Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.7 Pretoria Jul. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i7.229
REVIEW
Cerebral palsy and its medicolegal implications in low-resource settings - the need to establish causality and revise criteria to implicate intrapartum hypoxia: A narrative review
I BhoratI; E BuchmannII; P Soma-PillayIII; E NicolaouIV; L PistoriusV; I SmutsVI; S VelaphiVII
IFCOG, PhD; Subdepartment of Fetal Medicine, Department of Obstetrics and Gynaecology, Nelson R Mandela School of Medicine, College of Health Sciences., University of KwaZulu-Natal, Durban, South Africa; Council Member and Chair of Expert Opinion Panel, South African Society of Obstetrics and Gynaecology
IIFCOG, PhD; Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIFCOG, PhD; Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of Pretoria, South Africa; Hon. Secretary, South African Society of Obstetrics and Gynaecology
IVFCOG, PhD; Division of Fetal Medicine, Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg., South Africa
VFCOG, PhD; Division of Fetal Medicine, Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
VIFCP (Paed), PhD; Department of Paediatric Neurology, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa
VIIFCP (Paed), PhD; Department of Paediatrics, Faculty of Health Sciences, University of the "Witwatersrand, Johannesburg, South Africa
ABSTRACT
The objective of this study was to establish scientific causality and to devise criteria to implicate intrapartum hypoxia in cerebral palsy (CP) in low-resource settings, where there is potential for an increase in damaging medicolegal claims against obstetric caregivers, as is currently the situation in South Africa. For the purposes of this narrative review, an extensive literature search was performed, including any research articles, randomised controlled trials, observational studies, case reports or expert or consensus statements pertaining to CP in low-resource settings, medicolegal implications, causality, and criteria implicating intrapartum hypoxia. In terms of causation, there are differences between high-income countries (HICs) and low-resource settings. While intrapartum hypoxia accounts for 10 - 14% of CP in HICs, the figure is higher in low-resource settings (20 - 46%), indicating a need for improved intrapartum care. Criteria implicating intrapartum hypoxia presented for HICs may not apply to low-resource settings, as cord blood pH testing, neonatal brain magnetic resonance imaging (MRI) and placental histology are frequently not available, compounded by incomplete clinical notes and missing cardiotocography tracings. Revised criteria in an algorithm for low-resource settings to implicate intrapartum hypoxia in neonatal encephalopathy (NE)/ CP are presented. The algorithm relies first on specialist neurological assessment of the child, determination of the occurrence of neonatal encephalopathy (by documented or verbal accounts) and findings on childhood MRI, and second on evidence of antepartum and intrapartum contributors to the apparent hypoxia-related CP. The review explores differences between low-resource settings and HICs in trying to establish causation in NE/CP and presents a revised scientific approach to causality in the context of low-resource settings for reaching appropriate legal judgments.
Worldwide in the past half century, one medical malpractice claim, i.e. birth-related cerebral palsy (CP), has had a major impact on obstetric care, resulting in defensive practices to prevent litigation. CP litigation has arguably the highest quantum claims, resulting in skyrocketing insurance premiums for obstetricians, therefore placing service delivery under serious threat.[1,2] This phenomenon is not restricted to high-income settings. The impact is enormous in South Africa (SA), for example, where the public health service contingent liabilities for alleged CP-related medical negligence amounted to USD314 million[3] in the years 2018 - 2019. Litigants implicate an adverse event at birth in causation of CP, with the suggestion that the outcome could have been prevented by better intrapartum care.
The aim of this narrative review is to establish causality for intrapartum hypoxia in low-resource settings with their inherent contexts and challenges, and to present a more tailored and appropriate approach to the question of criteria implicating intrapartum hypoxia in neonatal encephalopathy (NE)/CP in low-resource settings. Criteria for intrapartum hypoxia presented for high-income countries (HICs) may not necessarily apply to a low-resource setting. The review will explore differences between low-resource settings and HICs in trying to establish causation in NE/CP, and the need to present a different approach in applying criteria in low-resource settings, so that appropriate scientifically based legal judgments can be made.
Search methods
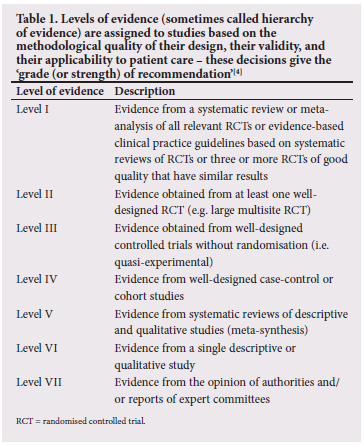
We performed an extensive literature search including any research articles, randomised controlled trials, observational studies, case reports or expert or consensus statements pertaining to CP in low-resource settings, medicolegal implications, causality, and criteria implicating intrapartum hypoxia. Electronic search strategies included searching the MEDLINE, Embase, Cochrane Library and PubMed databases over the period 1990 to the present. Literature other than English was included in the search. Twenty-one relevant papers were selected and included in this narrative review using lead words cerebral palsy in low-resource settings', 'medicolegal implications', causality' and criteria implicating intrapartum hypoxia'. Levels of evidence were assigned to studies based on the methodological quality of their design, their validity, and their applicability to patient care[4] (Table 1). CP and medicolegal implications in high-resource settings were excluded.
Intrapartum v. antenatal factors in CP causation in low-resource settings: The evidence
Only 10 - 14% of CP is caused by term or near-term intrapartum hypoxia in HICs[2] (level V evidence). Epidemiological studies in these settings show that most cases of CP are not related to intrapartum hypoxia[5,6] (level V evidence). Antenatal factors may initiate a causal pathway for perinatal brain injury[7] (level IV evidence) in HIC settings. The extent and role of antenatal factors in causation of CP in low-resource settings is not well understood. The figures indeed differ. The finding of higher numbers of children with spastic quadriplegia (a form of CP associated with intrapartum hypoxic-ischaemic injury) in African countries and in other low-resource settings[8-11] (level V - VI evidence) suggests a greater role for intrapartum complications than is the case in HICs. CP also occurs more frequently in low-income countries, and this excess could be due to factors affecting fetal and postnatal brain development, e.g. preterm birth, intrauterine growth restriction, obstetric complications, birth asphyxia, neonatal jaundice and cerebral infections. The associated mortality linked to these conditions is likely to affect the reported prevalence of CP.[12,13] Overall, in low-income countries, the CP prevalence varies from 2 to 10 per 1 000 live births, while in HICs the prevalence is 1.8 - 2.3 per 1 000 live births.[9,14] In low-resource settings, so-called perinatal asphyxia is implicated as the aetiological factor in 20 - 46% of cases of CP based on studies from Nigeria, North India and Tanzania[15] (level V evidence). Regarding NE as an antecedent to CP, a Nepalese study found that intrapartum monitoring detected evidence of intrapartum hypoxia in 43% of encephalopathic infants[16] (level V evidence). Data from a large referral hospital in SA showed a birth asphyxia incidence of 8.7 - 15.2 per 1 000 live births, higher than the HIC incidence of 1 - 5 per 1 000 live births[17] (level V evidence).
In a low-resource setting, Lally et αl.[18] investigated cerebral injury in South India, and found no evidence for established brain lesions in infants with NE, suggesting potentially treatable perinatal origins (level IV evidence). Ellis et αl.,[16] considering risk factors for NE in Nepal, found that independent intrapartum factors included non-cephalic presentations, prolonged rupture of membranes, use of oxytocin, obstructed labour, cord prolapse and uterine rupture (level IV evidence). There was evidence of intrapartum hypoxia in 60% of encephalopathic infants.
Fetal priming factors that may contribute to NE/hypoxic ischaemic encephalopathy (HIE) include chorioamnionitis,[19,20] placental-mediated disease,[21-23] primary placental disorders,[24-26] metabolic diseases such as diabetes, [27] toxic factors, substance abuse, postmaturity, maternal factors[28] and genetics.[29] There is a dearth of information on the role of fetal priming factors in low-resource settings, although the study by Ellis et al.[16] (level VI evidence) has alluded to a few, including inadequate antenatal care (especially attention to risk assessment) as a risk factor. More studies are needed on fetal priming factors in low-resource settings so that preventive and therapeutic strategies can be devised. A study in Uganda[14] revealed that independent risk factors for NE are maternal and newborn infection, and inflammation, based on blood cultures, molecular assays and placental histology.
The role of fetal priming, i.e. antenatal factors priming the fetal brain for serious neurological injury intrapartum, has been highlighted in the controversial HELIX trial, performed in low-to middle-income countries (LMICs),[30] and rebuttals to this trial.[31] In essence, the HELIX trial suggested that all LMICs should immediately suspend cooling in neonates owing to the reported absence of benefit and increased mortality in neonates who were cooled. This was in contrast to the experience of academic hospitals in SA that use therapeutic hypothermia to treat NE with suspected HIE and showed benefit,[31] in contrast to the HELIX trial - a major difference in outcome. The differences could be related to how intrapartum hypoxia was defined. In the HELIX trial, there was no report of neonatal blood gases within the first hour after delivery and only 11% of the neonates had cord pH measurements. We believe cord pH to be one of the main criteria/ parameters to implicate intrapartum hypoxia in NE, i.e. a cord pH <7 and/or base deficit >12 mmol/L,[32] and a recommendation for universal cord pH to be performed in all deliveries[33] has been made by the BetterObs programme of the South African Society of Obstetricians and Gynaecologists. Sepsis, for example, which is an important antenatal priming factor, limits the benefit of cooling (data for chorioamnionitis in the HELIX trial were absent), but funisitis was noted in 16% of neonates, which implies that antenatal infection could explain the predominant white-matter damage that was detected by magnetic resonance imaging (MRI)). The controversy over the HELIX trial, with different outcomes compared with results in other LMICs with therapeutic cooling, highlights the need to establish scientific causation using specific criteria[32] for acute intrapartum hypoxia in NE/HIE, not only for medicolegal reasons, but to identify infants who will benefit from therapeutic hypothermia, which has been noted to be beneficial in infants who have sustained acute intrapartum hypoxia.
The fetus may therefore be primed for hypoxia antenatally, and the intrapartum period may be just the final link in a series of pathophysiological processes that began or had their roots in the antenatal period long before birth. And even if NE/HIE can be linked to an intrapartum incident, this does not necessarily imply poor care, or suggest that the incident was preventable.
Cardiotocography and neuroimaging: Differences Detween low-resource settings and HICs in establishing causation
With regard to the use of cardiotocography (CTG), there is a point of divergence between HICs and low-resource settings. The frequent or routine use of CTG in HIC settings should not and cannot be copied in low-resource conditions. If CTG is to be used, correct interpretation needs to be applied. If skills to interpret CTG are not available in a low-resource setting, CTG should not be used. For example, the national maternity care guidelines in SA advocate the use of CTG only in high-risk clinical scenarios in referral hospitals where interpretive skills for CTG are available, with intermittent auscultation recommended in low-risk situations monitored in outlying clinics. The CTG is widely used in the medicolegal setting to pronounce on causation and liability, but this is a retrospective analysis with the outcome already known, that lends itself to bias. On its own, electronic fetal monitoring using the CTG is often open to biased interpretation, this being a subjective modality with large inter-observer and sometimes intra-observer variability[34] Furthermore, in low-resource settings, CTG is not always easily available. In a systematic review investigating strategies for intrapartum fetal surveillance in low-resource settings, Housseine et al.[35] (level V evidence) concluded that use of the partogram with intermittent auscultation was associated with a lower caesarean section rate (10 - 15%) but similar perinatal outcomes compared with CTG. Use of CTG was associated with higher caesarean section rates without proven benefit, so the authors recommended against routine CTG use in such settings.
With regard to neuroimaging, there is a distinct difference between low-resource settings and HICs in its use to diagnose neurological lesions in the aetiology and/or prediction of CP. Whereas in HICs early neonatal MRI may be the norm, in low-resource settings the norm would be late MRI, mainly in childhood, often as part of medicolegal litigation. Imaging patterns are usually classified into deep nuclear grey-matter injury, i.e. basal ganglia-thalamus (BGT) injury, watershed (WS) injury, white-matter (WM) injury and vascular lesions. According to Elsingergy et al.,[36] a database of SA children showed the most common lesion in hypoxic ischaemic brain injury in late MRI to be isolated BGT injury (27.6%), followed by combined BGT-WS injury (20.7%) and isolated WS injury (16.4%). Nakao et al[37] in Japan found that among 672 term infants with CR 76% had BGT-dominant injury, 5.4% WM injury, 1.2% WS injury and 1.6% stroke; 1.9% were normal, and 14% unclassified. However, timing of neurological lesions in late MRI predicting when the insult occurred will always be an issue. Radiological findings describing the evolution of these lesions have merit and can correctly identify the injury, but it is unlikely that a direct extrapolation to the labouring mother in the maternity ward can always be made, as there are so many variables to consider, including the possibility of an already hypoxic primed fetus as a result of proximal and distal antenatal factors. Clinical correlation with neuroimaging will be imperative in this scenario. The radiological perspective on its own without clinical correlation is unacceptable with late MRI. The use of isolated radiological confirmation of hypoxic-ischaemic injury, as seems so often to be the practice in medicolegal cases in SA courts, does not necessarily implicate the intrapartum period.
On the other hand, neonatal MRI is relatively accurate in timing of the neurological lesion and thus identifying intrapartum hypoxia as a probable aetiological factor.[38,39]
BGT injury, i.e. the acute profound injury pattern, although linked to an intrapartum sentinel event, may also occur in the absence of clinically obvious sentinel events, and this is well described in the literature.[40] A single sudden hypoxic event of unknown cause presenting as an acute onset of fetal bradycardia in a monitored fetus, can result in a BGT injury pattern.[0]
Another neuroimaging modality that may have relevance in low-resource settings is the use of intracranial ultrasound in the neonatal period, which is more accessible and less expensive than MRI. However, compared with MRI, it is far less sensitive and accurate in diagnosing the neurological lesion and predicting CP,[41] and it is unlikely to be utilised as a useful metric in a medicolegal context.
Discussion
Low-resource settings are different from those in HICs, making the discussion of NE and CP of special interest with regard to medicolegal review. CTG may not be available because of resource constraints,[35] and cord blood gas analysis requires technology that is not affordable in all birthing centres.[42] Assigned Apgar scores may be incorrect,[43] and the need for neonatal resuscitation may at times be a more feasible marker of the newborns condition at birth.[44] In our experience of an SA low-resource setting, retrospective review of events is hampered by incomplete clinical notes and missing CTG tracings. Placental histology is rarely considered, and neonatal brain MRI is almost never available. The determination of causality many years after birth then depends on retrospective maternal accounts in the face of incomplete clinical documentation and the findings of childhood MRI. Courts may be urged by civil society groups to confirm birth asphyxia as causal and preventable,[45] and may then determine liability based on HIC obstetric care standards. Establishing causation and liability for CP in the low-resource setting is as much a challenge as in HICs, if not more so, and must go beyond the wish to assign intrapartum asphyxia as the sole cause. Civil court judgments on a balance of probabilities' applied to a complex medical condition such as CP may hinge on eloquent legal argument rather than robust scientific analysis. The resulting judgments with high-quantum payouts present financial burdens that threaten to overwhelm low-resourced and overburdened healthcare systems.
Criteria implicating intrapartum hypoxia in NE/CP in low-resource settings
The National Collaborative Perinatal Project study noted that the risk of CP is increased by the presence of abnormal neurological signs in the neonatal period, most notably poor respiratory effort, subnormal level of consciousness, seizures and inability to suck, which indicate the syndrome of NE.[46] The American College of Obstetricians and Gynecologists (ACOG) defines NE as a disturbance in neurological function in the earliest days of life in an infant born at >35 weeks of gestation, manifested by a subnormal level of consciousness or seizures, and often accompanied by depression of tone and reflexes.[38] NE is often assumed to be the consequence of hypoxic-ischaemic brain damage, yet it can occur in the absence of markers of intrapartum hypoxia, and may even have a closer relationship to prelabour events.[7,47] Furthermore, NE could be due to factors other than hypoxia-ischaemia, especially infections, which are more common in low-resource settings. The ACOG has recommended that confirmed cases of NE with Apgar scores <5 at 5 and 10 minutes, fetal umbilical artery acidaemia (pH <7.0 or base deficit > 12 mmol/L), presence of multisystem organ failure and neuroimaging evidence of acute brain injury on MRI (done in the neonatal period <3 weeks after delivery) will determine the likelihood that an acute peripartum or intrapartum event was a contributor to the development of NE.[38] This is, however, a product of an HIC discussion using modalities available in that setting.
The ACOG highlights that the first mandatory step in an assessment of NE is to confirm whether a specific infant meets the case definition, and that this must be based on reliable and accurate observations made by trained staff.[38] One of the signs of NE is seizures, which might present as sucking movements and pedalling of limbs. These movements can also be normal for newborns, and their diagnosis is improved by use of the electroencephalogram. In low-resource settings, assessment of newborns is often performed by junior staff in facilities where there is limited equipment available to confirm seizures and monitor cerebral function. Infants may therefore be labelled as having seizures, with resulting overdiagnosis of NE in low-resource settings. In our experience, lawyers often use this diagnosis made by junior doctors to implicate intrapartum hypoxia as a contributor to CP. Other criteria to implicate intrapartum injury namely umbilical artery pH, presence of multisystem organ failure, neuroimaging and placental histology, require laboratory testing and specialised equipment that are often not available in low-resource settings. Along the same lines, exclusion of other causes of NE such as infection requires lumbar puncture, blood cultures and other blood tests, which may also not be available. A diagnosis of NE being due to an intrapartum event as recommended by the ACOG therefore poses a major challenge in low-resource settings.
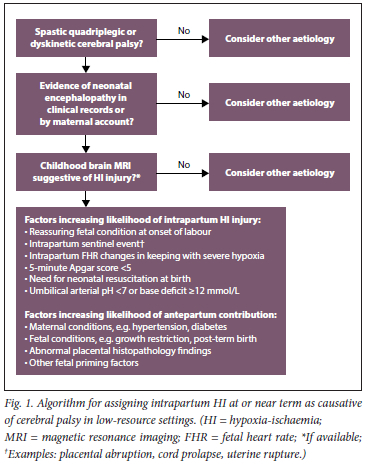
We therefore contend that, in low-resource settings, modalities recommended by the ACOG 2014 guidelines[38] to diagnose intrapartum hypoxia as causative of NE often do not realistically apply. These deficiencies complicate the process of determining causation. Courts therefore need to rely on maternal accounts, limited clinical records, CTGs (if done and available) and childhood MRI reports, with the benefit of hindsight, to reconstruct events surrounding the birth of the child. Individual modalities such as the CTG and MRI should not be considered in isolation. A systematic approach to make best use of the limited available information is needed. We suggest revised criteria in an algorithm for low-resource settings to implicate intrapartum hypoxia in CP/NE (Fig. 1). The algorithm relies first on specialist neurological assessment of the child, determination of the occurrence of NE (by documented or verbal accounts) and findings on childhood MRI, and second on evidence of antepartum and intrapartum contributors to the apparent hypoxia-related CP.
Conclusion
In low-resource settings, expert medical evidence in CP litigation requires a context-appropriate and focused approach to the question of criteria implicating intrapartum hypoxia in causation. This review has explored differences between low-resource settings and HICs in trying to establish causation in NE/CP and the need to present a different approach, one that would be crucial for reaching fair and correct judgments in a legal setting. There is a dearth of data on CP, its causation and its medicolegal implications in low-resource settings, requiring new focus in these areas to devise strategies in dealing with this condition, especially in the medicolegal setting.
Declaration. None.
Acknowledgements. None.
Author contributions. IB, EB, PS-P, EN, LP, IS, SV: protocol/project development, data collection and management, data analysis, manuscript writing/editing.
Funding. None.
Conflicts of interest. None.
References
1. Sartwelle TP, Johnston JC. Cerebral palsy litigation. Change course or abandon ship. J Child Neurol 2015;30(7):828-841. https://doi.org/10.1177/0883073814543306 [ Links ]
2. MacLennan A, Nelson KB, Hankins G, Speer M. Who will deliver our grandchildren? Implications of cerebral palsy litigation. JAMA 2005;294(13):1688-1690. https://doi.org/10.1001/jama.294.13.1688 [ Links ]
3. Taylor B, van Waart J, Ranchod S, Taylor A. Medicolegal storm threatening maternal and child healthcare services. S Afr Med J 2018;108(3):149-150. https://doi.org/10.7196/SAMJ.2018.vl08i3.13139 [ Links ]
4. Ackley BJ, Swan BA, Ladwig G, Tucker S. Evidence-based Nursing Care Guidelines. Medical-surgical Interventions. St Louis, Mo.. Mosby Elsevier, 2008:7. [ Links ]
5. Jacobsson B, Hagberg G. Antenatal risk factors for cerebral palsy. Best Pract Res Clin Obstet Gynaecol 2004;18(3):425-436. https://doi.org/10.1016/j.bpobgyn.2004.02.011 [ Links ]
6. Mclntyre S, Taitz D, Keogh J, Goldsmith S, Badawi N, Blair E. A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol 2013;55(6):499-508. https://doi.org/10.1111/dmcn.12017 [ Links ]
7. Badawi N, Kurinczuk JJ, Keogh JM, et al. Intrapartum risk factors for newborn encephalopathy. The Western Australian case-control study. BMJ 1998;317(7172):1554-1558. https://doi.org/10.1136/bmj.317.7172.1554 [ Links ]
8. Donald K, Samia P, Kakooza-Mwesige A, Bearden D. Pediatric cerebral palsy in Africa. A systematic review. Semin Pediatr Neurol 2014;21(1):30-35. https://doi.org/10.1016/j.spen.2014.01.001 [ Links ]
9. Karumuna JM, Mgone CS. Cerebral palsy in Dar es Salaam. Cent Afr J Med 1990;36( 1):8-10. [ Links ]
10. Van Toorn R, Laughton P, van Zyl N. Aetiology of cerebral palsy presenting at Tygerberg Hospital. S Afr J Child Health 2007;1(2):74-77. [ Links ]
11. Singhi P, Saini AG. Changes in the clinical spectrum of cerebral palsy over two decades in North India - an analysis of 1212 cases. J Trop Pediatr 2013;59(6):434-440. https://doi.org/10.1093/tropej/fmt035 [ Links ]
12. Mclntyre S, Taitz D, Keogh J, Goldsmith S, Badawi N, Blair E. A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol 2013;55(6):499-508. https://doi.org/10.1111/dmcn.12017 [ Links ]
13. Wang H, Liddell CA, Coates MM, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990 - 2013. A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384(9947):957-979. https://doi.org/10.1016/S0140-6736(14)60497-9 [ Links ]
14. Kakooza-Mwesige A, Andrews C, Peterson S, Mangen FW, Eliasson AC, Forssberg H. Prevalence of cerebral palsy in Uganda. A population-based study. Lancet Glob Health 2017;5(12):e1275-e1282. https://doi.org/10.1016/S2214-109X(17)30374-1 [ Links ]
15. Gladstone M. A review of the incidence and prevalence, types and aetiology of childhood cerebral palsy in resource-poor settings. Ann Trop Paediatr 2010,30(3).181-196. https://doi.org/10.1179/146532810X12786388978481 [ Links ]
16. Ellis M, Manandhar N, Manandhar DS, Costello AM. Risk factors for neonatal encephalopathy in Kathmandu, Nepal, a developing country. Unmatched case-control study. BMJ 2000;320(7244):1229-1236. https://doi.org/10.1136/bmj.320.7244.1229 [ Links ]
17. Bruckmann EK, Velaphi S. Intrapartum asphyxia and hypoxic ischaemic encephalopathy in a public hospital. Incidence and predictors of poor outcome. S Afr Med J 2015;105(4):298-303. https://doi.org/10.7196/SAMJ.9140 [ Links ]
18. Lally PJ, Price DL, Pauliah SS, et al. Neonatal encephalopathic cerebral injury in South India assessed by perinatal magnetic resonance biomarkers and early childhood neuro developmental outcome. PLoS ONE 2014;9(2):e87874. https://doi.org/10.1371/journal.pone.0087874 [ Links ]
19. Wu YW, Escobar GJ, Grether JK, et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA 2003;290(20):2677-2684. https://doi.org/10.1001/jama.290.20.2677 [ Links ]
20. Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA 1997;278(3):207-211. [ Links ]
21. Figueras F, Gratacos E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol Fetal Diagn Ther 2014;36(2):86-98. https://doi.org/10.1159/000357592 [ Links ]
22. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The great obstetrical syndromes' are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204(3):193-201. https://doi.org/10.1016/j.ajog.2010.08.009 [ Links ]
23. Figueras F, Caradeux J, Crispi F, Eixarch E, Peguero A, Gratacos E. Diagnosis and surveillance of late-onset fetal growth restriction. Am J Obstet Gynecol 2018;218(2S):S790-S802.el. https://doi.org/10.1016/j.ajog.2017.12.003 [ Links ]
24. Volpe JJ. Placental assessment provides insight into mechanisms and timing of neonatal hypoxic-ischemic encephalopathy. J Neonatal Perinatal Med 2019;12(2):113-116. https://doi.org/10.3233/NPM-190270 [ Links ]
25. Redline RW. Classification of placental lesions. Am J Obstet Gynecol 2015;213(4 Suppl):S21-S28. https://doi.org/10.1016/j.ajog.2015.05.056 [ Links ]
26. Redline RW. Placental pathology. A systematic approach with clinical correlations. Placenta 2008;29(Suppl A):S86-S91. https://doi.org/10.1016/j.placenta.2007.09.003 [ Links ]
27. Bhorat IE, Bagratee JS, Pillay M, Reddy T. Use of the myocardial performance index as a prognostic indicator of adverse fetal outcome in poorly controlled gestational diabetic pregnancies. Prenat Diagn 2014;34(13):1301-1306. https://doi.org/10.1002/pd.4471 [ Links ]
28. Nelson KB. Causative factors in cerebral palsy. Clin Obstet Gynecol 2008;51(4):749-762. https://doi.org/10.1097/GRF.0b013e318187087c [ Links ]
29. MacLennan AH, Thompson SC, Gecz J. Cerebral palsy. Causes, pathways, and the role of genetic variants. Am J Obstet Gynecol 2015;213(6):779-788. https://doi.org/10.1016/j.ajog.2015.05.034 [ Links ]
30. Thayyil S, Pant S, Montaido P, et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX). A randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health 2021;9(9):el273-el285. https://doi.org/10.1016/S2214-109X(21)00264-3 [ Links ]
31. Ballot DE, Ramdin TD, Bandini RM. Therapeutic hypothermia for neonatal hypoxic ischaemic encephalopathy should not be discontinued in low- and middle-income countries. S Afr Med J 2021;111(12):1168-1169. https://doi.org/10.7196/SAMJ.2021.v111i12.16180 [ Links ]
32. Bhorat I, Buchmann E, Soma-Pillay P, Nicolaou E, Pistorius L, Smuts I. Cerebral palsy and criteria implicating intrapartum hypoxia in neonatal encephalopathy - an obstetric perspective for the South African setting. S Afr Med J 2021;lll(3b):280-288. https://doi.org/10.7196/SAMJ.2021.v111i4b.15399 [ Links ]
33. Bhorat IE, Pistorius L, Soma-Pillay P, Smuts I. The case for the routine use of umbilical cord pH in all deliveries. O&G Forum 2017;27(3):33-33. [ Links ]
34. Nelson KB, Sartwelle TP, Rouse DJ. Electronic fetal monitoring, cerebral palsy, and caesarean section. Assumption versus evidence. BMJ 2016;355:i6405. https://doi.org/10.1136/bmj.i6405 [ Links ]
35. Houseinne N, Punt MC, Brown JL, et al. Strategies for intrapartum foetal surveillance in low- and middle-income countries. A systematic review. PLoS ONE 2018;13(10):e0206295. https://doi.org/10.1371/journal.pone.0206295 [ Links ]
36. Elsingergy MM, Worede F, Venkatakrishna S, Curie J, Andronikou S. Magnetic resonance imaging diagnosis of causes of cerebral palsy in a developing country. A database of South African children. S Afr Med J 2021;111(9):910-916. https://doi.org/10.7196/SAMJ.2021.v111i9.15666 [ Links ]
37. Nakao M, Nanba Y, Okumura A, et al. Correlation between heart rate evolution patterns and MRI findings in severe cerebral palsy. A longitudinal study. BJOG 2022;129(9):1542-1582. https://doi.org/10.1111/1471-0528.17089 [ Links ]
38. Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists' Task Force on Neonatal Encephalopathy. Obstet Gynecol 2014;123(4):896-901.https://doi.org/10.1097/01.AOG.0000445580.65983.d2 [ Links ]
39. Mirmiran M, Barnes PD, Keller K, et al. Neonatal brain MRI before discharge is better than serial cranial ultrasound predicting cerebral palsy in very low birthweight infants. Pediatrics 2004;114(4):992-998. https://doi.org/10.1542/peds.2003-0772-L [ Links ]
40. Pasternak JF, Gorey MT. The syndrome of acute near-total intrauterine asphyxia in the term infant. Pediatr Neurol 1998;18(5):391-398. https://doi.org/10.1016/s0887-8994(98)00002-2 [ Links ]
41. Medina-Alva P, Duque KR, Zea-Vera A, et al. Combined predictors of neurodevelopment in very low birthweight preterm infants. Early Hum Dev 2019;130:109-115. https://doi.Org/10.1016/j.earlhumdev.2019.01.019 [ Links ]
42. Omo-Aghoja L. Maternal and fetal acid-base chemistry. A major determinant of perinatal outcome. Ann Med Health Sci Res 2014;4(1):8-17. https://doi.org/10.4103/2141-9248.126602 [ Links ]
43. Miltenburg AS, Kiritta RF, Meguid T, Sundby J. Quality of care during childbirth in Tanzania. Identification of areas that need improvement. Reprod Health 2018;15(1):14. https://doi.org/10.1186/sl2978-018-0463-1 [ Links ]
44. Wall SN, Lee ACC, Carlo W, et al. Reducing intrapartum-related neonatal deaths in low-and middle-income countries - what works? Semin Perinatal 2010;34(6):395-407. https://doi.org/10.1053/j.semperi.2010.09.009 [ Links ]
45. Brodie M. Why TAC wants a say in landmark medical negligence case. Spotlight, 10 December 2019. https://www.spotlightnsp.co.za/2019/12/10/why-tac-wants-a-say-in-major-medical-negligence-case/ (accessed 27 July 2020). [ Links ]
46. Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. Multivariate analysis of risk. N Engl J Med 1986;315(2):81-86. https://doi.org/10.1056/NEJM198607103150202. [ Links ]
47. Badawi N, Kurinczuk JJ, Keogh JM, et al. Antepartum risk factors for newborn encephalopathy. The Western Australian case-control study. BMJ 1998;317(7172):1549-1553. https://doi.org/10.1136/bmj.317.7172.1549 [ Links ]
 Correspondence:
Correspondence:
I Bhorat
bhorat@worldonline.co.za
Accepted 2 February 2023














