Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.6 Pretoria jun. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i6.442
RESEARCH
Microbiological analysis and predictors of gallbladder infection with antimicrobial susceptibility patterns in an HIV setting
R SinghI; S Mewa KinooII, III; P RamjathanIV; K Swe Swe-HanV; B SinghVI
IFCS (SA), MMed (Surg); Department of General Surgery, King Edward VIII Hospital, Durban, South Africa
IIMMed (Surg), PhD; Department of General Surgery, King Edward VIII Hospital, Durban, South Africa
IIIMMed (Surg), PhD; Department of Surgery, Nelson R Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IVFC Path (SA) Micro, MMed (Microbiol); National Health Laboratory Service, King Edward VIII Hospital and Department of Medical Microbiology, Nelson R Mandela School of Medicine., College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
VMMed (Microbiol), PhD; National Health Laboratory Service, Inkosi Albert Luthuli Central Hospital Academic Complex and Department of Medical Microbiology, Nelson R Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
VIFCS (SA), MD; Department of Surgery, Nelson R Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: South Africa (SA) has a high prevalence of HIV infection, which has been shown to affect the prevalence and severity of other infections and of sepsis, particularly in gallbladder (GB) disease. Empirical antimicrobial (EA) therapy for acute cholecystitis is based largely on bacterial colonisation of bile (bacteriobilia) and antimicrobial susceptibility patterns (antibiograms) obtained from the developed world, where the prevalence of HIV infection is very low. In an era of ever-increasing antimicrobial resistance, it is of vital importance to monitor and update local antibiograms
OBJECTIVES: Owing to the paucity of data available locally to guide treatment, our objectives were to examine GB bile for bacteriobilia and to assess antibiograms in a setting with a high prevalence of HIV infection, in order to determine the need for review of our local antimicrobial policies for both EA therapy for GB infections and preoperative antimicrobial prophylaxis (PAP) for laparoscopic cholecystectomy (LC
METHODS: A retrospective observational descriptive study was undertaken at King Edward VIII Hospital, Durban, SA. Hospital records were reviewed for all patients undergoing LC over a 3-year period. GB bacteriobilia and antibiograms were assessed and compared between people living with HIV (PLWH) and people who were HIV uninfected (HIV-U). The preoperative parameters of age, endoscopic retrograde cholangiopancreatography (ERCP), procalcitonin (PCT), C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR) were assessed as predictors for bacteriobilia. Statistical analyses were performed using R for statistical computing, andp-values <0.05 were considered statistically significant
RESULTS: There were no differences in bacteriobilia or antibiograms between PLWH and HIV-U. There was >30% resistance to amoxicillin/ clavulanate and cephalosporins. Aminoglycoside-based therapy had good susceptibility patterns, while carbapenem-based therapy demonstrated the lowest resistance levels. ERCP and age were predictors of bacteriobilia (p<0.001 and p<0.002, respectively), but PCT, CRP and NLR were not
CONCLUSION: PLWH should follow the same PAP and EA therapy recommendations as HIV-U. For EA therapy, we recommend a combination of amoxicillin/clavulanate with aminoglycoside-based therapy (amikacin or gentamicin), or piperacillin/tazobactam as monotherapy. Carbapenem-based therapy should be reserved for drug-resistant species. For elective LC, elderly patients or patients who have previously undergone ERCP, we recommend selective use of cefazolin for PAP
Gallstone disease (GD) occurs in up to 20% of the population in developed countries and is the leading cause of acute cholecystitis (AC) and cholecystectomy[1] In South Africa (SA), recent evidence indicates that the cholecystectomy rate has almost doubled in the past decade[2] The actual cost of GD to the already overburdened healthcare system in SA is not known. However, in developed countries it is rated as the second-highest gastrointestinal cost burden to the healthcare system.[3] Gallbladder (GB) bile is sterile in the absence of pathology[4] Pathological conditions of the GB result in bacterial colonisation of bile (bacteriobilia), with GD the major risk factor.[5
The latest published management guidelines for AC are the Tokyo Guidelines 2018 (TG18).[6] The standard of care for patients presenting with AC is early laparoscopic cholecystectomy (LC) (within 72 hours of onset of symptoms), as it has similar outcomes compared with delayed or interval laparoscopic cholecystectomy
(ILC).[6] However, these 'standard of care' recommendations stem from institutions in high-income countries. In Durban, KwaZulu-Natal (KZN) Province, SA, Mbatha and Anderson[7] and Makatini et al.[8] showed in two separate studies in two different state hospitals that >85% of patients presented late and management therefore mainly consisted of ILC. Timeous empirical antimicrobial (EA) therapy is therefore of paramount importance to tide these patients over to an ILC. Omission of treatment with timeously appropriate EAs in these patients results in bacteriobilia, which may progress to infection and sepsis with fatal consequences.
The choice of antimicrobials for the treatment of AC varies. The 2013 Tokyo Guidelines (TG)[9] stated that 'such therapy [antimicrobial therapy] depends upon knowledge of both local microbial epidemiology and patient-specific factors that affect selection of appropriate agents'. Knowledge of 'local microbial epidemiology' for
AC is severely lacking in SA. Furthermore, SA has a unique 'patient-specific factor in that it was estimated in 2021 that 7.52 million (13.1%) of its population of 57.7 million were people living with human immunodeficiency virus (PLWH), a higher rate than any other country[10] Of PLWH in SA in 2021, 26% lived in KZN and 3.9 million were on antiretroviral therapy (ART).[10] Compared with HIV-uninfected people (HIV-U), PLWH have an increased risk of developing GB disease and tend to develop cholelithiasis at a younger age [10,11] has also been shown to affect the prevalence and severity of sepsis.[12] EA therapy for AC is based largely on the most common causative organisms and susceptibility patterns obtained from the developed world, where the prevalence of PLWH is very low; however, local practice remains hostage to these international guidelines, such as the TG, as there is a paucity of data available to guide treatment locally[9]
Considering that the management approach for our patients is largely ILC, and that this is a critical step in definitive management of AC, the use of preoperative antimicrobial prophylaxis (PAP) for LC is another area of antimicrobial discussion. Previously, during the era of open cholecystectomy, PAP was standard routine practice. However, since LC has become the gold-standard operation for elective GB removal, the incidence of infectious complications has significantly decreased compared with the open operation, giving rise to doubt about the necessity for routine PAP for LC on the part of surgeons.[13] Although many surgeons still routinely use PAP today despite this reservation, this routine use is constantly debated in the interests of antimicrobial stewardship and limiting unnecessary exposure and expense.
In a clinical setting where there is a dearth of information on bacteriobilia and antibiograms to guide treatment locally, especially in an era of ever-increasing antimicrobial resistance, the importance of monitoring and updating local antimicrobial susceptibility patterns (antibiograms) is underscored. We therefore examined GB bile for bacteriobilia in a setting with a high prevalence of PLWH, to determine whether there are differences in microbiology in our local population compared with what has been reported internationally, and whether this situation may demand review of our local EA policies for GB infections. We also examined the use of various preoperative parameters, viz. age, endoscopic retrograde cholangiopancreatography (ERCP), neutrophil-lymphocyte ratio (NLR), C-reactive protein (CRP) and procalcitonin (PCT), in patients undergoing cholecystectomy to predict which patients are at risk of bacteriobilia, especially higher-risk individuals such as PLWH. In addition to reviewing EA therapy, the study therefore also aimed to help guide the use of PAP, minimise antimicrobial overuse and curb resistance.
Methods
Study setting
A retrospective observational descriptive study was undertaken at King Edward VIII Hospital, Durban, over the 3-year period January 2018 - December 2020.
Sampling
Hospital records were reviewed for all patients undergoing cholecystectomy (for indications including biliary colic, AC, obstructive jaundice and gallstone pancreatitis). Patients presenting with AC were graded into mild, moderate and severe according to the TGI8, and patients who were hospitalised within a month of presenting with AC were classified as having healthcare-associated biliary infections. Clinical demographic data included age, gender, ethnicity, HIV status and the use of ART, which were obtained from the patient charts. Other relevant clinical information, including preoperative use of ERCP, was also obtained from the patient charts. Preoperative viral loads, CD4 counts, white cell counts (WCCs), neutrophil counts, lymphocyte counts, NLR, CRP PCT, and postoperative GB bile microbiology (bacteriobilia) and antimicrobial susceptibility (antibiograms) were obtained from the National Health Laboratory Service LABTRAK website. All intermediate results on antibiograms were regarded as resistant. The method of bile sampling was obtained from theatre records. Patients without documented HIV status, patients whose bile was not sampled, and patients whose bile was sampled by methods other than intraoperative aseptic GB aspiration prior to cholecystectomy were all excluded from the study.
Ethical considerations
Ethics approval was granted by the University of KwaZulu-Natal Biomedical Research Ethics Committee (ref. no. BE429/18). Permission to conduct the research was granted by the KZN Department of Health (ref. no. KZ-201810-030) and King Edward VIII Hospital (ref. no. KE 2/7/1/51/2018).
Data analysis
Statistical analyses were performed using R for statistical computing, version 4.0.0, release 2020 (R Core Team and R Foundation for Statistical Computing, Austria). Descriptive statistics such as frequencies and percentages were used to summarise categorical variables. Central tendency and dispersion of data were measured using means and standard deviations for normally distributed variables and medians and interquartile ranges for skewed variables. Associations between categorical variables were tested using either Fisher's exact test where 80% of cells had an expected count <5 or Pearsons χ2 test where 80% of cells had an expected count >5. Similarly, with regard to the testing of associations between continuous variables, for normally distributed data means were compared using independent t-tests, while for non-normally distributed data, the Mann-Whitney U-test was used. Statistical tests were two-sided, and p-values <0.05 were considered statistically significant.
Results
Demographic profile
A total of 400 patients underwent cholecystectomy during the study period. After reviewing the patient charts and theatre records, 72 patients were excluded (N=328). The mean age of the patients was 45 years (range 19 - 79). Of the patients, 266 (81.1%) were female and 62 (18.9%) were male; 227 (69.2%) were black, 60 (18.3%) were Indian, 25 (7.6%) were white and 16 (4.9%) were of mixed race; and 264 (80.5%) were HIV-U and 64 (19.5%) were PLWH. Of the 64 PLWH, 61 (95.3%) were on ART. For the PLWH, the median CD4 count was 586 cells/µL and the median viral load was 0 copies.
Preoperative parameters predicting bacteriobilia
Of the 328 patients, 90 had bacteriobilia of GB bile. A number of preoperative parameters were analysed for predicting bacteriobilia of GB bile. Patients with positive cultures had a higher median WCC, a higher median neutrophil count and a higher NLR than those with negative cultures, but none of these variables reached statistical significance (Table 1). For patients with positive cultures, the median preoperative PCT level of 0.04 μg/L and CRP level of 12.0 mg/L despite being only marginally higher than the normal reference range values of <0.01 μg/L and <10.0 mg/L, respectively, were statistically significantly higher than the median values of patients with negative cultures (Table 1). Older age was a significant predictor of bacteriobilia of GB bile (Table 1). The preoperative parameter that demonstrated the greatest statistical significance in predicting a positive bile culture was preoperative ERCP (Fig. 1). There was no statistically significant difference in the preoperative parameters assessed between PLWH and HIV-U, and these are therefore not presented. Preoperative parameters of all patients are summarised in Table 1.
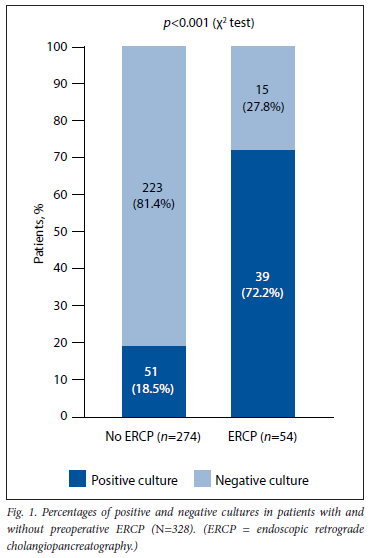
ERCP as a predictor of bacteriobilia
Fig. 1 shows that 72.2% of patients who underwent preoperative ERCP had a positive GB bile culture, while only 18.6% of patients who did not have a preoperative ERCP procedure had a positive GB bile culture. ERCP was therefore a strong predictor of positive GB bile culture (p<0.001). The median time between ERCP and cholecystectomy was 21 days.
Comparison of organisms between PLWH and HIV-U
Among the 90 patients who had positive GB bile cultures, a comparison was made between HIV-U and PLWH. Seventy-four were HIV-U, with a 28.0% positive culture rate, and 16 were PLWH, with a 25% positive culture rate. There was no statistically significant difference in culture rates between HIV-U, PLWH and the overall culture rate of 27.4%. The differences in organism groups between HIV-U and PLWH are summarised in Fig. 2, which shows that there were also no statistically significant differences between them. The organisms with the highest overall culture rate were Gram-negative organisms, viz. Escherichia coli, Klebsiella pneumoniae, Enterococcus species and Enterobacter species (Fig. 2). The most frequently isolated enterococcal species were Enterococcus faecium (6/22), Enterococcus faecalis (5/22), Enterococcus gallinarum (4/22) and Enterococcus casseliflavus (4/22). Three other enterococcal species were isolated.
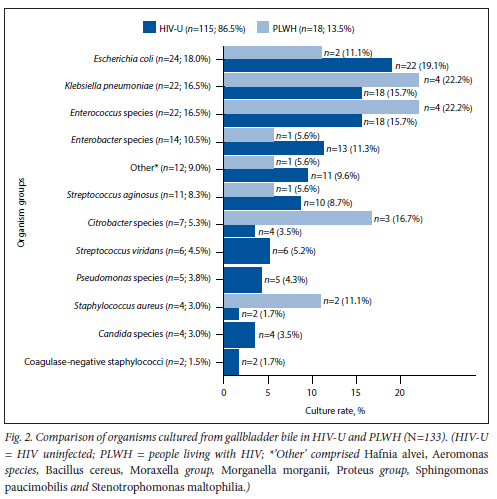
Sixty-seven patients cultured a single organism, 17 patients cultured two organisms, and 6 patients cultured three organisms. Only 2 PLWH cultured more than one organism. Of the 4 patients who cultured Candida species, none were PLWH. Of the total positive bile cultures (n=90), 32 were from patients with AC (3 of these cases were acalculous), 24 from patients with bilary colic, 23 from patients with obstructive jaundice, and 11 from patients with gallstone pancreatitis. Of the 32 cases of AC, 25 were of grade 1 severity, 5 of grade 2 severity and 2 of grade 3 severity according to the TG grading system of severity. Twenty-nine of the 32 cases were community acquired, as these patients had no recent history of hospital admission.
Comparison of antibiograms of GB isolates between PLWH and HIV-U
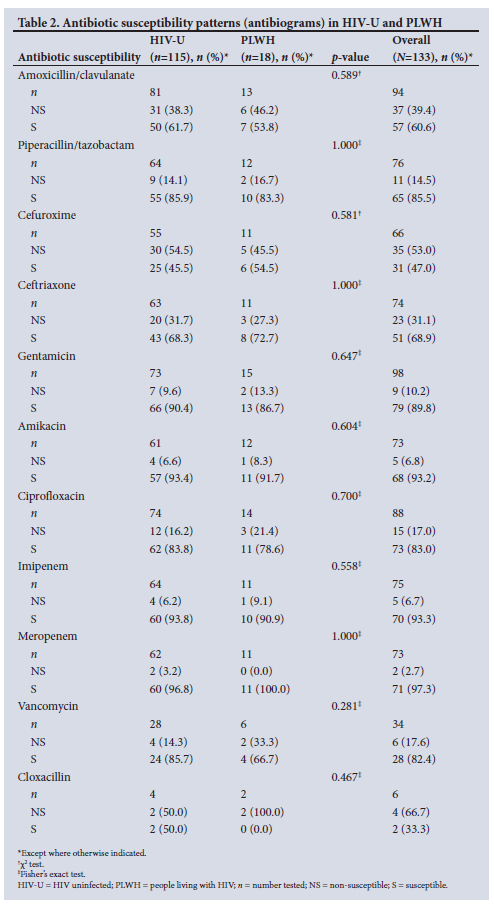
A comparison of antibiograms between HIV-U and PLWH revealed no statistically significant difference for any of the antimicrobials tested (Table 2). There was an overall high level of antimicrobial resistance (>30%) to penicillin-based therapy in the form of amoxicillin/clavulanate and cephalosporin-based therapy (second-generation cefuroxime and third-generation ceftriaxone), with resistance levels of 39.4%, 53.0% and 31.1%, respectively. Bacterial isolates had a low overall resistance level of 14.5% to penicillin-based therapy in the form of piperacillin/ tazobactam; however, carbapenem-based therapy (imipenem and meropenem) demonstrated the lowest resistance levels of 6.7% and 2.7%, respectively. Aminoglycoside-based therapy (amikacin and gentamicin) showed good susceptibility patterns with only 6.8% and 10.2% resistance, respectively and fluoroquinolone-based therapy (ciprofloxacin) showed 17.0% resistance. Three out of 4 Staphylococcus aureus isolates (75%) were resistant to cloxacillin (methicillin-resistant S. aureus), and 1 out of 2 coagulase-negative staphylococci (50%) was resistant to cloxacillin. All Staphylococcus species were 100% susceptible to vancomycin. Among the Enterococcus isolates with susceptibility results, 3 out of 4 (75%) of the E. faecalis isolates were susceptible to ampicillin. The single ampicillin-resistant E. faecalis isolate was susceptible to vancomycin. Of the E. Jaecium isolates with susceptibility results, 2 out of 4 (50%) were susceptible to ampicillin. Of the ampicillin-resistant E. faecium isolates, 1 was susceptible to vancomycin and 1 was resistant to vancomycin. Of the fungibilia (bile colonised by fungi) cultures (4 Candida species - 3 Candida albicans and 1 Candida parapsilosis), 50% were resistant to fluconazole.
Discussion
Omission of EA therapy for AC, or incorrect choice of EA for AC due to lack of local sensitivity patterns, can lead to catastrophic outcomes such as multiorgan failure followed by death. In patients suspected to have AC, current prompt appropriate EA therapy is mandatory[14] The choice of EA therapy however, varies between treatment guidelines and is largely guided by local antimicrobial susceptibility data.[14] For example, penicillin-based therapy in the form of ampicillin/ sulbactam has been removed from the North American guidelines because it has little activity left against E. coli in that region. [15] In addition to antimicrobial resistance in patients with community-acquired intra-abdominal infections being widely reported, the spectrum of microbiology of bile may also change over time.[16,17] Periodic evaluation and updating of local antibiograms is therefore important in ensuring that EA regimens remain effective and relevant. Locally there are no treatment guidelines based on susceptibility data, and we therefore follow the TG, which are guided by a prospective study of AC involving 116 institutions worldwide.[18] Furthermore, although the incidence of opportunistic infections has decreased with the roll-out of ART, PLWH are at particular risk of bacterial bloodstream infections even with ART, as HIV-induced disorders of the immune system are only partially restored by combination ART[19] In the present study, we factored in PLWH and compared them with HIV-U with regard to the spectrum of microbiology of bile, and found no statistically significant differences. When comparing the overall proportions of organisms isolated from bile cultures with the microbiology in the TG, the present study mirrored the frequency of bacteriobilia, with E. coli, K. pneumoniae, and Enterococcus and Enterobacter species being among the most common cultured. We can therefore conclude that the spectrum of overall proportions of microbiology of bile in our local population of both PLWH and HIV-U is not dissimilar to what is reported internationally. Furthermore, in a local KZN study that reviewed bacteriobilia from the bile duct during ERCP, the authors also concluded that there was no difference in bacteriobilia rates and no difference in the spectrum of organisms between PLWH and HIV-U. [20]
The overall antimicrobial susceptibility patterns in the present study when comparing PLWH and HIV-U also did not reach statistical significance. For community-acquired grade 1 AC, which was the predominant presentation in the present study, the TG recommend penicillin-based therapy in the form of ampicillin/ sulbactam or cephalosporin-based therapy as initial EA therapy only if local antimicrobial susceptibility pattern (antibiogram) resistance rates are <20%.[14] If this recommendation is applied to the findings of the present study penicillin-based therapy in the form of amoxicillin/clavulanate and cephalosporin-based therapy (second-generation cefuroxime and third-generation ceftriaxone) may be poor choices as initial EA therapy for both PLWH and HIV-U presenting with AC locally, owing to their >30% resistance rates in the present study. Both penicillin in the form of ampicillin/sulbactam and cephalosporins are proving high-resistance antimicrobial choices for bacteriobilia in several countries, whereas penicillin-based therapy in the form of piperacillin/tazobactam is not, which is in keeping with the present study findings.[21,22] In fact, the Surgical Infection Society and the Infectious Diseases Society of America reported penicillin and cephalosporins to be among the antimicrobials with the highest resistance against Enterobacteriaceae 13 years ago in their 2010 guidelines.[23]
Resistance to fluoroquinolones (ciprofloxacin) was reasonably low in the present study, but according to both the TG and the WorldSociety of Emergency Surgery (WSES), its use is only recommended as an alternative agent in combination with metronidazole for patients with beta-lactamase allergies when the susceptibility of cultures is known, as the antimicrobial resistance has been reported to be increasing significantly worldwide. [16,24] In the present study, aminoglycoside-based therapy (amikacin and gentamicin) showed good susceptibility patterns, and in view of the favourable cost, they may be used as part of combination EA therapy together with a beta-lactam antimicrobial for AC locally. Carbapenem-based therapy (imipenem and meropenem) demonstrated the lowest resistance levels in the present study, and in view of the higher cost and for antibiotic stewardship purposes, it is suggested that this therapy be reserved for multidrug-resistant cases. The findings and recommendations of the present study are not dissimilar to those of a recent large cohort study of 2 288 patients with 492 cultures, demonstrating similar culture and antibiogram results.[25]
For grade 3 community-acquired AC, EA therapy against Pseudomonas species is recommended owing to the high virulence of these organisms and the risk of mortality [14] In the present study, however, the culture rate of Pseudomonas species was very low (3.8%), and the majority of patients presented with a much less severe form of AC in the form of grade 1 community-acquired AC. The study therefore does not have sufficient data to recommend against the use of EA therapy for Pseudomonas species. Vancomycin has been recommended as the EA of choice against Enterococcus for grade 3 community-acquired AC, but because an increasing prevalence of vancomycin-resistant Enterococcus has been reported, treatment should be guided by local antibiograms.[23] In the present study, in view of the low numbers of grade 3 community-acquired AC and varying susceptibility patterns of vancomycin against ampicillin-resistant E. faecium and E. faecalis isolates we suggest targeted therapy for severe cases of AC where Enterococcus may be suspected. Owing to the low prevalence of fungibilia in AC and the high resistance to fluconazole in the present study, fungi should be treated according to susceptibility.
In the recent WSES guidelines, there has been a suggestion that biliary penetration of antimicrobial agents for AC be considered when making a choice of EA, especially in patients with obstruction, where there is evidence that as obstruction occurs, secretion of antimicrobials into the bile decreases.[24] The WSES reports that for aminoglycoside-based therapies, amikacin has a better antibiotic bile-to-serum concentration ratio (ABSCR) than gentamicin, for penicillin-based therapies piperacillin/tazobactam has abetter ABSCR than amoxicillin/clavulanate, and for carbapenem-based therapies imipenem has a better ABSCR than meropenem.[24] However, the TG report a lack of randomised trials comparing antimicrobial biliary penetration to determine the clinical relevance of these ratios, particularly in acute infection of the GB, and has therefore not factored them into EA choice at present.[14]
The role of PAP for LC in uncomplicated GB disease has been debated. In a recent meta-analysis, which included 21 randomised clinical trials, it was observed that PAP for LC was safe and effective in reducing surgical site infections and global infections during hospitalisation.[13] Notwithstanding this finding, current guidelines do not support the routine use of PAP for elective LC for uncomplicated GD.[26,27] The TG recommend PAP for AC and cholangitis only[28] However, a more recent double-blind, placebo-controlled, randomised study found that PAP did not affect the risk of postoperative infectious complications (PICs), even in patients undergoing LC for AC.[29]
The American Society of Health-System Pharmacists guidelines only advocate PAP for complicated GD such as AC and jaundice, and if there is a risk of intraoperative GB rupture and open conversion.[30] A retrospective study in Sweden showed that the only significant risk factor for PICs was a positive bile culture.[31] In view of the Swedish study and the combined global widespread overuse of antibiotics and increasing resistance patterns, the present study placed some emphasis on the importance of assessing possible predictors of bacteriobilia that may guide the use of PAP. In this regard, preoperative age, ERCP, NLR, CRP and PCT were assessed as predictors and markers of subclinical inflammation and infection.
Age is known to be a predictor of declining function of the immune system, leading to an increased incidence of infection. [32] In the present study, patients with positive bile cultures were of statistically significantly older age, similar to a prospective trial in another developing country[5] The definition of advanced age varies among individuals, cultures and countries, but according to the World Health Organization it is defined as an age>65 years.[33] Interestingly, in a review of 163 patients presenting with AC and bacteriobilia, >65 years was found to be a cut-off age for predicting bacteriobilia.[34]
ERCP involves entering the sterile biliary system via the unsterile duodenum, and it is therefore not surprising that it is a known risk factor for bacteriobilia.[35,36] Patients who have had previous biliary tree manipulation with ERCP have a six times increased risk of presenting with bacteriobilia, and use of antimicrobials during the ERCP procedure has not been convincingly shown to decrease the incidence of bacteriobilia.[35,36] ERCP has been found be an independent factor affecting positive bile culture in a multivariate analysis.[22] This finding was also confirmed in the present study We therefore recommend that in our population group, including both PLWH and HIV-U, PAP should be considered in older patients and all patients among whom preoperative ERCP was undertaken, to potentially decrease PICs. If PAP is to be used, current guidelines suggest the use of cefazolin.[37]
In the present study, the NLR was raised in patients with GB bacteriobilia, but this did not reach statistical significance. The sensitivity of CRP for the diagnosis of GB bacteriobilia was calculated to be 12 mg/L in the present study, which is remarkably low even though statistically significantly higher than negative culture specimens, and therefore gives rise to some doubt about the diagnostic potential of this laboratory marker in predicting GB bacteriobilia. The sensitivity of PCT for the diagnosis of GB bacteriobilia was calculated to be 0.04 μg/L in the present study, which like CRP is remarkably low and also gives rise to doubt about the diagnostic potential of this laboratory marker in predicting GB bacteriobilia. Furthermore, in a recent local study, CRP and PCT were unable to differentiate between patients with and without bacteriobilia of bile sampled via ERCP in both PLWH and HIV-U.[20] The absence of any significant impact therefore speaks against the routine use of preoperative NLR, CRP and PCT in predicting bacteriobilia for the use of PAP in patients undergoing LC.
In keeping with curbing resistance patterns, EA therapy should be discontinued postoperatively following LC for AC, as there is sufficient evidence from a randomised clinical trial demonstrating no benefit postoperatively after removing uncomplicated GBs.[38]
A major limitation of the present study is that it was a retrospective study of patient charts and laboratory results. Missing clinical data and laboratory results will lead to gaps in the study. Despite there being a big discrepancy in numbers between HIV-U and PLWH in the study, we considered that the numbers were representative of the population in general, as 19.5% of patients in the study were PLWH and in the population as a whole 13.1 % are PLWH.
Conclusion
PLWH in the present study were found to have similar bacteriobilia and antibiograms to HIV-U and should therefore follow the same EA and PAP recommendations. For EA therapy, penicillin-based monotherapy in the form of amoxicillin/clavulanate should be used with caution owing to its low susceptibly rate in the present study. We recommend the use of piperacillin/tazobactam as an alternative to penicillin-based EA monotherapy in view of its superior drug susceptibility rate in the present study. If amoxicillin/clavulanate is to be used as initial EA therapy, we highly recommend combining it with an aminoglycoside (amikacin or gentamicin) as a cost-beneficial alternative treatment strategy because of the high rate of susceptibility to aminoglycosides in the present study and the relatively low expense of these drugs. Cephalosporin-based therapy should also be used with caution in view of poor susceptibility rates in the present study We recommend that carbapenem-based therapy should be reserved for drug-resistant species owing to cost and in the interests of antibiotic stewardship. Fluoroquinolone-based therapy (ciprofloxacin in combination with metronidazole) should be reserved for patients with beta-lactamase allergies. In view of the high bacteriobilia rates in older patients and patients who had preoperative ERCP in the present study, we recommend the selective use of cefazolin as PAP in these specific groups of patients undergoing elective LC. Finally, the study indicates that use of the preoperative laboratory markers CRP, PCT and NLR for predicting GB bacteriobilia and electing to use PAP in patients undergoing LC cannot be recommended.
Declaration. The research for this study was done in partial fulfilment of the requirements for RS's MMed (Surg) degree at the University of KwaZulu-Natal.
Acknowledgements. The authors acknowledge the contributions of Dr V V Ramkelawon, Dr C G Bhula and Dr S Ebrahims.
Author contributions. The research idea was conceptualised by SMK and BS. Data were collected and captured by RS. Data were analysed by RS. SMK, PR and KSS-H. All authors contributed equally to the write-up of the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am 2010;39(2):157-169.https://doi.org/10.1016/j.gtc.2010.02.003 [ Links ]
2. Khan ZA, Khan MU, Brand M. Increases in cholecystectomy for gallstone related disease in South Africa. Sei Rep 2020;10(1):13516. https://doi.org/10.1038/s41598-020-69812-3 [ Links ]
3. Myer PA, Mannaiithara A, Singh G, Singh G, Pasricha PJ, Ladabaum U. Clinical and economic burden of emergency department visits due to gastrointestinal diseases in the United States. Am J Gastroenterol 2013;108(9):1496-1507. https://doi.org/10.1038/ajg.2013.199 [ Links ]
4. Elkeles G, Mirizzi PL. A study of the bacteriology of the common bile duct in comparison with the other extrahepatic segments of the biliary tract. Ann Surg 1942;116(3):360-366. https://doi.org/10.1097/00000658-194209000-00007 [ Links ]
5. De Oliveira RS, da Silva P, Queiroz CAS, Terra-Junior JA, Crema E. Prevalence of bacteriobilia in patients undergoing elective colecystectomy. Arq Bras Cir Dig 2018;31(3):e1392. https://doi.org/10.1590/0102-672020180001e1392 [ Links ]
6. Mayumi T, Okamoto K, Takada T, et al. Tokyo Guidelines 2018. Management bundles for acute Cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 2018;25(1):96-100. https://doi.org/10.1002/jhbp.519 [ Links ]
7. Mbatha SZ, Anderson E Outcomes in laparoscopic cholecystectomy in a resource constrained environment. S Afr J Surg 2016;54(3):8-12. [ Links ]
8. Makatini GM, MewaKinooS, Singh B. An audit of interval cholecystectomy for acute cholecystitis in alow resource healthcare system. S Afr J Surg 2020;58(1):10-13. [ Links ]
9. Gomi H, Solomkin JS, Takada T, et al. TG13 antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary PancreatSci 2013;20(1):60-70. https://doi.org/10.1007/s00534-012-0572-0 [ Links ]
10. Kinoo SM, Nagiah S, Chuturgoon A, Singh B. Symptomatic gallstones and HIV in black South African women. Changing trends of gallstone disease? South Afr J HIV Med 2021;22(1):1208. https://doi.org/10.4102/sajhivmed.v22il.l208 [ Links ]
11. Nash JA, Cohen SA. Gallbladder and biliary tract disease in AIDS. Gastroenterol Clin North Am 1997;26(2):323-335. https://doi.org/10.1016/s0889-8553(05)70297-1 [ Links ]
12. Huson MAM, Grobusch MP, van der Poll T. The effect of HIV infection on the host response to bacterial sepsis. Lancet Infect Dis 2015;15(1):95-108. https://doi.org/10.1016/S1473-3099(14)70917-X [ Links ]
13. Liang B, Dai M, Zou Z. Safety and efficacy of antibiotic prophylaxis in patients undergoing elective laparoscopic cholecystectomy. A systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31(5):921-928.https://doi.org/10.1111/jgh.13246 [ Links ]
14. Gomi H, Solomkin JS, Schlossberg D, et al. Tokyo Guidelines 2018. Antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 2018;25(1):3-16. https://doi.org/10.1002/jhbp.518 [ Links ]
15. Mazuski JE, TessierJM, May AK, et al. The Surgical Infection Society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt) 2017;18(1):1-76. https://doi.org/10.1089/sur.2016.261 [ Links ]
16. Sung YK, Lee JK, Lee KH, Lee KT, Kang C-I. The clinical epidemiology and outcomes of bacteremic biliary tract infections caused by antimicrobial-resistant pathogens. J Am Gastroenterol 2012;107(3):473-483. https://doi.org/10.1038/ajg.2011.387 [ Links ]
17. Kwon W, Jang J-Y, KimE-Q et al. Changing trend in bile microbiology and antibiotic susceptibilities. Over 12 years of experience. Infection 2013;41(1):93-102. https://doi.org/10.1007/sl5010-012-0358-y [ Links ]
18. Coccolini F, Sartelli M, Catena F, et al. Antibiotic resistance pattern and clinical outcomes in acute cholecystitis. 567 consecutive worldwide patients in a prospective cohort study. Int J Surg 2015;21:32-37. https://doi.org/10.1016/j.ijsu.2015.07.013 [ Links ]
19. Jordano Q, Faicó V, Almirante B, et al.. Invasive pneumococcal disease in patients infected with HIV. Still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis 2004;38(11):1623-1628. https://doi.org/10.1086/420933 [ Links ]
20. Chiliza KS, Madela F, Tlou B, Anderson F. Obstructive jaundice. Studies on predictors of biliary infection and microbiological analysis in an HIV setting. S Afr Med J 2021;111(8):803-808. https://doi.org/10.7196/SAMJ.2021.v111i8.15255 [ Links ]
21. Parekh PM, Shah NJ, Suthar PP, et al. Bacteriological analysis of bile in cholecystectomy patients. Int J Res Med Sci 2017;3(11):3091-3096. https://doi.org/10.18203/2320-6012.ijrms20151142 [ Links ]
22. Yun SP, Seo H-I. Clinical aspects of bile culture in patients undergoing laparoscopic cholecystectomy. Medicine (Baltimore) 2018;97(26):ell234. https://doi.org/10.1097/MD.0000000000011234 [ Links ]
23. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children. Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010;11(1):79-109. https://doi.org/10.1089/sur.2009.9930 [ Links ]
24. Ansaioni L, Pisano M, Coccolini F, et al. 2016 WSES guidelines on acute calculous cholecystitis. World J Emerg Surg 2016;11:25. https://doi.org/10.1186/sl3017-016-0082-5 [ Links ]
25. Shafagh S, Rohani SH, Hajian A. Biliary infection. Distribution of species and antibiogram study. Ann Med Surg (Lond) 2021;70:102822. https://doi.org/10.1016/j.amsu.2021.102822 [ Links ]
26. Vohra RS, Hodson J, Pasquali S, Griffiths EA. Effectiveness of antibiotic prophylaxis in non-emergency cholecystectomy using data from a population-based cohort study. World J Surg 2017;41(9):2231-2239. https://doi.org/10.1007/s00268-017-4018-3 [ Links ]
27. Sarkut P, Kilicturgay S, Aktas H, Ozen Y, Kaya E. Routine use of prophylactic antibiotics during laparoscopic cholecystectomy does not reduce the risk of surgical site infections. Surg Infect (Larchmt; 2017;18(5):603-609. https://doi.org/10.1089/sur.2016.265 [ Links ]
28. Takada T, Strasberg SM, Solomkin JS, et al. TG13. Updated Tokyo Guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 2013;20(1):1-7. https://doi.org/10.1007/s00534-012-0566-y [ Links ]
29. Jaafar G, Sandblom G, Lundell L, Hammarqvist F. Antibiotic prophylaxis in acute cholecystectomy revisited. Results of a double-blind randomised controlled trial. Langenbecks Arch Surg 2020;405(8): 1201-1207. https://doi.org/10.1007/s00423-020-01977-x [ Links ]
30. Bratzier DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14(1):73-156. https://doi.org/10.1089/sur.2013.9999 [ Links ]
31. DarkahiB, Sandblom G, Liljeholm H, Videhuit P, Melhus A, Rasmussen IC. Biliary microflora in patients undergoing cholecystectomy. Surg Infect (Larchmt) 2014;15(3):262-265. https://doi.org/10.1089/sur.2012.125 [ Links ]
32. Stahl EC, Brown BN. Cell therapy strategies to combat immunosenescence. Organogenesis 2015;11(4):159-172. https://doi.org/10.1080/15476278.2015.1120046 [ Links ]
33. Yasuda H, Takada T, Kawarada Y, et al. Unusual cases of acute cholecystitis and cholangitis. Tokyo Guidelines. J Hepatobiliary Pancreat Surg2007;14(1)98-113. https://doi.org/10.1007/s00534-006-1162-9 [ Links ]
34. Asai K, Watanabe M, Kusachi S, et al. Bacteriological analysis of bile in acute cholecystitis according to the Tokyo Guidelines. J Hepatobiliary Pancreat Sci 2012;19(4):476-486. https://doi.org/10.1007/s00534-011-0463-9 [ Links ]
35. Rupp C, Bode K, Weiss KH, et al. Microbiological assessment of bile and corresponding antibiotic treatment. A stro be-compliant observational study of 1401 endoscopic retrograde cholangiographies. Medicine (Baltimore) 2016;95(10):e2390. https://doi.org/10.1097/MD.0000000000002390 [ Links ]
36. Gr an el-Villach L, Gil-Fortuno M, Fortea-Sanchis C, Gamon-Giner RL, Martinez-Ramos D, Escrig-Sos VJ. Factors that influence bile fluid microbiology in cholecystectomised patients. Rev Gastroenterol Mex 2020;85(3):257-263. https://doi.org/10.1016/j.rgmx.2019.07.006 [ Links ]
37. Steccanella F, Amoretti P, Barbieri MR, Bellomo F, Puzziello A. Antibiotic prophylaxis for hepato-biliopancreatic surgery - a systematic review. Antibiotics (Basel) 2022;11(2):194. https://doi.org/10.3390/antibioticsll020194 [ Links ]
38. Regimbeau JM, Fuks D, Pautrat K, et al. Effect of postoperative antibiotic administration on postoperative infection following cholecystectomy for acute calculous cholecystitis. A randomized clinical trial. JAMA 2014;312(2):145-154. https://doi.org/10.1001/jama.2014.7586 [ Links ]
 Correspondence:
Correspondence:
S Mewa Kinoo
smewakinoo@gmail.com
Accepted 28 March 2023














