Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.6 Pretoria Jun. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i6.16765
RESEARCH
Periprosthetic joint infection: A South African perspective
J S HiddemaI; A L J du ToitII; D R van der JagtIII, †; H WilsonIV; C A HugoV; A R SekeittoVI
IMB ChB, FC Orth (SA); Department of Orthopaedic Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
IIMB ChB, FC Orth (SA); Orthopaedic Practice, Johannesburg, South Africa
IIIMB BCh, FC Orth (SA); Arthroplasty Unit, Department of Orthopaedic Surgery, Faculty of Health Sciences, University of the Witwatersrand; Charlotte Maxeke Johannesburg Academic Hospital
IVMB ChB; Department of Orthopaedic Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
VMB ChB; Department of Surgery, Frere Provincial Hospital, East London
VIMB ChB, FC Orth (SA); Arthroplasty Unit, Department of Orthopaedic Surgery, Faculty of Health Sciences, University of the Witwatersrand; Charlotte Maxeke Johannesburg Academic Hospital
ABSTRACT
BACKGROUND: South African (SA) data on the bacteriology and sensitivity profile of periprosthetic joint infection are lacking. Current regimens for systemic and local antibiotic therapy are based on international literature. These regimens are different for the USA and Europe, and therefore might not be relevant to SA
OBJECTIVES: To determine the characteristics of periprosthetic joint infection in an SA clinical setting by identifying the most common organisms cultured, and establishing their antibiotic sensitivities in order to propose the most appropriate empiric antibiotic treatment regimen
In the case of two-stage revision procedures, we aim to compare the organisms cultured during the first stage v. organisms cultured during the second stage in second-stage procedures that had positive cultures. Furthermore, in these culture-positive second-stage procedures we aim to correlate the bacterial culture with the erythrocyte sedimentation rate/C-reactive protein result.
METHODS: We performed a retrospective cross-sectional study looking at all hip and knee periprosthetic joint infections in patients >18 years treated at a government institution and a private revision practice in Johannesburg, SA between January 2015 and March 2020. Data were collected from the Charlotte Maxeke Johannesburg Academic Hospital hip and knee and the Johannesburg Orthopaedic hip and knee databanks
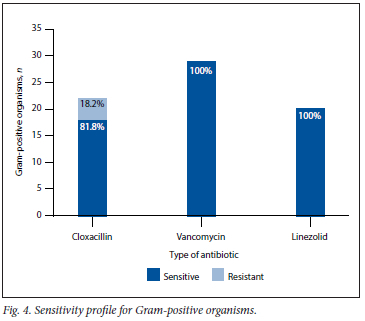
RESULTS: We included 69 patients who underwent 101 procedures relating to periprosthetic joint infection. Positive cultures were found in 63 samples, and 81 different organisms were identified. The most common organisms cultured were Staphylococcus aureus (n=16, 19.8%) and coagulase-negative Staphylococcus (n=16, 19.8%), followed by Streptococci species (n=l 1, 13.6%). The positive yield in our cohort was 62.4% (n=63). A polymicrobial growth was found in 19% (n=12) of the culture-positive specimens. Of all the micro-organisms cultured, 59.2% (n=48) were Gram-positive, v. 35.8% (n=29) Gram-negative. The remainder were fungal and anaerobic organisms, at 2.5% (n=2) each. Gram-positive cultures displayed 100% sensitivity to vancomycin and linezolid, whereas Gram-negative organisms displayed 82% sensitivity towards gentamicin and 89% sensitivity towards meropenem, respectively
CONCLUSION: Our study identifies the bacteriology of periprosthetic joint infections and their sensitivities in a SA setting. We recommend that empiric antibiotic-loaded cement spacers and systemic antibiotic regimens should consist of meropenem or gentamicin, or vancomycin and rifampicin to achieve the broadest spectrum of coverage and most likely success in eradicating infection
Primary total joint arthroplasty (TJA) is one of the most common orthopaedic procedures performed worldwide. According to Sloan et al.,[1] total hip arthroplasty (THA) will grow by 71%, to 635 000 procedures per year, whereas total knee arthroplasty (TKA) will grow by 85%, to 1.26 million procedures per year by 2030 in the USA alone. One of the most common complications of TJA, requiring revision surgery, is periprosthetic joint infection (PJI).[2] The incidence of PJI is 1 - 2% in primary and 4% in revision arthroplasties, respectively[3] With the increase in TJA procedures being performed worldwide, there will also be the inevitable increase in PJI.[4] This creates a significant financial burden on global healthcare with the cost for revision arthroplasty being up to 76% higher than for primary TJA.[5] Klouche et al.[6] demonstrated that the cost of revision for infection is 2.57 times higher than the cost of revision for non-infective causes. There is also a five-fold increase in mortality in revision procedures for PJI v. revision procedures for aseptic failures.[7] The 5-year survival rate for PJI is lower than that of female breast cancer.[8,9] Helwig et al.[10] have shown that subjective quality of life in patients following PJI is significantly reduced.
Current operative methods for treating PJI include debridement, antibiotics and implant retention (DAIR) for acute and acute delayed PJI, whereas chronic PJI is most commonly treated with either a single-stage revision procedure, or the gold standard two-stage revision procedure.[11]
According to the Infectious Diseases Society of America (IDSA), the medical treatment following DAIR, one-stage revision, two-stage revision or resection arthroplasty entails the initiation of intravenous (IV) broad-spectrum antibiotics if the organism and anti-microbial sensitivities are not known. Once the causative organism and antimicrobial sensitivities are known, the treatment can be adjusted accordingly. For staphylococcal PJI, the recommended treatment is 2-6 weeks of IV antibiotics in combination with oral rifampicin twice daily[11] The duration of antibiotic therapy is, however, controversial. A recent article by Bernard et al[12] showed that 12 weeks' duration of antibiotic therapy was superior to 6 weeks' duration.
After completion of systemic therapy, antibiotics are stopped for 2 weeks, which is also commonly known as an antibiotic holiday whereafter serological markers of inflammation and nutrition are obtained (erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and serum albumin). In the event that these markers have normalised, the second stage can usually be performed by inserting a new cemented prosthesis.[13] IDSA recommends that a suitable oral antibiotic, such as ciprofloxacin or levofloxacin, in combination with oral rifampicin is then used for an additional 3 months in THA, whereas TKA requires treatment for 6 months. For non-staphylococcal PJI, 4-6 weeks of targeted IV antibiotics or highly bio-available oral antibiotics is recommended.[11]
The chances of successful treatment of PJIs are greatly increased when the causative organism is correctly identified and treated with the appropriate antibiotics. However, in 2 - 36% of cases, the causative organism cannot be identified.[14] Culture-negative PJI is defined as a PJI, according to the Musculoskeletal Infection Society criteria, where no organism has been cultured. Hersh et al.[15] performed an observational study on culture-negative PJIs treated with irrigation and debridement (I&D). Failure of treatment was defined as the need for any subsequent surgery or a positive culture within 2 years of the initial I&D. Of these failures, 53.33% became culture positive. Staphylococcus species were the causative organisms in 62.5% of all these cases.[16] When the organism is unknown, a typical empiric IV antibiotic regimen consists of carbapenem and vancomycin.[17,18] This broad-spectrum regimen is aimed at effective coverage of resistant organisms.
The most common antibiotics used in antibiotic-loaded cement (ALC) spacers are gentamicin, vancomycin, tobramycin and clindamycin.[19] These antibiotics comply with the prerequisites of an antibiotic to be used in an ALC spacer: it must be heat stable, hydrophilic, bactericidal and have high elusion rates from polymethyl methacrylate (PMMA) that is maintained above the minimum inhibitory concentration, and must be available in powder form. Furthermore, it must be safe at high tissue concentrations and have a broad spectrum of antimicrobial coverage, or be effective against the most likely organisms involved.[20] A typical broad-spectrum mixture can consist of 3 g of vancomycin and 2 g of gentamicin added to 40 g of Palacos cement.[13] Examples of commercially available ALC are Copal G+C and Copal G+V from Heraeus Medical, which consists of 40 g of PMMA bone cement mixed with 1 g of gentamicin and 1 g of clindamycin, or 0.5 g of gentamycin and 2 g vancomicin, respectively.
Until now, the epidemiology of PJI in South Africa (SA) has not been studied. Local treatment guidelines are derived from international literature, and it is unknown if the local microbiological aetiology of PJI is similar to those of the international community. The aim of this study was to determine the characteristics of PJI in an SA clinical setting by identifying the most common micro-organisms cultured, their antibiotic sensitivities as well as the appropriate use of ALC spacers and intravenous antibiotic therapy. In the case of two-stage procedures, we aimed to compare the organisms cultured during the first stage v. the second stage, and also the bacterial culture during the second stage with the ESR/CRP result.
Methodology
We performed a retrospective cross-sectional study of all adult patients (>18 years of age) treated surgically for PJI at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) orthopaedic unit and a private revision arthroplasty practice (Mediclinic Sandton), from 1 January 2015 to 31 March 2020.
Patients treated for infections not related to joint arthroplasty, and patients in whom the organisms cultured were described on the microbiology report as likely being a contaminant, were excluded from the study.
Ethics approval was obtained from the University of the Witwatersrand Human Research Ethics Committee (Medical) for the Johannesburg Orthopaedic hip and knee databank, as well as the CMJAH hip and knee arthroplasty databanks, (ref. no. M200838)
Furthermore, permission was obtained from the chief executive officers of CMJAH and Mediclinic Sandton, respectively, to conduct research at these facilities.
Data were collected from the CMJAH hip and knee arthroplasty databanks, as well as the Johannesburg orthopaedic hip and knee databank. Only data from patients who were diagnosed with PJI were collected. These data included the patients' personal details such as age, gender, type of surgery and stage. The patients' microbiology results, antibiotic sensitivities, as well as their CRP and ESR results were collected from the aforementioned databanks. The data were then captured in an Excel (Microsoft, USA) spreadsheet for comparison and statistical analysis. All patients were assigned to unique participant numbers to maintain confidentiality and anonymity.
The data were then transferred to the Stata version 14.0 statistics software package (Stata Corp., USA), which was used for data cleaning and analysis.
Descriptive statistics were used to analyse the demographic profile of the participants, common organisms and sensitivities of organisms. These were reported as percentages and frequency.
Inferential statistics was carried out using Pearson's χ- test for the following: to compare the number of organisms cultured in a public hospital to those cultured in a private hospital; to compare the organisms cultured during the first stage v. the second stage in cases of two-stage revisions; and to correlate bacterial culture during the second stage with the ESR and CRP result. P<0.05 was considered significant.
Results
Within our study period, 69 patients met the inclusion criteria: 40 females and 29 males. A combined total of 101 surgical procedures were performed for PJI, of which 65 were related to knees and 36 to hips (Fig. 1).
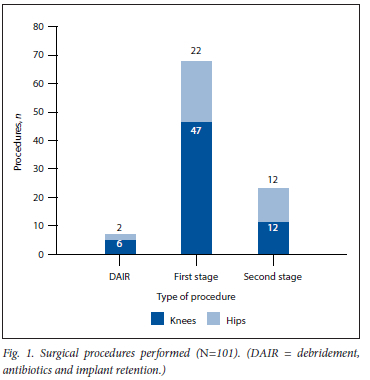
Eight patients underwent a DAIR revision procedure, while 93 staged revision procedures were done. All staged revision procedures were part of a two-stage technique, and no single-stage revisions were done. Of these two-stage procedures, 69 were first-stage and only 24 were second-stage procedures (Fig. 1). Six patients had one or more repeat first-stage procedures. Only 19 patients completed both their first- and second-stage revision procedures at our institutions during the specified study period.
Of the 101 procedures, 63 had a positive bacterial growth on microscopy, culture and sensitivity (MC+S) and 38 had a negative growth, making an overall positive culture yield in our cohort of 62.4%. The majority of the positive bacterial cultures were from first-stage revision procedures (n=48), while second-stage revision procedures yielded 11 positive cultures. DAIR procedures yielded 4 positive cultures. The culture-positive yield for first- and second-stage revision procedures were 69.6% and 45.8%, respectively. DAIR procedures demonstrated a 50% positive yield (n=4). A total of 81 organisms were cultured from the 63 culture-positive specimens (Table 1).
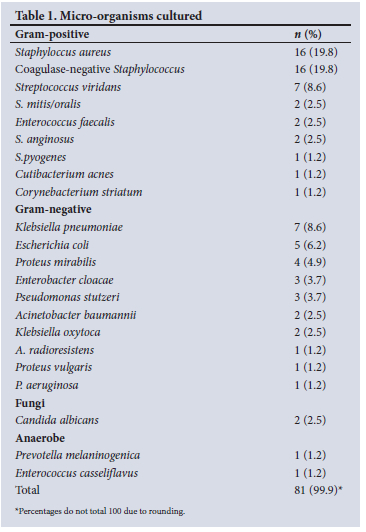
Gram-positive organisms were found in 59.2% (n=48) of cultures, v.35.8% (n=29) Gram-negative. The remainder were fungal and anaerobic organisms at 2.5% (n=2) each (Fig. 2).
Overall, the most common organisms cultured were Staphylococcus aureus (n=16, 19.8%) and coagulase-negative Staphylococcus (CoNS) (n=16, 19.8%), followed by Streptococci species (n=12, 14.8%).
Of the Gram-negative organisms cultured, Klebsiella pneumoniae was the most prevalent, and represented 9% (n=7) of all cultures.
Twelve of the samples yielded a polymicrobial growth (19%). As shown in Table 2, more polymicrobial growth was found in the private sector (n=10) than the public sector (n=2; p=0.024). Further logistic regression analysis showed that samples from the private sector were six times more likely to yield a polymicrobial growth compared with the samples from the public sector (odds ratio 6.1, p=0.028, 95% confidence interval 1.2 - 30.6).
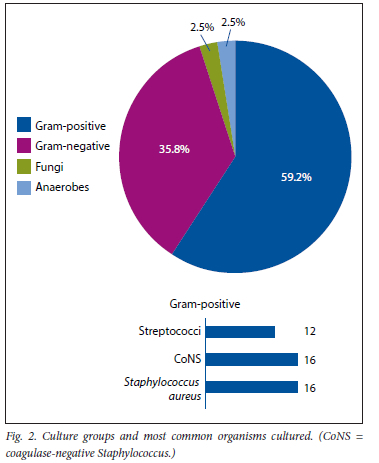
Of the 29 Gram-negative organisms cultured, only 11 were tested for gentamicin sensitivity, of which 81.8% (n=9) were sensitive and 18.2% (n=2) were resistant. Amikacin had a similar pattern, with 81.3% sensitivity among Gram-negative organisms tested (Table 3, Fig. 3). Tobramycin sensitivity was only reported in one case, which was a multidrug resistant strain of Acinetobacter baumannii, and only sensitive to Colistin. Notably, of the samples tested for ciprofloxacin sensitivity, a mere 47.4% were sensitive in the Gram-negative cohort. Meropenem proved to be the most efficacious antibiotic towards Gram-negative organisms, with a sensitivity of 88.9% (Table 3, Fig. 3). Again, the two organisms that displayed resistance to meropenem were two multidrug resistant Λ. baumannii strains.
When comparing organisms cultured during the first stage v. the second stage of the two-stage procedures, there was no statistically significant difference (Table 5).
Of the 19 patients who completed both stages of their two-stage revision procedures during the specified study period, 8 yielded a culture-positive specimen during the second stage. Six of these 8 patients cultured different micro-organisms during the first stage v. the second stage, with the remaining two patients showing recurrent growth of the same micro-organism.
We also found no correlation between the ESR/CRP level and the organism cultured during the second stage (p= 1.000).
Of the 11 culture-positive second stage procedures, eight CRP results and five ESR results were available (Table 6). The CRP result was abnormal (>10 mg/L) in 87.5% (n=7) of these patients, and the ESR result abnormal (>30 mm/hr) in 80% (n=4).
Discussion
Multiple international studies have established the commonly isolated organisms in PJL Throughout these studies, S. aureus was the most prevalent organism, followed by CoNS and Streptococci[17,20-23] In keeping with the international literature, we have found the Gram-positive organisms (S. aureus, CoNS and Streptococci species) to be the most prevalent, followed by the Gram-negative K. pneumoniae.
We found a 2.5% incidence of fungal growth, with Candida albicans being the only fungus cultured (n=2). Internationally, the incidence is reported to be between 1 and 3%, with C. albicans also being the most common.[24-27] Although rare, there is an increase in fungal PJI worldwide, and these infections are particularly difficult to treat, with high failure rates.[28] This is partly due to the fact that fungi form a very complex biofilm, and also because these patients are usually immunocompromised.[29]
Antimicrobial resistance is of great concern in PJI and the medical fraternity as a whole.[30] Internationally, there is a rise in antibiotic resistance, which may require a change in the choice of antibiotics used.[31,32] This change in empiric antibiotic therapy should, however be made with antibiotic stewardship in mind.[33] The incidence of methicillin resistance varies greatly in the literature.[20-23] In a study by Peel et al[22] conducted in Australia, almost half of the S. aureus isolates were methicillin resistant, which was in keeping with a US study done by Pulido et al[21] Benito et al.[23] found a 28% methicillin resistance among S. aureus PJL Another study conducted by Moran et al[17] in the UK had just over 15% incidence of methicillin resistance among their S. aureus isolates. This is also in keeping with our findings of 18.8% methicillin resistance. None of the Gram-positive isolates showed resistance to vancomycin.
With regard to Gram-negative organisms, gentamicin sensitivity was 81.8%. Interestingly, we observed a significant resistance towards ciprofloxacin by Gram-negative organisms, of 47.4%. This might be a significant finding, as ciprofloxacin is a commonly used antibiotic for enteral continuation therapy in PJI due to its favourable reduction in biofilm production.[34] Meropenem had the best sensitivity profile against Gram-negative organisms, at 88.9%. Meropenem is a suitable antibiotic for use in ALC spacers because it comes in powder form, is heat stable and has good elusion characteristics.[35,36] Meropenem might thus be the antibiotic of choice for empiric Gram-negative coverage.
An increased ESR/CRP result at the time of the second-stage revision procedure has been associated with an increased reinfection rate.[37] A normal ESR/CRP result, however, does not always exclude PJI.[38] We therefore tried to determine whether certain organisms were more likely to be cultured with a normal ESR/CRP during the second stage of the two-stage revision procedures. There was, however, no relationship found. Despite this, we still support and recommend a 2-week antibiotic holiday, followed by a repeat joint aspiration and tissue biopsy prior to commencing the second stage.
Furthermore, in cases of two-stage revision procedures, there was also no statistically significant difference between organisms cultured during the first stage v. organisms cultured during the second stage.
The culture-negative PJI rate in our study was 37.6%, which is slightly higher than the expected range reported in the literature of 2 - 36%.[14] The high culture-negative rate could possibly be due to an inadequate number of specimens, the use of a suboptimal culture medium in specimens taken for MC+S during surgery or antibiotic therapy by referring physicians prior to sampling. It is recommended that at least three, but ideally five to six, tissue samples be taken during surgery for MC+S to increase the chances of a positive yield.[11] We would like to emphasise the importance of this to increase the culture-positive yield.
When looking at the positive culture yield during second-stage procedures, it was found to be 45.8% (n=ll), which is much higher than we expected, and than the 12 - 25% incidence reported in the literature.[39-41] There could be many contributing factors to this finding. One reason could be that the organisms cultured during the second stage were skin contaminants, i.e. S. aureus, which was the most common organism cultured in our cohort. Another possible reason could be that the infection was not completely eradicated by the time of re-implantation. This was, however, not the case in our study as there was no statistically significant correlation between organisms cultured during the first v. the second stage procedures (Table 5).
With our study being multicentred in order to present the whole demographic of our area, samples were sent to private as well as government laboratories. When comparing the samples cultured in the different laboratories, samples cultured in private laboratories were six times more likely to result in a polymicrobial growth than organisms cultured in the government laboratory. The reasons for this discrepancy are unknown and will need further investigation and research; however, we postulate that this finding could possibly be due to a shorter incubation time in the government laboratories due to systemic constraints, whereas longer incubation times are common practice in private laboratories. Differences in sampling protocols between institutions, i.e. the number of samples taken for MC+S and the culture medium in which samples are sent to the laboratory may, once again, explain this finding. We did not find an increase in polymicrobial growth among samples that underwent sonication in the laboratory v. those that did not.
According to our knowledge, this is the first study on the bacteriology and the characteristics of PJI in SA. The study, however has a few limitations. One of the limitations of our study was the small sample size, which might compromise statistical significance. One such example is the resistance profile of meropenem. Only one organism was resistant to meropenem, but this represented 11% of all Gram-negative organisms tested for meropenem sensitivity. Furthermore, this organism was a multidrug-resistant A. baumannii strain, sensitive to only Colistin. Thus, the efficacy of meropenem might in reality be much higher than the reported 89%. Despite the small sample size, however, our findings were still very similar to international studies with much larger sample sizes. Another limitation was the low number of patients who completed both stages of their two-stage revision procedures during the study period (n=19, 27.5%). This might be attributed to patients lost to follow-up or receiving their second-stage procedure at a different institution, the cross-sectional nature of the study and the incomplete capturing of data in our data banks.
Owing to these limitations, we believe that there is definitely a need for future research on PJI and antibiotic sensitivity in SA with larger sample sizes. We recommend that laboratories adopt a standard set of antibiotics to test sensitivities of PJI organisms against, as we have found that many organisms were not tested for sensitivity against the most commonly used antibiotics in ALC spacers.[19] One such example is tobramycin, where only one Gram-negative organism was tested against, out of a possible 29. We further recommend that ceftazidime/avibactam linezolid, tigecycline and rifampicin be tested in addition to the standard battery of antibiotics for PJL
Conclusion
According to our results and findings, we recommend that empiric ALC spacers and empiric IV antibiotic regimens should consist of meropenem or gentamicin, vancomycin and rifampicin to achieve the broadest spectrum coverage and most likely success in eradicating infection. We believe that knowing the bacteriology profiles in your demographic area is of utmost importance because of the high negative-yield rate from culture specimens in PJI, in which case empiric antibiotic strategies are implemented.
Declaration. This article has been submitted and passed for JSHs degree of Master of Medicine in the branch of Orthopaedic Surgery at the University of the Witwatersrand, Johannesburg.
Acknowledgements. We would like to thank Kaeri van der Jagt for her help in the retrieval of data from the Johannesburg Orthopaedic hip and knee databanks.
Author contributions. JSH: substantial contributions to the conception and design of the work, and the acquisition, analysis and interpretation of data for the work, drafting the work and revising it critically for important intellectual content. ARS and ALJduT: substantial contributions to the conception and design of the work, and the acquisition, analysis and interpretation of data for the work and revising it critically for important intellectual content. DRvdJ: Substantial contributions to study conceptualisation and design and data collection and contribution. HW and CAH contributed equally.
Funding. None.
Conflicts of interest. None.
References
1. Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the US, 2014 to 2030. J Bone Joint Surg Am 2018;100(17):1455-1460. https://doi.org/10.2106/JBJS.17.01617 [ Links ]
2. National Joint Registry. 2018 15th Annual Report UK. NJR 15th Annual Report 2018. www.njrreports.org.uk (accessed 25 February 2020). [ Links ]
3. Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection. Current concepts and outlook EFORT Open Rev 2019;4(7):482-494. https://doi.org/10.1302/2058-5241.4.180092 [ Links ]
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89(4):780-785. https://doi.org/10.2106/JBJS.F00222 [ Links ]
5. Weber M, Renkawitz T, Voellner F, et al. Revision surgery in total joint replacement is cost-intensive. Biomed Res Int 2018;2018:8987104. https://doi.org/10.1155/2018/8987104 [ Links ]
6. Kiouche S, Sariali E, Mamoudy P. [Analyse du cout des reprises des protheses totales de hanche infectées]. Revue de Chirurgie Orthopedique et Traum at ologique 2010;96(2):167-175. https://doi.org/10.1016/j.rcot2010.02.005 [ Links ]
7. Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am 2013;95(24):2177-2184. https://doi.org/10.2106/JBJS.L.00789 [ Links ]
8. National Cancer Institute. SEER Cancer Stat Facts. Female breast cancer. Bethesda. National Cancer Institute, 2020. https://seer.cancer.gov/statfacts/html/breast.html (accessed 25 February 2020). [ Links ]
9. Kurtz SM, Lau EC, Son MS, Chang ET, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection. Trendsin periprosthetic joint infection and mortality riskfor the Medicare population. J Arthroplast 2018;33(10):3238-3245. https://doi.org/10.1016/j.arth.2018.05.042 [ Links ]
10. Heiwig P, Morlock J, Oberst M, et al. Periprosthetic joint infection - effect on quality of life. Int Orthop 2014;38(5):1077-1081. https://doi.org/10.1007/s00264-013-2265-y [ Links ]
11. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection. Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56(1):e1-e25. https://doi.org/10.1093/cid/cis803 [ Links ]
12. Bernard L, Arvieux C, Brunschweiler B, et al. Antibiotic therapy for 6 or 12 weeks for prosthetic joint infection. N Engl J Med 2021;384(21):1991-2001. https://doi.org/10.1056/NEJMoa2020198 [ Links ]
13. Haddad FS, Sukeik M, Alazzawi S. Is single-stage revision according to a strict protocol effective in treatment of chronic knee arthroplasty infections? Clin Orthop Relat Res 2015;473(1):8-14. https://doi.org/10.1007/s11999-014-3721-8 [ Links ]
14. Vanhegan IS, Morgan-Jones R, Barrett DS, Haddad FS. Developing a strategy to treat established infection in total knee replacement. A review of the latest evidence and clinical practice. J Bone Joint Surg Br 2012;94(7):875-881.https://doi.org/10.1302/0301-620X94B7.28710. [ Links ]
15. Hersh BL, Shah NB, Rothenberger SD, Zlotnicki JP, Kiatt BA, Urish KL. Do culture negative periprosthetic joint infections remain culture negative? J Arthroplast 2019;34(11):2757-2762. https://doi.org/10.1016/j.arth.2019.06.050 [ Links ]
16. Qasim SN, Swann A, Ashford R. The DAIR (debridement, antibiotics and implant retention) procedure for infected total knee replacement - a literature review. SICOT J 2017;3.2. https://doi.org/10.1051/sicotj/2016038. [ Links ]
17. Moran E, Masters S, Berendt AR, McLardy-Smith P, Byren I, Atkins BL. Guiding empirical antibiotic therapy in orthopaedics. The microbiology of prosthetic joint infection managed by debridement; irrigation and prosthesis retention. J Infect 2007;55(1):1-7. https://doi.org/10.1016/j.jinf.2007.01.007 [ Links ]
18. Sousa R, Pereira A, Massada M, et al. Empirical antibiotic therapy in prosthetic joint infections. Acta Orthopaedica Belgica 2010;76(2):254-259. [ Links ]
19. Van Vugt TAG, Arts JJ, Geurts JAP. Antibiotic-loaded polymethylmethacrylate beads and spacers in treatment of orthopedic infections and the role of biofilm formation. Front Microbiol 2019;10.1626. https://doi.org/10.3389/fmicb.2019.01626 [ Links ]
20. Carrega G, Bartolacci V, Burastero G, et al. Etiology of prosthetic joint infections in a tertiary care centre in Italy. Infez Med 2008;16(4):204-208. [ Links ]
21. Pulido L, Ghanem E, Joshi A, Purtili JJ, Parvizi J. Periprosthetic joint infection. The incidence, timing and predisposing factors. Clin Orthop Relat Res 2008;466(7):1710-1715. https://doi.org/10.1007/s11999-008-0209-4 [ Links ]
22. Peel TN, Cheng AC, Choong PF, Buising KL. Early onset prosthetic hip and knee joint infection. Treatment and outcomes in Victoria, Australia. J Hosp Infect 2012;82(4):248-253. https://doi.org/10.1016/j.jhin.2012.09.005 [ Links ]
23. Benito N, Franco M, Ribera A, et al. Time trends in the aetiology of prosthetic joint infections. A multicentre cohort study. Clin Microbiol Infect 2016;22(8):732.el-8. https://doi.org/10.1016/j.cmi.2016.05.004 [ Links ]
24. Azzam K, Parvizi J, Jungkind D, et aL Microbiological, clinical, and surgical features of fungal prosthetic joint infections. A muiti-institutional experience. J Bone Joint Surg Am 2009;91(Suppl 6):S142-S149. https://doi.org/10.2106/JBJS.I.00574 [ Links ]
25. Kuo FC, Goswami K, Shohat N, Blevins K, Rondon AJ, Parvizi J. Two-stage exchange arthroplasty is a favorable treatment option upon diagnosis of a fungal periprosthetic joint infection. J Arthroplast 2018;33(11):3555-3560. https://doi.org/10.1016/j.arth.2018.07.024 [ Links ]
26. Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res 2013;471(9):3002-3009. https://doi.org/10.1007/si1999-013-3007-6 [ Links ]
27. Phelan DM, Osmon DR, Keating MR, Hanssen AD. Delayed reimplantation arthroplasty for candidal prosthetic joint infection. A report of 4 cases and review of the literature. Clin Infect Dis 2002;34(7):930-938. https://doi.org/10.1086/339212 [ Links ]
28. Gross CE, Delia Valie CJ, Rex JC, Traven SA, Durante EC. Fungal periprosthetic joint infection. A review of demographics and management. J Arthroplast 2021;36(5):1758-1764. https://doi.org/10.1016/j.arth.2020.11.005 [ Links ]
29. Nace J, Siddiqi A, Taimo CT, Chen AF. Diagnosis and management of fungal periprosthetic joint infections. J Am Acad Orthop Surg 2019;27(18):e804-e818. https://doi.org/10.5435/JAAOS-D-18-0033] [ Links ]
30. Fulkerson E, Valie CJ, Wise B, Walsh M, Preston C, Di Cesare PE. Antibiotic susceptibility of bacteria infecting total joint arthroplasty sites. J Bone Joint Surg Am 2006;88(6):1231-1237. https://doi.org/10.2106/JBJS.E.00004 [ Links ]
31. Ludwick L, Chisari E, Wang J, Clarkson S, Collins L, Parvizi J. Emergence of antibiotic resistance across two-stage revision for periprosthetic joint infection. J Arthroplast 2021;36(8):2946-2950. https://doi.org/10.1016/j.arth.2021.04.007 [ Links ]
32. Isturiz R. Global resistance trends and the potential impact on empirical therapy. Int J Antimicrob Agents 2008;32(Suppl 4):S201-S206.https://doi.org/10.1016/S0924-8579(09)70003-2 [ Links ]
33. Myers TG, Lipof JS, Chen AF, Ricciardi BF. Antibiotic stewardship for total joint arthroplasty in 2020. J Am Acad Orthop Surg 2020;28(18):e793-e802. https://doi.org/10.5435/JAAOS-D-19-00850 [ Links ]
34. Rodriguez-Pardo D, Pigrau C, Lora-Tamayo J, et al. Gram-negative prosthetic joint infection, outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin Microbiol Infect 2014;20(11):0911-0919. https://doi.org/10.1111/1469-0691.12649 [ Links ]
35. Báez LA, Längsten C, Givaruangsawat S, McLaughlin R. Evaluation of in vitro serial antibiotic elution from meropenem-impregnated polymethylmethacrylate beads after ethylene oxide gas and autoclave sterilization. Vet Comp Orthop Traumatol 2011;24(1):39-44. https://doi.org/10.3415/VCOT-10-05-0070 [ Links ]
36. Schmid M, Steiner O, Fasshold L, Goessler W, Holl AM, Kühn KD. The stability of carbapenems before and after admixture to PMMA-cement used for replacement surgery caused by Gram-negative bacteria. Eur J Med Res 2020;25(1):34. https://doi.org/10.1186/s40001-020-00428-z [ Links ]
37. Klemt C, Padmanabha A, Esposito JG, Laurencin S, Smith EJ, Kwon YM. Elevated ESR and CRP prior tc second-stage reimplantation knee revision surgery for periprosthetic joint infection are associated with increased reinfection rates. J Knee Surg 2023;36(4):354-361. https://doi.org/10.1055/s-0041-1733902 [ Links ]
38. Pérez-Prieto D, Portilio ME, Puig-Verdié L, et al. C-reactive protein may misdiagnose prosthetic ioint infections, particularly chronic and low-grade infections. Int Orthop 2017;41(7):1315-1319. https://doi.org/10.1007/s00264-017-3430-5 [ Links ]
39. Theii C, Freudenberg SC, Gosheger G, Schmidt-Braekling T, Schwarze J, Moelienbeck B. Do positive cultures at second stage re-implantation increase the risk for reinfection in two-stage exchange for periprosthetic joint infection? J Arthroplast 2020;35(10):2996-3001. https://doi.org/10.1016/j.arth.2020.05.029 [ Links ]
40. Akgün D, Müller M, Perka C, Winkler T. A positive bacterial culture during re-implantation is associated with a poor outcome in two-stage exchange arthroplasty for deep infection. Bone Joint J 2017;99-B(11):1490-1495. https://doi.org/10.1302/0301-620X.99B11.BJJ-2017-0243-R1 [ Links ]
41. Tan TL, Gomez MM, Manrique J, Parvizi J, Chen AF. Positive culture during reimplantation increases the risk of subsequent failure in two-stage exchange arthroplasty. J Bone Joint Surg Am 2016;98(15).1313-1319. https://doi.org/10.2106/JBJS.15.01469 [ Links ]
 Correspondence:
Correspondence:
J S Hiddema
jshiddema@gmail.com
Accepted 22 March 2023
† Deceased














