Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.7 Pretoria Jul. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i7.271
IN PRACTICE
Namibian spitting cobra, Naja nigricincta nigricincta (zebra snake): Oral flora and antibiotic sensitivity, a cross-sectional study
E SaaimanI; C BuysII; F TheartIII
IMB ChB, MMed (Anaest); Anaesthetist, private practice, Windhoek, Namibia; member of the Namibian Snakebite Interest Group
IIMB ChB, MMed (ORL); Ear, nose and throat surgeon, private practice, Windhoek, Namibia; Namibian snakebite management expert; founding member and head of the Namibian Snakebite Interest Group
IIIBSc (Natural Resource Management); Snake expert and handler; member of the Namibian Snakebite Interest Group
ABSTRACT
This was a cross-sectional study with the aim of characterising Naja nigricincta nigricinctas oral bacterial flora as well as accompanying sensitivities and resistance towards antibiotics. Naja nigricincta nigricincta (zebra snake) is a spitting cobra indigenous to Namibia. Nasopharyngeal and venom swabs for bacterial culture and antibiotic sensitivity were taken from 37 native zebra snakes originating from the Khomas region that were captured for removal and relocation. Enterococcus faecalis, Proteus spp., Morganella morganii and Pseudomonas spp. were the organisms most often cultured. The antibiotic sensitivity profiles of these organisms suggest ciprofloxacin or a third-generation cephalosporin plus gentamicin or piperacillin-tazobactam as prophylactic antibiotics in case of Naja nigricincta nigricincta bites.
African spitting cobras, such as Naja nigricollis, Naja mossambica and Naja nigricincta nigricincta, most often bite at night while the victims are asleep. Spitting cobra bites frequently result in local necrosis and secondary infection, often culminating in disfigurement, loss of function and amputation.[1-6] Small children and babies are often bitten. Children are particularly vulnerable to snake envenomation, and suffer a high morbidity and mortality from these severely cytotoxic bites.
Naja nigricincta nigricincta (Western barred spitting cobra/zebra snake) (Fig. 1) is endemic to central and northern Namibia and southern Angola, and accounts for most of the venomous bites encountered in these areas of Namibia.[1,2,7,8]

This cytotoxic venom typically results in a severe dermonecrosis. This resembles a type of venom-induced necrotising fasciitis, with fast-spreading necrosis in the fascial planes between skin and deeper-lying muscles.[1,2]
Prophylactic antibiotics are not recommended following snakebite in southern Africa.[9] In Namibia, antibiotics are routinely part of the treatment of all cytotoxic bites (Namibian Medical Snakebite Management guidelines - Drs PJC Buys and EL Saaiman, unpublished). An increasing number of studies suggest that soft-tissue infection is one of the most substantial complications of cytotoxic snakebites, and that pre-emptive antibiotics should be considered in patients with severe local envenomation.'4,10-15-
The extensive tissue destruction and devitalisation, caused by local cytotoxic envenomation, predispose the wound to bacterial infection. Inoculation of bacteria originating from the snake's indigenous oral flora, the environment or the victim's surrounding skin can occur during a bite. Venom-induced dermonecrosis may thus expand and exacerbate into an accompanying soft-tissue infection, and even progress to infective necrotising fasciitis.[6,16-19]
Comprehensive identification of the microbiology of bite wounds and oral flora of culprit snakes is pertinent in selecting suitable empirical and prophylactic antibiotics, preventing secondary infection and reducing morbidity.[15,19-21]
No data exist on Naja nigricincta nigricincta's oral microbiome. Very few case reports of Naja nigricincta nigricincta snakebites have been recorded. Microscopy, culture and sensitivity (MC&S) results on 10 Naja nigricincta nigricincta bite wound swabs (2012 - 2020) were recovered from case files and analysed. The swabs were all taken at different times post bite. There were no data regarding the swabbing procedures, the specific wound areas swabbed or the indications for taking the wound swabs. Enterococcus faecalis, Morganella morganii and Proteus spp. were cultured most often. Other Gram-negative bacteria cultured included Klebsiella pneumoniae, Escherichia coli, Salmonella enterica, Serratia marcescens, Schewanella algae and Chryseobacterium indologenes. The Gram-negative organisms were sensitive to third- and fourth-generation cephalosporins, ciprofloxacin, gentamicin and piperacillin-tazobactam (Table 1).
Two other regional publications originating from KwaZulu-Natal (KZN), South Africa, examining the microbiology from infected cytotoxic snakebite wounds.[15,22] Blaylock[22] studied wound swabs taken from 14 cytotoxic snakebite victims, with associated necrosis, abscesses and haematomas. The snakes were mostly unidentified. In this study, Morganella morganii, Proteus spp., Citrobacter and Serratia spp. were cultured most often. No antibiotic profiles on the organisms were done (Table 2).
Wagener et al.[4] analysed the microbiology results of 42 snakebite patients who required surgical debridement for extensive skin and soft-tissue necrosis after snakebite. The snakes responsible were not identified. Snakes most likely responsible for cytotoxic bites in this area of KZN are Naja mossambica (Mozambique spitting cobra), Bitis arietans (puff adder) and Hemachatus haemachatus (rinkhals).[8] At the time of debridement, tissue samples of necrotic or infected tissue were sent for bacteriological analysis. Morganella morganii, Enterococcus faecalis and Proteus spp. were most often encountered. Other organisms cultured were Salmonella enterica, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae and Citrobacter freundii (Table 2). The Gram-negative Enterobacteriaceae showed a resistance to penicillins and first- and second-generation cephalosporins, but were sensitive to third-generation cephalosporins, ciprofloxacin and aminoglycosides.[4]
Both the above KZN study (Wagener et al.) and the Naja nigricincta nigricincta bite wound swab results are very similar to Taiwanese and Chinese publications on wound infections secondary to snakebite, where Morganella morganii and Enterococcus spp. were the most common pathogens found. All these Gram-negative organisms displayed a similar resistance to penicillins and first and second-generation cephalosporins (Table 2).[4,14,17,23]
According to above bite wound microbiology, piperacillin-tazobactam, a quinolone, or second- or third-generation cephalosporin are proposed as empirical therapy following snakebite.[4,14,17,23]
The possibility that primary infections are caused by the inoculation of the snake's oral flora during a bite is illustrated by a recent case report. A 2-and-a-half-year-old boy presented with an infective ( Proteus vulgaris) necrotising fasciitis following Naja nigricincta nigricincta bite with rapid deterioration into multi-organ failure and death. A Proteus vulgaris with the same antibiotic profile was cultured from the mouth of the culprit snake (Table 3).[6]
This case underscored the lack of current data on Naja nigricincta nigricincta's oral biome, the limited data on post-Naja nigricincta nigricincta bite wound microbiology and the inadequacy of the post-snakebite antibiotic protocol then in place.
Study
This was a cross-sectional study. The aim was to characterise the patterns of oral bacterial flora in Naja nigricincta nigricincta, to determine the antibiotic sensitivity and resistance of above pathogens and to develop rational guidelines for antimicrobial prophylaxis after Naja nigricincta nigricincta snakebite injury in Namibia.
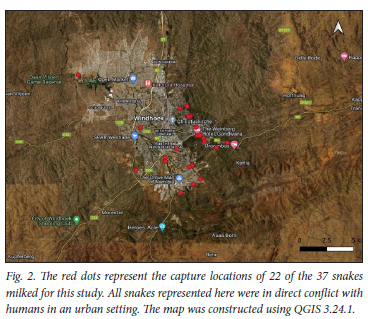
The study was conducted between 18 November 2020 and 15 April 2021. A total of 37 zebra snakes (Naja nigricincta nigricincta) that were caught for removal and relocation were used in the study. All snakes came from the Khomas region of Namibia, with the GPS location of where the snake was caught recorded in accordance with the Ministry of Environment, Forestry and Tourism's Human-Snake-Conflict-Mitigation programme (Fig. 2). The snakes were identified and measured, and the gender established, and milked by a local expert. Both an oropharyngeal and venom swab were taken from each snake using aseptic techniques by a medical professional. The swabs were sent for MC&S using conventional culture methods.
All the snakes were relocated into the wild.
Permit
In line with regulations imposed by the Namibian Ministry of Environment, Forestry and Tourism regarding handling of animals for scientific purposes, the study protocol was submitted for study permission from the National Commission on Research Science and Technology (NCRST) (Section 4 of Research Science and Technology Act, Act No. 23 of 2004).
Permit AN20200222 was granted in accordance with Section 45 of the Nature Conservation Ordinance 4 of 1975 of the Republic of Namibia, regarding animals for scientific purposes.
Catching and swabbing
Expert snake handlers caught the snakes. The GPS location of where the snake was caught was recorded using the Epicollect app (Fig. 2).
Cloacal probing with a blunt sexting probe[24] to establish gender, as well as snout-to-vent measurements using the tube restraint method, were done by a local snake expert.[24]
The snakes were milked using the acknowledged method of voluntary injection of the venom into a receptacle through a rubber or para-film membrane (Fig. 3).[25] The para-films were cleaned with 90% ethanol spray. A first milking was followed immediately by a second milking through another ethanol-cleaned para-film. This was done to achieve a more representative sample of uncontaminated venom - rather like a midstream urine sample. A swab was taken from the second sample.

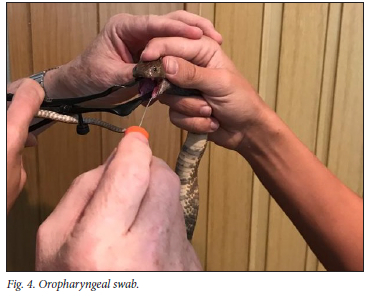
After milking, the snakes' mouths were opened by a small sterile speculum and an oropharyngeal swab taken (Fig. 4). All swabs were taken by a medical professional and were kept refrigerated before being sent to NAMPath laboratory.
Bacterial identification and antibiotic susceptibility
A Gram smear was made aseptically onto a slide, prior to inoculation, which was then stained and viewed under 100x objective lenses. The swab was inoculated onto 5% blood agar, McConkey agar and chocolate agar plates. After inoculating the plates, the swab was transferred into a tube containing thio-glycolate enrichment medium. Both the inoculated plates and thyoglycolate medium with the swab inside were then incubated at 37°C. The 5% blood agar and chocolate agar were incubated under CO2 conditions.
Plates were read after 24 hours of incubation, and identification was by use of the manual API 10S method for lactose fermenters. For non-lactose fermenters and Gram-positive cocci, the automated Vitek 2 system was utilised. Antibiotic susceptibilities were determined either by the manual Kirby-Bauer method or the automated Vitek system (minimum inhibitory concentration assay).
After 24 hours, the swabs that were inoculated into the thioglycolate enrichment medium were re-inoculated onto 5% blood agar, chocolate agar and McConkey agar and incubated at 37°C.
After 48 hours of incubation, plates were read for identification and antibiotic susceptibility (as described above). Previously inoculated plates from the thioglycolate medium were checked for new bacterial growth not obtained from the initial culture plates. If new bacteria had grown, identification and antibiotic susceptibility were done as a follow-up.
Results from the Gram stain, identification and antibiotic susceptibility were entered into the laboratory information system, and laboratory reports generated.
Results
Sex and length
Twenty-one adult snakes, of which 15 were male (adult length >80 cm),[26] measuring 90 - 165 cm, and 16 juvenile snakes, ranging from 28 - 35 cm, were caught.
Bacteria cultured
Organisms were cultured from all venom and oral samples. No anaerobic organisms were cultured. In clinical practice, juvenile Naja nigricincta nigricincta snakes are very seldom responsible for bites. The results from adult and juvenile snakes were differentiated and recorded into separate tables (Tables 4, 5 and 6).
Antibiotic profile
The antibiotic profiles of organisms cultured from adult and juvenile Naja nigricincta nigricincta oropharynges and venom are detailed in Table 7.
Discussion
Although venom is thought to be sterile with strong antimicrobial properties,[22] archaea, algae, bacteria, fungi, protozoa and viruses have all been found to be present in certain venom microenvironments. [27,28] The anatomy of the envenomation apparatus, i.e. an open duct attached to a liquid vessel with intermittent flow, may allow for the colonisation and facilitation of bacterial persistence and adaptation within antimicrobial venom.[29] All the venom samples yielded organisms, and eight of the adult venom samples cultured different organisms than those from the same snake's oropharynx, suggesting venom colonisation. Further research in this field is needed.
The aim of this study was to identify oral snake pathogens with their specific antibiotic profile that can be inoculated during snakebite. Whether these organisms originate from the venom or the oropharynx is not of clinical relevance. In analysing the findings of the study, the results of each snake's venom and oropharyngeal swabs were thus combined.
No anaerobic organisms were cultured. The most frequent organisms cultured from Naja nigricincta nigricincta adult venom and oropharynx were Enterococcus faecalis and Proteus spp., both in 71.4% of cases, with Morganella spp. and Pseudomonas spp. in 19%. Also cultured were Salmonella, Acinetobacter and Citrobacter spp. (Tables 4 and 6). Pathogens were present in the oral flora (venom and/or oropharynx) of even the smallest snake. Enterococcus faecalis (43.7%) and Proteus spp. (87.5%) were most often cultured from the juvenile snakes (Tables 5 and 6).
In line with other publications, the similarity of the organisms cultured between individual Naja nigricincta nigricincta's hints at the possibility of a species-specific or host-specific oral microbiome.[30] Further studies comparing the oral microbiome of different species will clarify this issue.
Male zebra snakes have a much larger home range with possibly more prey diversity than their female counterparts (unpublished data, Mr F Theart, 2022). This may account for the larger number of adult males caught in this study. These differences could potentially influence variations between male and female oral microbacteria. The sample size from this study was too small to identify any substantial differences between the oral flora of the different sexes.
Whether the bacteria cultured can benefit the host by playing a role in enhancing venom effects or digestion is unclear.[30,31] Enterococcus faecalis and the Gram-negative bacteria cultured in our study have all been implicated in necrotising fasciitis.[32] Inoculation of these bacteria into an area of extensive venom-induced destruction and tissue devitalisation, with a resultant soft-tissue infection, will further expand and exacerbate tissue damage, and may even culminate in an infective necrotising fasciitis.[16-18] In the 'weaponised bacteria theory', Auffenberg[33] postulated that bacteria in Komodo dragon (Varanus komodoensis) venom are a mechanism for prey debilitation and mortality, because of the absence of said bacteria in the reptile's oral microbiome. This raises the question of whether the 'weaponized bacteria theory' may be true for the Naja nigricincta nigricincta oral microbiome.[34]
All the Enterococcus faecalis cultured were sensitive to penicillins, all cephalosporins, ciprofloxacin and gentamicin. The Gram-negative bacteria from the adult snakes displayed a resistance of 35.8% against amoxy-clavulanic acid, 78.5% against cephalothin (first-generation cephalosporin) and 60% against cefuroxime (second-generation cephalosporin). Sensitivity of 92% was displayed towards ceftriaxone (third-generation cephalosporin), 96.3% to piperacillin-tazobactam, and 100% sensitivity to ciprofloxacin and gentamicin (Table 7). The pathogens cultured from the juvenile snakes showed less overall resistance and greater sensitivity towards penicillin and second-generation cephalosporins than their counterparts from adult snakes (Table 7).
Juvenile Naja nigricincta nigricincta snakes are very seldom responsible for bites (authors' own clinical experience). Only the data from the adult snake samples were taken into consideration for antibiotic selection suggestions. In view of above results, recommended antibiotic prophylaxis after Naja nigricincta nigricincta bites is ciprofloxacin or a third-generation cephalosporin plus gentamicin or piperacillin-tazobactam. Since controversy surrounds the use of ciprofloxacin in children, a third-generation cephalosporin plus gentamicin may be a safer option for the paediatric population.[35] Piperacillin-tazobactam should be reserved for severely ill patients.
Limitations
The snakes that were swabbed were not snakes responsible for bites.
This study was conducted from mid-November to mid-April. Seasonal variations in snake oral flora are unknown, and current results may not reflect other time periods. The sample size of 37 snakes is not large, and all the snakes originated from the Khomas region in Namibia. The results may therefore not be representative of the oral flora of all Naja nigricincta nigricinctas from all geographical areas, or of other spitting cobras.
Practical issues and financial constrains necessitated conventional culture methods, although sequencing techniques would have been able to identify more bacterial species.
Conclusion
Although subject to several limitations, this study has provided an initial baseline database on the oral microbiology of Naja nigricincta nigricincta with concomitant antibiotic sensitivities and resistance. This was an important first step in the quest to identify suitable prophylactic and empirical antimicrobial therapy secondary to Naja nigricincta nigricincta snakebite. The next step will be to perform a comprehensive standardised study on the microbiology of Naja nigricincta nigricincta snakebite wounds.
The antibiotic profile from Naja nigricincta nigricincta's oral microbiome, as cultured in this study, is very similar to the profile of the Gram-negative Enterobacteriaceae cultured from both the KZN study and the Naja nigricincta nigricincta bite wounds (Tables 1 and 7).[4] Based on these findings, prophylactic antimicrobial therapy after Naja nigricincta nigricincta bites should comprise of ciprofloxacin or a third-generation cephalosporin plus gentamicin or piperacillin-tazobactam.
Declaration. None.
Acknowledgements. The authors thank Mr. Esegiel Gaeb, Mr. Elton Afrikaner and Mr. Melusi Ndlovu at NamPath Laboratory for their assistance, advice and support on this project.
Author contributions. ES: literature review, protocol, supervise milking procedure, swab venom and snake's oropharynx, data analysis, article compilation. PB : supervisor, oversee data analysis and article compilation. FT: identification, catching, handling, milking and releasing snakes.
Funding. Funds were donated by the Dean Heunis De Wet Trust Fund.
Conflicts of interest. None.
References
1. Buys PJC. Medical management of snakebite in Namibia. Windhoek: Gamsberg Macmillan, 2003. [ Links ]
2. Saaiman E, Buys P. Spitting cobra (Naja nigricincta nigricincta) bites complicated by rhabdomyolysis, possible intravascular haemolysis and coagulopathy. S Afr Med J 2019;109(10):736. https://doi.org/10.7196/SAMJ.2019.v109i10.14103 [ Links ]
3. Tilbury CR. Observations on the bite of the Mozambique spitting cobra (Naja mossambica mossambica). S Afr Med J 1982;61(9):308-313. https://hdl.handle.net/10520/AJA20785135_14484 (accessed 15 May 2023). [ Links ]
4. Wagener M, Naidoo M, Aldous C. Wound infection secondary to snakebite. S Afr Med J 2017;107(4):315-319. https://doi.org/10.7196/SAMJ.2017.v107i4.12084 [ Links ]
5. Warrell DA, Greenwood BDNOLPC. Necrosis, haemorrhage and complement depletion following bites by the spitting cobra. QJM 1976;45(1):1-22. https://doi.org/10.1093/oxfordjournals.qjmed.a067448 [ Links ]
6. Saaiman EL, Buys PJC. Fatal infective necrotising fasciitis: Complication following Naja nigricincta nigricincta bite (western barred spitting cobra/zebra snake). S Afr Med J 2022;112(12):892-896. https://doi.org/10.7196/SAMJ.2022.v112i12.16689 [ Links ]
7. Griffen M. The Zebra snake antivenom project. Travel News Namibia, 2001. https://www.travelnewsnamibia.com/news/stories/featured-stories/the-zebra-snake-antivenom-project/ (accessed 8 October 2018). [ Links ]
8. Marais J. A Complete Guide to the Snakes of Southern Africa. 2nd ed. Cape Town: Struik, 2004. [ Links ]
9. Blaylock RS. The identification and syndromic management of snakebite in South Africa. S Afr Family Pract 2005;47(9):46-53. https://doi.org/10.1080/20786204.2005.10873288 [ Links ]
10. Tsai Y-H, Hsu W-H, Huang K-C, Yu P-A, Chen C-L, Kuo LT. Necrotizing fasciitis following venomous snakebites in a tertiary hospital of southwest Taiwan. Int J Infect Di. 2017;63:30-36. https://doi.org/10.1016/j.ijid.2017.08.005 [ Links ]
11. Gutierrez JM, Rucavado A, Chaves F, Diaz C, Escalante T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 2009;54(7):958-975. https://doi.org/10.1016/j.toxicon.2009.01.038 [ Links ]
12. Houcke S, Resiere D, Lontsingoula GR, et al. Characteristics of snakebite-related infection in French Guiana. Toxins 2022;14(2):89. https://doi.org/10.3390/toxins14020089 [ Links ]
13. Saravia-Otten P, Gutierrez J, Arvidson S, Thelestam M, Flock J-I. Increased infectivity of Staphylococcus aureus in an experimental model of snake venom-induced tissue damage. J Infect Dis 2007;196(5):748-754. https://doi.org/10.1086/520537 [ Links ]
14. Huang L-W, Wang J-D, Huang J-A, Hu S-Y, Wang L-M, Tsan Y-T. Wound infections secondary to snakebite in central Taiwan. J Venomous Animals Toxins incl Trop Dis 2012;18(3):272-276. https://doi.org/10.1590/S1678-91992012000300004 [ Links ]
15. Resiere D, Gutierrez JM, Neviere R, Cabie A, Hossein M, Kallel H. Antibiotic therapy for snakebite envenoming. J Venom Anim Toxins incl Trop Dis 2020;26. https://doi.org/10.1590/1678-9199-jvatitd-2019-0098 [ Links ]
16. Sadeghi M, Barazandeh M, Zakariaei Z, et al. Massive cutaneous complications due to snakebite: A case report and literature review. Clin Case Rep 2021;9(5):e04129. https://doi.org/10.1002/ccr3.4129 [ Links ]
17. Garg A, Sujatha S, Garg J, Acharya NS, Parija SC. Wound infections secondary to snakebite. J Infect Dev Ctries 2009;3(3):221-223. https://doi.org/10.3855/jidc.39 [ Links ]
18. Lin JH, Sung WC, Mu HW, Hung DZ. Local cytotoxic effects in cobra envenoming: A pilot study. Toxins 2022;14(2):122. https://doi.org/10.3390/toxins14020122 [ Links ]
19. Chuang P-C, Lin W-H, Chen Y-C, Chien C-C, Chiu I-M, Tsai T-S. Oral bacteria and their antibiotic susceptibilities in Taiwanese venomous snakes. Microorganisms 2022;10(5):951. [ Links ]
20. Mao Y-C, Chuang H-N, Shih C-H, et al. An investigation of conventional microbial culture for the Naja atra bite wound, and the comparison between culture-based 16S Sanger sequencing and 16S metagenomics of the snake oropharyngeal bacterial microbiota. PLoS Negl Trop Dis 2021;15(4):e0009331. https://doi.org/10.1371/journal.pntd.0009331 [ Links ]
21. Yak R, Lundin A-C, Pin P, Sebastin S. Oral bacterial microflora of free-living reticulated pythons (Python reticulatus) in Singapore. J Herpetol Med Surg 2015;25:40-44. https://doi.org/10.5818/1529-9651-25.1.40 [ Links ]
22. Blaylock RS. Antibiotic use and infection in snakebite victims. S Afr Med J 1999;89(8):874-876. [ Links ]
23. Chen CM, Wu KG, Chen CJ, Wang CM. Bacterial infection in association with snakebite: A 10-year experience in a northern Taiwan medical center. J Microb Immunol Infect 2011;44(6):456-460. https://doi.org/10.1016/j.jmii.2011.04.011 [ Links ]
24. Atkins MCP, Larsen KW. Measuring snakes across the decades. Are tube-restraint measurements compatible with an earlier method? Herpetol Conserv Biol 2020;15(2):350-353. [ Links ]
25. Chippaux JP, Williams V, White J. Snake venom variability: Methods of study, results and interpretation. Toxicon 1991;29(11):1279-1303. https://doi.org/10.1016/0041-0101(91)90116-9 [ Links ]
26. Spawls S, Branch B. The Dangerous Snakes of Africa. London: Bloomsbury Publishing, 2020. [ Links ]
27. Samy RP, Stiles BG, Franco OL, Sethi G, Lim LHK. Animal venoms as antimicrobial agents. Biochem Pharmacol 2017;134:127-138. https://doi.org/10.1016/j.bcp.2017.03.005 [ Links ]
28. Ul-Hasan S, Rodriguez-Román E, Reitzel AM, et al. The emerging field of venom-microbiomics for exploring venom as a microenvironment, and the corresponding Initiative for Venom Associated Microbes and Parasites (iVAMP). Toxicon X 2019;4:100016. https://doi.org/10.1016/J.TOXCX.2019.100016 [ Links ]
29. Esmaeilishirazifard E, Usher L, Trim C, et al. Microbial adaptation to venom is common in snakes and spiders. bioRxiv 2018;348433. https://doi.org/10.1101/348433 [ Links ]
30. Zancolli G, Mahsberg D, Sickel W, Keller A. Reptiles as reservoirs of bacterial infections: Real threat or methodological bias? Microb Ecol 2015;70(3):579-584. [ Links ]
31. Smith SN, Colston TJ, Siler CD. Venomous snakes reveal ecological and phylogenetic factors influencing variation in gut and oral microbiomes. Front Microbiol 2021;12:657754. https://doi.org/10.3389/fmicb.2021.657754 [ Links ]
32. Li X, Du Z, Tang Z, Wen Q, Cheng Q, Cui Y. Distribution and drug sensitivity of pathogenic bacteria in diabetic foot ulcer patients with necrotizing fasciitis at a diabetic foot center in China. BMC Infect Dis 2022;22(1):1-10. https://doi.org/10.1186/s12879-022-07382-7 [ Links ]
33. Auffenberg W. The Behavioral Ecology of the Komodo Monitor. Gainesville: University Press of Florida, 1981. [ Links ]
34. Fry B. Venomous Reptiles and their Toxins: Evolution, Pathophysiology and Biodiscovery. Oxford: Oxford University Press, 2015. [ Links ]
35. Masoumi B, Eslami G, Alizadeh-Navaei R, Mondal P, Rezai MS. Safety profile of using ciprofloxacin in paediatrics: A systematic review and meta-analysis. J Pediatr Rev 2019;7(3):129-140. http://doi.org/10.32598/jpr.7.3.129 [ Links ]
 Correspondence:
Correspondence:
E Saaiman
msnyman99@gmail.com
Accepted 13 April 2023














