes in BC and OSCC patients, and the majority (62.5%) were HR-HPV genotypes. The prevalence of HR-HPV in BC was 64.0%, compared with 64% in India,[32] 61% in Syria,[33] 48.6% in Iran[34] 42% in Venezuela[35] and 42% in the UK,[36] with relatively high prevalence rates, and 25% in Morocco,[37] 21% in Japan,[38] 17.5% in Korea,[39] 17.3% in China[22] and 5.8% in Australia,[40]' with relatively low rates. The most common HPV genotypes were 16, 70, 51, 35 and 82, where 16 and 70 accounted for the majority (72.2%). The 36.6% HPV 16 prevalence rate is similar to 35% reported in Mexico[41] and 33% in Argentina,[42] but contrasts with 19.3% in Japan[38] and 69% in India,[32] which were much lower and higher, respectively. The low 5% HPV 18 prevalence contrasts with a high prevalence in Australian BC patients (55%).[40] Interestingly, the high HPV 51 prevalence in our BC patients (21.8%) was also noted in Venezuela (30.8%)[35] and Korea (63.6%).[39] HPV 70, which was as prevalent as HPV 16 (35.6% and 36.6%, respectively) in this study, has not been prominent in BC studies performed elsewhere. It was noted that HPV 70 was also very prominent in OSCC patients in the present study, as was HPV 16, which probably reflects a regional population HPV infection pattern.
Many BC patients (31.7%) were infected by >2 HR-HPV genotypes, with 13.9% having 3 or 4 co-infections. A high prevalence of multiple HR-HPV genotype infections has been reported in SA women with cervical cancer, but the predominant HPV genotype profile of HPV 16, 56, 18 and 39 was different from the profile found in BC in the present study[43] This contrasts with concordant HPV infection profiles in Australian BC patients with prior cervical cancer.[44]
There was a very high prevalence of HR-HPV (70.0%) in OSCC patients. This is in contrast to the generally much lower HPV infection rates reported in many other geographical areas, especially in the West.[12,13] This high HPV prevalence rate may partly account for the endemically high rate of OSCC in SA. High HPV prevalence has also been reported in other high OSCC prevalence countries such as China[15] and Iran.[16]
The 60.0% rate of co-infection with multiple HR-HPV genotypes in OSSC patients in this study was much higher than that in BC women (31.7%), but their HPV genotype profiles were qualitatively similar although quantitatively different. This finding may reflect the HPV genotype infection pattern in the SA population, since a similar HPV profile was reported in asymptomatic young women in Cape Town and Johannesburg.[29] In the present study, HPV 51 and 70 accounted for the majority of genotypes in OSSC (62.0% and 48.0%, respectively), while HPV 16 and 70 were most common in BC (36.6% and 35.6%, respectively). It was further noted that the prevalence of HPV 82 was proportionately double in OSSC compared with BC (34.0% v. 15.8%, respectively). Interestingly, a case control study from Johannesburg analysed serum anti-HPV-16 antibody levels in a cohort of patients with different cancers including OSCC and BC.[45] The BC patients' results were pooled together with those of other cancers that were not thought to be associated with HPV infection as a putative 'negative' case comparison. The study found a significantly high rate of anti-HPV-16 seropositivity in OSCC patients, but not in the putative 'negative' case control patients, which included BC patients. The BC patients constituted 50% of the 'negative control' cancers. It would have been salutary to have analysed the BC patients' results separately to see whether the findings would still indicate a non-significant anti-HPV-16 seropositivity.
The profile of HR-HPV in OSSC differs significantly from that previously reported in SA, where there was predominance of HPV 11 (an LR-HPV genotype), HPV 16 and HPV 39.[26,27]It also contrasts with that reported for Chinese and US patients, who predominantly had HPV 16, 57 and 26,[46] and with Japanese[47] and Iranian[16] patients, who predominantly had HPV 16 and 18.
Co-infection with multiple HPV genotypes in BC and OSCC has been reported previously in other studies from various countries and regions.[26,27,35,38] Such co-infections have been shown to be associated with more advanced or high-grade squamous intraepithelial neoplasia in the uterine cervix.[48] Multiple HR-HPV infections probably act synergistically to induce malignant transformation of target tissue cells, since their mechanism or intensity of action on the cell cycle may differ; for example, HPV 16 E6 inhibits p53 tumour suppressor gene protein[19,20] and HPV 18 E7 targets pRb[21,22] in the cell cycle checkpoints. The transforming effect of HPV is enhanced or facilitated by the growth effect of oestrogen on its target breast and reproductive tissues.[20] It has been reported that patients who develop cervical and breast cancers associated with HPV are younger than those with cancers that are not, and their cancers are more aggressive, probably as a result of synergy between HPV and oestrogen.[44]
Although HPV infection has most frequently been associated with squamous cell carcinoma of the uterine cervix and the oesophagus, it has also been demonstrated in adenocarcinoma of the uterine cervix[49] and of the oesophagus, including the premalignant Barrett's dysplasia of the oesophagus.[12,17,50] It is therefore not surprising that HPV infection is also associated with adenocarcinoma of the breast.
Despite the fact that oncogenesis is a multistep process that involves a variety of risk factors, such as oestrogen for BC[20] and lifestyle factors, such as diet and smoking, in addition to oncogenic viral infections working sequentially in tandem or synergistically contemporaneously, HPV-induced cancers are preventable by mass HPV vaccination.[51] A national HPV vaccination campaign was introduced in SA in 2014 to provide free vaccination to all girls aged >9 years with a bivalent HPV 16/18 vaccine aimed at cervical cancer prevention.[30] The finding of multiple HPV co-infections in patients with breast and oesophageal cancers in the present study highlights the need to broaden the scope of HPV vaccine to include both girls and boys. A tailor-made polyvalent vaccine targeted at this population should therefore be designed to include HPV 70, 51, 82 and 35 genotypes in addition to HPV 16 and 18 in a nanovalent HPV vaccine,[52] or at the very least a pentavalent HPV vaccine incorporating HPV 16, 51, 70, 82 and 35, for it to be completely effective in the prevention of breast or oesophageal cancer in our region. The paucity of HPV 18 in both BC (5.0%) and OSCC (2.0%) in the present study is noteworthy and diminishes its relevance and importance in the design of vaccines specifically aimed at these cancer types in the region.
The limitation of the current study is that it examined HPV genotype profiles at a single academic institution, and its findings may therefore need to be confirmed in other parts of the country. However, while previous HPV genotyping in SA women with cervical squamous cell carcinoma showed a quantitatively and qualitatively different HPV genotype profile,[43] a recent study of HPV prevalence among sexually active but asymptomatic young women in Cape Town and Johannesburg found similar HPV genotype profiles to the ones demonstrated in the current study, indicating their widespread distribution in the country. [29] These authors also advocate for a vaccine with expanded HPV genotypes. The research on HPV genotype infection profiles in cancer patients, especially BC, needs to be confirmed in other southern African regions to demonstrate the generalisability of our findings.
Conclusion
The present study has demonstrated similar and novel HPV genotype infection profiles in SA BC and OSCC patients. In addition to HPV 16, described for cancer of the uterine cervix, the study identified HPV 51, 70, 82 and 35 as other prevalent HR-HPV genotypes in this patient population. This finding calls for the expansion of the current bivalent HPV 16/18 vaccination for girls to include boys, and the addition of HPV 51, 70, 35 and 82 genotypes as well in a vaccine for SA.
Declaration. The research for this study was done in partial fulfilment of the requirements for NM's MSc (Epidemiology) degree at the University of Pretoria.
Acknowledgements. None.
Author contributions. Conception and design: NM, TM, MK; molecular biology experiment/processing: NM, RL; histological confirmation: MB; statistical analysis: AM, NL; drafting of the manuscript: NM, TM. All authors read and approved the final draft.
Funding. The research was funded from internal resources of the departments of Surgery and Anatomical Pathology.
Conflicts of interest. None.
References
1. Bray F, Ferlay J, Soerjomataram I, Seigel RL, Torre LA, Jemal A. Global cancer statistics 2018. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394-424. https://doi.org/10.3322/caac21492 [ Links ]
2. Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017. A systematic analysis for the global burden of disease study JAMA Oncol 2019;5(12):1749-1768. https://doi.org/10.1001/jamaoncol.2019.2996 [ Links ]
3. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev 2003;16(1):1-17. https://doi.org/10.1128/CMR16.1.1-17.2003 [ Links ]
4. Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30 Suppl 5:F12-F23. https://doi.org/10.1016/j.vaccine.2012.07.055 [ Links ]
5. Li N, Bi X, Zhang Y, Zhao P, Zheng T, Dai N. Human papillomavirus infection and sporadic breast carcinoma risk. A meta-analysis. Breast Cancer Res Treat 2011;126(2):515-520. https://doi.org/10.1007/sl0549-010-1128-0 [ Links ]
6. Bae J-M, Kim EH. Human papillomavirus infection and risk of breast cancer. A meta-analysis of case-control studies. Infect Agent Cancer 2016;11:14. https://doi.org/1186/sl3027-016-0058-9 [ Links ]
7. Kan C-Y, Iacopetta BJ, Lawson JS, Whitaker NJ. Identification of human papillomavirus DNA gene sequences in human breast cancer. Br J Cancer 2005;93(8):946-948. https://doi.org/10.1038/sj.bjc.6602778 [ Links ]
8. Wrede D, Luqmani YA, Coombes RC, Vousden KH. Absence of HPV 16 and 18 DNA in breast cancer. Br J Cancer 1992;65(6):891-894. https://doi.org/10.1038/bjc.l992.186 [ Links ]
9. Zhou Y, Li J, Ji Y, et al. Inconclusive role of human papillomavirus infection in breast cancer. Infect Agent Cancer 2015;10:36. https://doi.org/10.1186/sl3027-015-0029-6 [ Links ]
10. Bonlokke S, Blaakaer J, Steiniche T, et al. Evidence of no association between human papillomavirus and breast cancer. Front Oncol 2018;8:209. https://doi.org/10.3389/fonc.2018.00209 [ Links ]
11. Livanage SS, Rahman B, Ridda I, et al. The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma. A meta-analysis. PLoS ONE 2013;8(7):e69238. https://doi.org/10.1371/journal.pone.0069238 [ Links ]
12. Rajendra S, Pavey D, McKay O, Merrett N, Gautam SD. Human papillomavirus infection in esophageal squamous cell carcinoma and esophageal adenocarcinoma. A concise review. Ann N Y Acad Sci 2020:1482(1).36-48. https://doi.org/10.1111/nyas.l4509 [ Links ]
13. Sur M, Cooper K. The role of the human papillomavirus in esophageal cancer. Pathology 1998;30(4):348-354. https://doi.org/10.1080/00313029800169616 [ Links ]
14. Petrik JL, Wyss AB, Butler AM, et al. Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases. Systematic review and meta-analysis. Br J Cancer 2014;110(9):2369-2377. https://doi.org/10.1038/bjc.2014.96 [ Links ]
15. Zhang S-K, Guo L-W, Chen Q, et al. The association between human papillomavirus 16 and esophageal cancer in Chinese population. A meta-analysis. BMC Cancer 2015;15:99. https://doi.org/10.1186/S12885-015-1096-1 [ Links ]
16. Mohammadpour B, Rouhi S, Khodabandehloo M, Moradi M. Prevalence and association of human papillomavirus with esophageal squamous cell carcinoma in Iran. A systematic review and metaanalysis. Iran J Public Health 2019;48(7):1215-1226. [ Links ]
17. Li X, Gao C, Yang Y, et al. Systematic review with meta-analysis. The association between human papillomavirus infection and oesophageal cancer. Aliment Pharmacol Ther 2014;39(3):270-281. https://doi.org/10.1111/apt.12574 [ Links ]
18. Alsop BR, Sharma P. Esophageal cancer. Gastroenterol Clin North Am 2016;45(3):399-415. https://doi.org/10.1016/j.gtc.04.001 [ Links ]
19. Feller L, Wood NH, Khammissa RAG, Lemmer J. Human papillomavirus-mediated carcinogenes and HPV-associated oral and oropharyngeal squamous cell carcinoma. Part 1. Human papiliomavirus-mediated carcinogenesis. Head Face Med 2010;6:.14. https://doi.org/10.1186/1746-160X-6-14 [ Links ]
20. Shai A, Pitot HC, Lambert PF. p53 Loss synergizes with estrogen and papillomaviral oncogenes to induce cervical and breast cancer. Cancer Res 2008;68(8):2622-2631. https://doi.org/10.1158/0008-5472.CAN-07-5266 [ Links ]
21. Ameliem O, Sandvik JA, Stokke T, Pettersen EO. The retinoblastoma protein-ass ο dated cell cycle arrest in S-phase under moderate hypoxia is disrupted in cells expressing HPV18 E7 oncoprotein. Br J Cancer 1998;77(6):862-872. https://doi.org/10.1038/bjc.l998.143 [ Links ]
22. Wang Y-W, Zhang K, Zhao S, et al. HPV status and its correlation with BCL2, p21, p53, Rb, and survivin expression in breast cancer in a Chinese population. Biomed Res Int 2017;2017:6315392. https://doi.org/10.1155/2017/6315392 [ Links ]
23. Zhou C, Tuong ZK, Frazer IH. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front Oncol 2019;9:682. https://doi.org/10.3389/fonc.2019.00682 [ Links ]
24. Litwin TR, Clarke MA, Dean M, Wentzensen N. Somatic host cell alterations in HPV carcinogenesis. Viruses 2017;(8):206. https://doi.org/10.3390/v9080206 [ Links ]
25. Ohba K, Ichiyama K, Yajima M, et al. In vivo and in vitro studies suggest a possible involvement of HPV infection in early stage of breast carcinogenesis via APOBEC3B induction. PLoS ONE 2014;9(5):e97787. https://doi.org/10.1371/journai.pone.0097787 [ Links ]
26. Cooper K, Taylor L, Govind S. Human papillomavirus DNA in oesophageal carcinomas in South Africa. J Pathol 1995;175(3):273-277. https://doi.org/10.1002/path.l711750304 [ Links ]
27. Matsha T, Erasmus R, Kafuko AB, Mugwanya D, Stepien A, Parker MI. Human papillomavirus associated with oesophageal cancer. J Clin Pathol 2002;55(8):587-590. https://doi.org/10.1136/jcp.55.8.587 [ Links ]
28. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;719(3):209-249. https://doi.org/10.3322/caac21660 [ Links ]
29. Mbulawa ZZA, van Schalkwyk C, Hu N-C, et al. High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. PLoS ONE 2018;13(l):e0190166. https://doi.org/10.1371/journai.pone.0190166 [ Links ]
30. Delany-Moretlwe S, Kelley KF, James S, et al. Human papillomavirus vaccine introduction in South Africa. Implementation lessons from an evaluation of the national school-based vaccination campaign. Glob Health Sci Pract 2018;6(3):425-438. https://doi.org/10.9745/GHSP-D-18-00090 [ Links ]
31. Qiagen QIAamp DNA Mini and Blood Mini Handbook Fifth Edition, May 2016. https://www.bing.com/ck/a?!&&p=ldbc8bl7e3e72bddJmltdHM9MTY4NjUyODAwMCZpZ3VpZD0xMjljOTA3OClhMzkwLTYyNDktMTNlMC04MzU2YTJhMzYzY2UmaW5zaWQ9NTE4Mw&ptn=3&hsh=3&fclid=129c9078-a390-6249-13e0-8356a2a363ce&psq=Qiagen+QIAamp+DNA+Mini+and+Blood+Mini+Handbook+Fifth+Ediüon%2c+May+2016.&u=alaHR0cHM6Ly93d3cucWlhZ2VuLmNvbS91cy9y-XNvdXJjZXMvZG93bmxvYWQuYXNweD9pZD02MmEyMDBkNilmYWY0LTQ2OWItYjUwZi0yYjU5Y2Y3Mzg5NjImbGFuZzllbg&ntb=1 (accessed 15 June 2023). [ Links ]
32. Islam S, Dasgupta H, Roychowdhury A, et al. Study of association and molecular analysis of human papillomavirus in breast cancer of Indian patients. Clinical and prognostic implication. PLoS ONE 2017;12(2):e0172760. http://doi.org/10.1371/journal.pone.0172760 [ Links ]
33. Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa A-E. High-risk human papillomavirus in breast cancer in Syrian women and their association with Id-1 expression. A tissue microarray study. Br J Cancer 2008;99(3):404-407. https://doi.org/10.1038/sj.bjc.6604503 [ Links ]
34. Khodabandehlou N, Mostafaei S, Etemadi A, et al. Human papilloma virus and breast cancer. The role of inflammation and viral expressed proteins. BMC Cancer 2019;19(1):61. https://doi.org/10.1186/sl2885-019-5286-0 [ Links ]
35. Fernandes A, Bianchi G, Feltri AP, Pérez M, Correnti M. Presence of human papillomavirus in breast cancer and its association with prognostic factors. Ecancermedicalscience 2015;9:548. https://doi.org/10.3332/ecancer.2015.548 [ Links ]
36. Salman NA, Davies G, Majidy F, et al. Association of high risk human papillomavirus and breast cancer. A UK based study. Sei Rep 2017;7:43591. https://doi.org/10.1038/srep43591 [ Links ]
37. ElAmrani A, Gheit T, Benhessou M, et al. Prevalence of mucosal and cutaneous human papillomavirus in Moroccan breast cancer. Papillomavirus Res 2018,5.150-155. https://doi.org/10.1016/j.pvr.2018.04.003 [ Links ]
38. Khan NA, Castillo A, Koriyama C, et al. Human papillomavirus detected in female breast carcinomas in Japan. Br J Cancer 2008τ99(3).408-414. https://doi.org/10.1038/sj.bjc.6604502 [ Links ]
39. Choi J, Kim C, Lee HS, et al. Detection of human papillomavirus in Korean breast cancer patients by real-time polymerase chain reaction and meta-analysis of human papillomavirus and breast cancer. J Pathol Translat Med 2016;60(6)442-450. https://doi.org/10.4132/jptm.2016.07.08 [ Links ]
40. Lawson JS, Glenn WK, Salyakina D, et al. Human papilloma viruses and breast cancer. Front Oncol 2015;5:277. https://doi.org/10.3389/fonc.2015.00277 [ Links ]
41. Herrera-Goepfert R, Vela-Chávez T, Carrilio-Garcia A, et al. High-risk human papillomavirus (HPV) DNA sequences in metaplastic breast carcinomas of Mexican women. BMC Cancer 2013;13:445. https://doi.org/10.1186/1471-2407-13-445 [ Links ]
42. Suarez ALP, Lorenzetti MA, Lucano RG, et al. Presence of human papilloma virus in a series of breast carcinoma from Argentina. PLoS ONE 2013;8(4):e61613. https://doi.org/10.1371/journal.pone.0061613 [ Links ]
43. Lebelo RL, Bogers JJ, Thys S, et al. Detection, genoty ping and quantitation of multiple HPV infections in South African women with cervical squamous cell carcinoma. J Med Virol 2015,87(9).1594-1600. https://doi.org/10.1002/jmv.24132 [ Links ]
44. Lawson JS, Glenn, WK, Saiyakina D, et al. Human papillomavirus identification in breast cancer patients with previous cervical neoplasia. Front Oncol 2016,5:298. https://doi.org/10.3389/fonc.2015.00298 [ Links ]
45. Sitas F, Urban M, Stein L, et al. The relationship between anti-HPV-16 IgG seropositivity and cancer of the cervix, anogenitai organs, oral cavity and pharynx, oesophagus and prostate in a black South African population. Infect Agent Cancer 2007;2:6 https://doi.org/10.1186/1750-9378-2-6 [ Links ]
46. Wang X, Tian X, Liu F, et al. Detection of HPV DNA in esophageal cancer specimens from different regions and ethnic groups. A descriptive study. BMC Cancer 2010;10:19. https://doi.org/10.1186/1471-2407-10-19 [ Links ]
47. Takalashi A, Ogoshi S, Ono H, et al. High-risk human papillomavirus infection and over expression of p53 protein in squamous cell carcinoma of the esophagus from Japan. Dis Esophagus 1998;11(3):162-167. https://doi.org/10.1093/dote/11.3.162 [ Links ]
48. Park E, Kim J-Y, Choi S, Kim DS, Oh YL. Carcinogenic risk of human papillomavirus (HPV) genotypes and potential effects of HPV vaccines in Korea. Sci Rep 2019;9(1):12556. https://doi.org/10.1038/s41598-019-49060-w [ Links ]
49. Castellsague X, Diaz M, de Sanjose S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its co-factors. Implications for screening and prevention. J Natl Cancer Inst 2006;98(5):303-315. https://doi.org/10.1093/jnci/djj067 [ Links ]
50. Wong MYW, Wang B, Yang A, Khor A, Xuan W, Rajenda S. Human papillomavirus exposure and sexual behaviour are signficant risk factors for Barrett's dysplasia/esophageal adenocarcinoma. Dii Esophagus 2018;31(12). https://doi.org/10.1093/dote/doy051 [ Links ]
51. Kamolratanakul S, Pitisuttithum P. Human papillomavirus vaccine efficacy and effectiveness against cancer. Vaccines (Basel) 2021;9(12):1413. https://doi.org/10.3390/vaccines9121413 [ Links ]
52. Costa APF, Cobucci RNO, da Silva JM, da Costa Lima PH, Giraldo PC, Goncalves AK. Safety of human papillomavirus 9-valent vaccine. A meta-analysis of randomised trials. J Immunol Res 2017;2017:3736201. https://doi.org/10.1155/2017/3736201 [ Links ]
 Correspondence:
Correspondence:
T Mokoena
taole.mokoena@up.ac.za
Accepted 25 April 2023














