Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.6 Pretoria Jun. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i5.16522
RESEARCH
Profile of adverse drug reaction reports in South Africa: An analysis of VigiBase for the year 2017
M F MatlalaI, II; M S LubbeIII; H SteynI
IBPharm, MPharm; Medicine Usage in South Africa (MUSA), Faculty of Health Sciences, North-West University, Potchefstroom, South Africa
IIBPharm, MPharm; Pharmacovigilance Unit, South African Health Products Regulatory Authority, Pretoria, South Africa
IIIBPharm, PhD; Medicine Usage in South Africa (MUSA), Faculty of Health Sciences, North-West University, Potchefstroom, South Africa
ABSTRACT
BACKGROUND: The South African Health Products Regulatory Authority (SAHPRA) monitors the safety of health products by collecting and evaluating adverse drug reaction (ADR) reports submitted by healthcare professionals, patients and pharmaceutical companies. The reports are shared with the World Health Organization (WHO) Programme for International Drug Monitoring. A demographic and clinical profile of ADR reports will improve our understanding of ADR reporting in SA to enhance training of reporters at all levels
OBJECTIVE: To describe the demographic and clinical profile of spontaneous ADR reports received by SAHPRA during the year 2017
METHODS: A retrospective, cross-sectional study was conducted to describe all ADR reports submitted by SA to VigiBase, the WHO global database of individual case safety reports (ICSRs), during 2017. The demographic profile included patient characteristics (age and sex), type of reporter and the vigiGrade completeness score for each ICSR. The clinical profile included characteristics of the case, medicine(s) and reaction(s
RESULTS: A total of 8 438 reports with a mean completeness score of 0.456 (standard deviation 0.221) were assessed. Females and males represented 61.96% and 33.05% of cases, respectively (if sex was reported). All age groups were represented; however, 76.28% involved adults (aged 19 - 64 years). Physicians submitted the most reports (39.66%). Consumers were the reporters in 29.39% of cases. Pharmacists submitted only 4.45% of the reports. Anti-infective medicines were the most reported anatomical therapeutic class (20.08%), while HIV was the top indication reported (10.27%). The highest number of Medical Dictionary for Regulatory Activities (MedDRA) preferred terms used to describe reactions belonged to the system organ class, general disorder and administration site conditions. In 55.87% of the reports, the cases were reported as serious, and 12.47% were fatal. Death was the most reported MedDRA preferred term used to describe a reaction (5.17%
CONCLUSION: This was the first study that described ADR reports received by SAHPRA, and improves our understanding of reporting in the country. The core clinical elements that are important in signal detection were often not included in reports. The findings demonstrated that patients were more active contributors to the national pharmacovigilance database than pharmacists. Reporters should be trained in pharmacovigilance and ADR reporting processes to increase the quantity and completeness of reports
Medicines have modified how diseases are treated, prolonging and improving the quality of life. However, medicines are associated with adverse effects that may be detrimental to the patient and the health system at large.[1] This is despite clinical trials being conducted to ensure their safety and efficacy before marketing authorisation.[2] These studies take place in a highly controlled environment, where small and homogenous populations are monitored for a short period. Clinical trials cannot be powered adequately to detect rare, serious adverse events.[3] Therefore not all adverse effects are detected by the time of marketing authorisation.[4] Rare and very rare ADRs can only be detected when the drug is used by very large populations.[5] Additionally, individual polymorphisms lead to variability in drug metabolism, and can result in various patient responses to medications that may lead to ADRs.[6]
An ADR is defined as any response to a specific medicine that is unpleasant and unintended and occurs at doses used for prophylaxis, diagnosis or treatment of disease, or the modification of physiological function.[7] Several drugs have been subjected to regulatory decisions owing to ADRs that were only apparent after the drugs were marketed.[8] This highlights the essential role that pharmacovigilance plays in ensuring public health safety.[9] Pharmacovigilance is defined as 'the science and activities relating to the detection, assessment, understanding and prevention of adverse effects, or any other possible drug-related problems'.[10] Pharmacovigilance was established[11] to protect the public from the harmful effects of medicines following several tragic events around the globe, including the thalidomide tragedy.[12]
As part of pharmacovigilance, post-marketing surveillance uses different methods to generate complete safety and efficacy data regarding the profile of drugs.[13] These methods include a spontaneous reporting system (SRS), aggregate population-based data sources and computerised data collection from organised medical care programmes and post-marketing studies.[14] However, the SRS is most widely used.[10] It is also the primary source through which serious and unknown ADRs are detected,[15] and is often used to make regulatory decisions about marketed drugs. This method is also valuable for detecting delayed and rare ADRs, since it can be used to monitor all drugs throughout their lifetime.[16-19]
In 1987, an SRS was established in SA through the National Adverse Drug Events Monitoring Centre (NADEMC). This function was taken over by the National Department of Health through the previous Medicines Control Council, and was later adopted by the SA Health Products Regulatory Authority (SAHPRA). The critical role of the SRS is to monitor the safety profiles of medicines available in the country, and ensure they have a positive benefit-risk balance. The key activity of the SRS is the collection and evaluation of ADR reports submitted by healthcare professionals, patients and pharmaceutical companies.[20] The reports are shared with the Uppsala Monitoring Centre (UMC), the World Health Organization (WHO) Collaborating Centre for International Drug Monitoring, via VigiBase, the WHO global database of individual case safety reports (ICSRs).[21]
The minimum information required for a valid ADR report includes a suspected drug, a suspected reaction, a patient and an identifiable reporter.[22] Spontaneous ADR reporting in SA is based on the WHO recommended standard structured yellow form. Other forms include programmatic tuberculosis-HIV (TB-HIV) and the Council for International Organizations of Medical Sciences (CIOMS) forms. Pharmaceutical companies can also make use of direct reporting via E2B (XML) format. A separate case reporting form for adverse events following immunisation (AEFI) is used for vaccines. Case investigations are done for all severe AEFIs (serious and non-serious) during which a case investigation form is completed.
These forms are available in electronic format from the SAHPRA website.[23,24] During 2017, the Essential Medicines List (EML) clinical guide application (app) also had an attached ADR reporting module. Since April 2021, healthcare professionals and consumers can submit ADR and AEFI reports through the Med Safety App.[25] All reports received by SAHPRA are captured in VigiFlow, a web-based pharmacovigilance management system that was developed for national regulatory agencies to strengthen post-marketing surveillance. VigiFlow provides secure, controlled and easy sharing of adverse event reports to WHO through VigiBase.[26]
Several studies[27-32] have analysed national pharmacovigilance databases for different countries to describe the characteristics of ADR reports, but none has been done in SA to date. The present study is the first to describe the demographic and clinical profile of ADR reports in SA, and will improve our understanding of ADR reporting in the country and enhance training of reporters at all levels. Sharing the profile of reports received by SAHPRA with the public and healthcare professionals may improve awareness of ADRs to improve pharmacovigilance and reporting to ultimately increase patient safety.
Objective
To describe the demographic and clinical profile of ADRs reported in SA during 2017 by analysing the national ADR database of reports sent to VigiBase.
Methods
Study setting and design
Data were obtained from the SAHPRA Pharmacovigilance Unit, which maintains the central repository for all spontaneous reports in SA. The study was a retrospective, descriptive, cross-sectional analysis of spontaneous ADR reports submitted to VigiBase by SA from 1 January 2017 to 31 December 2017.
Data source
All ICSRs from SA sent to VigiBase for 2017 were extracted on 11 June 2019 (dataset date: 2019-06-09) using its search and analysis software known as VigiLyze. VigiLyze exports contain complete information for each case report, including the medications and reactions.
The study variables included in the analysis are listed in Table 1. The demographic profile was described according to the patient's characteristics (age and sex), type of reporter and vigiGrade completeness score.[33] With the exception of consumers/ non-healthcare professionals, the type of reporter is based on the qualification of the primary reporter, e.g. physician, pharmacist, other healthcare professional or lawyer. The data exported from VigiBase do not contain the type of sender (e.g. pharmaceutical company, healthcare professional or regional pharmacovigilance centre). Pharmaceutical companies submit the reports as received from healthcare providers and consumers on their behalf.
The vigiGrade completeness score ranges from 0.07 to 1, and is a measure of the amount of clinically relevant information in an ICSR as it appears in VigiBase, and reports with a completeness score >0.8 are considered well documented.[33]
The clinical profile includes characteristics of the case, medicine(s) and the reaction(s). Medicines were classified according to the WHODrug Dictionary[34] and anatomical therapeutic chemical (ATC) classification system.[35] The ADRs were classified according to the Medical Dictionary for Regulatory Activities' (MedDRA) preferred term and system organ class (SOC).[36]
There are five levels to the MedDRA hierarchy, arranged from very specific to very general. The most detailed level is called 'lowest level terms' (LLTs), and it reflects how an observation might be reported in practice. The next level, called 'preferred terms' (PTs), is a definite descriptor for a symptom, sign, disease diagnosis, therapeutic indication, investigation, surgical or medical procedure, and medical social or family history characteristic. Each LLT is linked to only one PT. Related PTs are grouped into 'high-level terms' (HLTs) based upon anatomy, pathology, physiology, aetiology or function. HLTs, related to each other by anatomy, pathology, physiology, aetiology or function, are linked to 'high-level group terms' (HLGTs), grouped into SOCs by aetiology, manifestation site or purpose.[36]
All reports that are received by the Pharmacovigilance Unit of SAHPRA are checked by technical staff (pharmacists) to ensure that all the information in the report is coded correctly according to MedDRA. They do a quality check to ensure that the paper-based/ E2B/XML reports were translated correctly into the VigiFlow system. The technical staff also confirm whether the reported reaction is expected and included in the package insert of the product. Reports in VigiFlow are committed to VigiBase, whether causality was determined by the country or not.
Ethical approval
The study was approved by the North-West University Health Research Ethics Committee (ref. no. NWU-00012-19-S1). Goodwill permission was also obtained from the interim/acting executive officer of SAHPRA.
Data analysis
The de-duplicated MedDRA version 22.0 coded data were exported onto an Excel package (Microsoft Corp., USA). Data were analysed using the Statistical Package for Social Sciences version 25 (IBM Corp., USA). Descriptive statistics, including frequencies (n) and proportions presented as percentages (%), arithmetic means and standard deviations (SDs), were used to summarise the demographic and clinical profile of ICSRs.
Results
A total of 8 438 unique ICSRs were extracted from VigiBase. The 8 438 cases contained 29 826 drug-event pairs, of which 20 438 (68.52%) were for suspect medicines. Concomitant and interacting medicines represented 31.25% (n=9 315) and 0.06% (n=18) of the drug-event pairs, respectively. The role of the medicine was not indicated for 0.18% (n=55) of the drug-event pairs.
Demographic profile
The majority of cases (61.96%, n=5 228) involved female patients, while 33.05% (n=2 789) involved males. Sex was not indicated on the remaining 4.99% (n=421) of the reports. The patients' age ranged between 0 and 99 (mean 47.18, SD 19.98) years. The highest number of reports were submitted for adult patients aged between 19 and 64 years (76.28%, n=4 837). The number and percentage of the reports per age group are provided in Table 2. The weight and height of the patient were only reported for 20.79% (n=1 754) and 10.27% (n=867) of the cases, respectively.
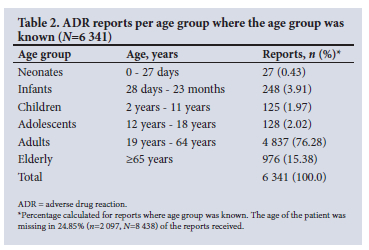
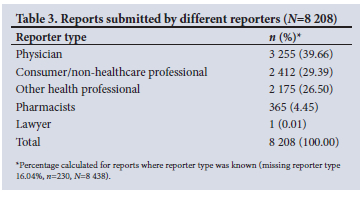
Type of reporter (Table 3) was indicated in 8 208 (97.27%) of all reports (N=8 438) received. Physicians submitted the highest number of reports (39.66%, n=3 255), while the smallest portion of reports came from pharmacists (4.45%, n=365).
The mean completeness score for the 8 438 reports received was 0.456 (SD 0.221). Only 11.29% (n=953) of reports had a completeness score >0.8 and are considered well documented.
Clinical profile
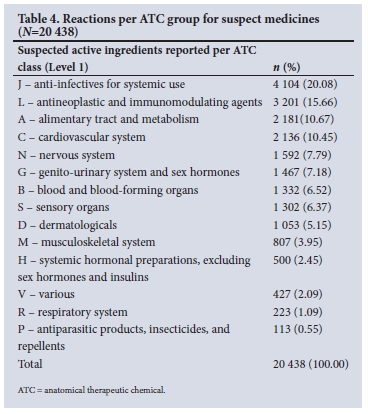
There were 797 different WHODrug active ingredients listed on ADR reports, of which 644 were the suspect drug in 20 438 drug-event pairs. The remaining 153 active ingredients were either reported as concomitant or interacting medicines. The suspect medicines belonged to all 14 ATC groups (Table 4), with anti-infectives for systemic use reported most frequently at 20.08% (n=4 104), followed by antineoplastic and immunomodulating agents (15.66%, n=3 201).
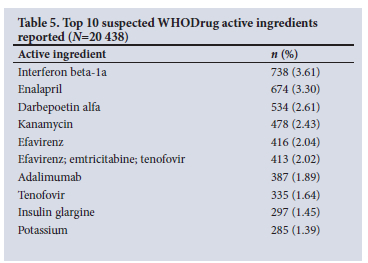
Tables 5 - 7 show the frequencies of the top 10 WHODrug active ingredients, indications and MedDRA preferred reaction terms. Interferon beta-1a was the active ingredient suspected to be the causative agent for the highest number of reactions at a frequency of 738 (3.61%), followed by enalapril (3.30%, n=674) and darbepoetin alfa (2.61%, n=534). The clinical indication for each suspect drug was indicated in 84% (n=17 160) of drug-event pairs. HIV was the most prevalent indication, reported in 1 763 (10.27%) cases, followed by hypertension (7.48%, n=1 284). Death was the most reported MedDRA preferred term in 1 056 (5.17%) of the drug-event pairs.
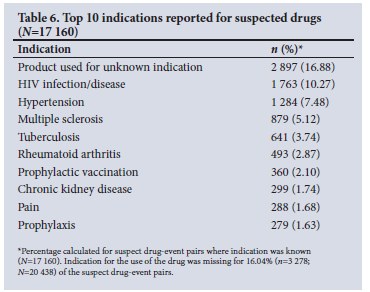
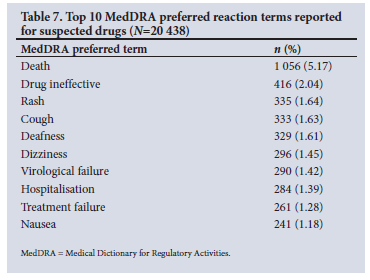
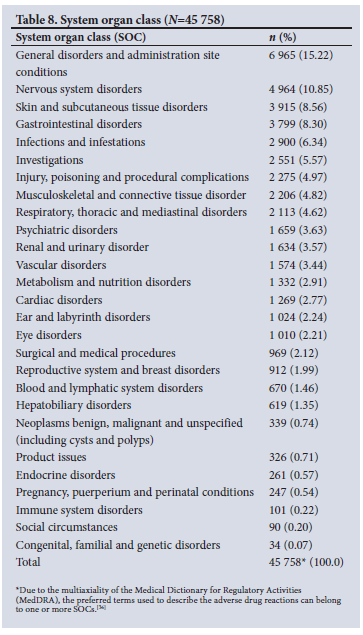
The highest number of MedDRA preferred terms used to describe ADRs belonged to the SOCs 'general disorders and administration site conditions' (15.22%, n=6 965), 'nervous system disorders' (10.85%, n=4 964) and 'skin and subcutaneous tissue disorders' (8.56%, n=3 915) (Table 8).
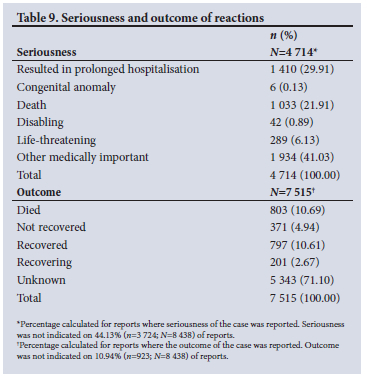
The cases that were reported as fatal and non-fatal represented 12.47% (n=1 052) and 87.53% (n=7 386) of 8 438 cases, respectively. According to CIOMS criteria, 55.87% (n=4 714) of the reported cases were classified as serious.[37] The outcome of the ADR was indicated on 7 515 (89.1%) reports. The seriousness criteria and outcome of the reaction can be viewed in Table 9.
The dose was reported for 49.67% (n=14 815) of drug-event pairs. In comparison, the temporal relationship between the start date of the suspected medicine and the onset date of the reaction was only reported for 47.18% (n=9 643) of events. The action taken with the drug was reported for 17 363 (84.95%) of the suspected drug-event pairs. The suspect drug was withdrawn in 40.50% (n=7 032) of the events for which action taken was reported. The dose was not changed in 8.03% (n=1 394) of the events, 'reduced' in 31 (0.18%) and 'increased' in 18 (0.10%) events. For the remaining 51.19% (n=8 888) suspected reactions, the action taken with the drug was reported as 'unknown action' or 'action not applicable.'
Discussion
Demographic profile
Age and sex
Our analysis revealed that (76.28%) of ADRs were reported for adults (aged 19 - 64 years), followed by the elderly (15.38%). This is in line with a Nigerian study that indicated the most prevalent age of reporting ADRs in Nigeria was between 21 and 40, at 45.6%.[28] The primary age for reporting ADRs was 35 years among patients on antiretroviral therapy (ART) in Nigeria.[38,39] Ampadu et al.[40] assessed ICSRs in VigiBase by national PV centres in Africa and compared it with the rest of the world. All member countries of the WHO-PIDM who contribute to VigiBase were included. They identified the dominant age group for reporting ADRs in Africa as 18 - 44 years (39.10%), compared with 45 - 64 years (24.13%) for the rest of the world.'401 The wider age range for adults and the fact that adult and elderly patients are more likely affected by chronic disease and multi-drug therapy may explain the higher numbers of reports.
Our results revealed more reports were received for female patients (61.9%) than males (33.0%). Ampadu et al.[40] also reported that more females (57%) were affected by ADRs than males (37%) for Africa and the rest of the world. Reports in Ethiopia's pharmacovigilance database contain 56.3% for females, compared with males (43.7%).[41] An analysis conducted by Watson et al.[42] on more than 18 million ICSRs in VigiBase during 2018 also indicated that women, from puberty and onwards and especially in their reproductive years, report more ADRs than men. This is a known phenomenon attributed to differences in biological, i.e. anatomical and physiological, functions between the two genders.[2] Watson et alls findings confirmed the importance of considering gender throughout the entire life-cycle of drug development and post-marketing surveillance to help understand the underlying reasons for reporting ADRs.[42]
Type of reporter
Healthcare professionals were the primary reporters in 68.86% of cases, of which physicians reported the highest amount (39.66%), while the lowest number of reports came from pharmacists (4.45%). Pharmacists are the most accessible healthcare professionals; therefore, community pharmacists have a crucial role in ensuring drug safety by detecting and reporting ADRs.[43] A study conducted among SA pharmacists showed that 57% of pharmacists indicated that ADR reporting is time-consuming, while 50% indicated that they lack clinical knowledge to detect ADRs.[44] A review of pharmacists' perspectives of spontaneous reporting revealed that knowledge, clinical competence and attitude towards ADR reporting are the main contributing factors towards under-reporting by pharmacists around the globe.[43] A study conducted in 48 primary healthcare clinics in the Tshwane District of Gauteng shows that only 16.0% of healthcare professionals surveyed (physicians, professional nurses, pharmacists and post-basic pharmacists' assistants) had ever reported a suspected ADR.[45] ADR reporting and ultimately patient care can be improved by the active involvement of a well-trained pharmacist for detecting ADRs, implementing pharmacovigilance programmes and training healthcare professionals regarding the need for ADR reporting.[46] Increasing pharmacovigilance awareness and improving and encouraging ADR reporting for all healthcare professionals, particularly pharmacists, is recommended to increase the number of reports received by SAHPRA.
Reports by consumers provide additional information that is not always included in healthcare professional reports, and therefore play a role in signal detection.[47] In the present study, 29.4% of reports were received from consumers, which is higher than what has been found in most European countries. During 2013 and 2014, patient-direct reporting accounted for 36% and 34% of reports received in Denmark, 23% and 20% in the Netherlands, and 18% and 21% for Sweden.[48] A survey of SA parents concluded that parents infrequently report ADRs, but that respondents had knowledge of where to find more information on ADR reporting and how ADRs can be reported.[49]
Completeness
The mean vigiGrade completeness score for the reports received was 0.456 (SD 0.221), and only 11.29% of reports had a completeness score >0.8 and are thus considered well documented. The completeness score starts at 1 for reports with information on time-to-onset, age, sex, indication, outcome, report type, dose, country, primary reporter type and comments. For each missing dimension, a penalty is detracted, which varies with clinical relevance.[33] The age and sex of the patient were not included in 24.85% and 4.99% of the received ICSR reports. The indication, outcome and type of reporter was missing for 16.04%, 10.94% and 2.41% of cases, respectively. The dose was reported for 49.67% of drug-event pairs, and time-to-onset of the reaction could only be calculated for 47.18% of events. The action taken with the suspect drug was unknown in 51.19% of the events, and the suspect drug was withdrawn in 40.50% of the events. Lack of this information in the reports limits the potential detection of unknown ADRs, and renders SRS futile. The incompleteness of reports is of concern, and makes it difficult to conduct causality assessments, which form a vital part of signal detection.
Clinical profile
Medicines reported
Medicines from all 14 ATC groups were suspected of causing ADRs. The most common classes were anti-infectives for systemic use (20.08%), antineoplastics and immunomodulating agents (15.66%), and alimentary tract and metabolism medicines (10.67%), which corresponds with the findings of other similar studies that were conducted in Jordan[50] and Turkey.[51] Anti-infectives, notably antiretrovirals and antibiotics, were identified as the main classes of drugs implicated in the ICSRs in Africa.[40] In a study conducted in SA, it was found that 8.4% of admissions in medical wards were attributable to ADRs, with anti-TB and antiretroviral drugs implicated in one-third of admissions.[52] A global review on patterns of ADRs indicated that anti-infectives were reported primarily in low- and middle-income countries.[29] The review characterised ADRs reported to VigiBase, related to national income level (in accordance with the World Bank definition), and SA was included as part of the upper-middle income group.[29]
In contrast, antineoplastic and immunomodulating agents were commonly reported in high-income countries.[29,53] The top 10 reported suspect drugs were dominated by medicines used to manage HIV and HIV co-infections. HIV was also the most reported indication (8.62%). In line with this finding, HIV was also the top reported indication (56.9%) on ADR reports submitted to the Nigerian National Pharmacovigilance Centre.[28] SA has the biggest HIV epidemic globally, and during 2019, the HIV prevalence in SA was 20.04% among the general population.[54] This may explain why HIV and anti-infectives were reported more frequently, and adults (19 - 64 years) were the largest group affected by ADRs.
Suspected ADRs
The most common SOCs involved in ADRs were general disorders and administration site conditions (15.22%), nervous system (10.85%), skin and subcutaneous tissue (8.56%), and gastrointestinal (GI) (8.30%) disorders. These findings are consistent with the analysis of ADRs submitted to VigiBase, which found that subcutaneous tissue disorders, nervous system and GI disorders were the most commonly reported[29]An analysis by Ampadu et αl.[40] revealed that African ICSRs were dominated by reports of skin and subcutaneous tissue (31.14%), general and administration site conditions (20.91%), nervous system (17.48%) and GI (16.10%) disorders, and were similar to the rest of the world.
ADRs reported by SAHPRA to VigiBase have not necessarily been assessed for causality, and the seriousness of the suspected reaction is determined by the reporter. Of the reports received, 55.87% were classified as serious. This is expected because marketing authorisation holders are mandated by law to report serious ADRs (SADRs).[55] Given the potentially debilitating impact of SADRs and the associated morbidity and mortality, it is encouraging to note a more significant proportion of reports received for SADRs. However, of concern is that the Pharmacovigilance Unit has identified no signals to date, and it can be recommended that SADRs be reviewed and analysed periodically to maximise signal detection.
Death was the most used MedDRA preferred term to describe reactions (5.17%; n=1 056; N=20 438). Reports that were classified as fatal represented 12.47% (n=1 052, N=8 438). Causality assessment has, however, not been performed for all cases, and therefore the outcome of the event or reaction cannot necessarily be linked to the reported drug. Death could be coincidental in an elderly patient, or due to other factors, such as multiple comorbidities.
Of the 8 438 ICSRs received, 89.1% had information on the outcome of the reaction(s). The outcome 'died' was indicated on 10.69% (n=803; n=7 515) of these reports, which is inconsistent with 1 033 cases where seriousness was classified as 'death', and the 1 052 cases that were classified fatal. The authors are unable to explain this discrepancy, as it could be the result of incorrect data capturing or robust data verification. Another concern is that the outcome was reported as unknown in 63.3% of the reports, which may indicate a lack of follow-up by the Pharmacovigilance Unit or lack of knowledge of reporting processes by the reporter. The reporter might understand the term 'outcome' incorrectly as the result of a possible investigation, and not the outcome of the ADR in the patient. This illustrates the need for training of reporters at all levels as well as staff of the Pharmacovigilance Unit at SAHPRA to improve the quality of reports and verification of data.
This is the first study in SA to analyse the country's data from the WHO's global pharmacovigilance database, VigiBase.
Study limitations
This study has several limitations that should be considered when interpreting the findings. The data in VigiBase are subject to reporting biases, confounding issues and heterogeneity. The information in VigiBase comes from various sources, and the probability that the suspected adverse effect is drug-related is not the same in all cases. This study could not analyse report characteristics according to senders; therefore, we could not differentiate ADR reporting between pharmaceutical companies and other sectors. The Pharmacovigilance Unit of SAHPRA did not conduct causality assessment on the reports before committing them to VigiBase, and therefore the outcome of the event or reaction cannot necessarily be linked to the reported drug. The study period was only 1 year, so the authors could not identify reporting trends over a longer study period. The wider range for the adult age group (18 - 64 years) makes it difficult to compare our findings with other studies.
Conclusion
The spontaneous reporting system's ultimate goal is to detect unknown ADRs through reviewing and analysing ADR reports. Therefore, it is paramount for the reporters to be aware of the clinical and demographic information essential for causality assessment and signal detection. The findings of this study demonstrate that core clinical elements that are important in signal detection and causality assessment are often not included in reports.
Reporting by pharmacists was lowest among healthcare professionals, and should be encouraged, because they have a crucial role in ensuring drug safety by detecting and reporting ADRs. Furthermore, patients have been identified as active contributors to the national pharmacovigilance database. An urgent need to increase public awareness and familiarity with ADR reporting processes to ensure both the quantity and completeness of reports has been identified.
Causality assessment is not performed for all reports that are received by the Pharmacovigilance Unit. We recommend the establishment of a signal detection working group within SAHPRA to build capacity and detect safety signals.
Inconsistencies in reports could be decreased if the technical staff in the Pharmacovigilance Unit are trained in data verification processes.
We recommend a follow-up study that evaluates reporting trends over a longer study period and evaluates the reporting between different types of senders (e.g. pharmaceutical company, healthcare professional or regional pharmacovigilance centre).
This demographic and clinical profile of ADR reports received by SAHPRA will improve our understanding of ADR reporting in the country to enhance training of reporters.
Declaration. This study was conducted as part of MFM's MPharm degree in Pharmacy Practice with Pharmacovigilance and Pharmacoepidemiology at North-West University.
Acknowledgements. Data from the WHO Collaborating Centre for International Drug Monitoring were used. The results of this study do not represent the opinion of the UMC or the WHO. The authors are indebted to SAHPRA for contributing data to the WHO Programme for International Drug Monitoring. The authors want to thank NorthWest University Statistical Consultation Services for assisting with data analysis.
Author contributions. MFM, MSL and HS were involved in the study design, data interpretation and manuscript writing.
Funding. None.
Conflicts of interest. MFM is the manager of the Pharmacovigilance Unit at SAHPRA, Pretoria, South Africa. To ensure objectivity, all data analyses were done by the Statistical Consultation Services of the NorthWest University.
References
1. Abubakar AR, Simbak NB, Haque M. Adverse drug reactions: Predisposing factors, modern classifications and causality assessment. Res J Pharm Technol 2014;7(9):1091-1098. [ Links ]
2. Alomar MJ. Factors affecting the development of adverse drug reactions. Saudi Pharm J 2014;22(2):83-94. https://doi.org/10.1016/j.jsps.2013.02.003 [ Links ]
3. Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther 1998;20:C40-C44. https://doi.org/10.1016/S0149-2918(98)80007-6 [ Links ]
4. Rolfes L, van Hunsel F, van der Linden L, et al. The quality of clinical information in adverse drug reaction reports by patients and healthcare professionals: A retrospective comparative analysis. Drug Saf 2017;40(7):607-614. https://doi.org/10.1007/s40264-017-0530-5 [ Links ]
5. Alomar M, Tawfiq AM, Hassan N, et al. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: Current status, challenges and the future. Ther Adv Drug Saf 2020;11:1-11. https://doi.org/10.1177%2F2042098620938595 [ Links ]
6. Weinshilboum R. Inheritance and drug response. N Engl J Med 2003;348(6):529-537. https://doi.org/10.1056/NEJMra020021 [ Links ]
7. World Health Organization. The importance of pharmacovigilance: safety monitoring of medicinal products. Geneva: WHO, 2002. https://apps.who.int/iris/handle/10665/42493 (accessed 23 September 2021). [ Links ]
8. Paludetto M-N, Olivier-Abbal P, Montastruc J-L. Is spontaneous reporting always the most important information supporting drug withdrawals for pharmacovigilance reasons in France; Pharmacoepidemiol Drug Saf 2012;21(12):1289-1294. https://doi.org/10.1002/pds.3333 [ Links ]
9. Giardina C, Cutroneo PM, Mocciaro E, et al. Adverse drug reactions in hospitalised patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study. Front Pharmacol 2018;9:350. https://doi.org/10.3389/fphar.2018.00350 [ Links ]
10. World Health Organization. Safety of medicines: A guide to detecting and reporting adverse drug reactions: Why health professionals need to take action. Geneva: WHO, 2002. https://apps.who.int/iris/handle/10665/67378 (accessed 23 September 2021). [ Links ]
11. Waller P, Harrison-Woolrych M. An Introduction to Pharmacovigilance. 2nd ed. New York: Wiley, 2017. [ Links ]
12. Fornasier G, Francescon S, Leone R, et al. An historical overview over pharmacovigilance. Int J Clin Pharm 2018;40(4):744-747. https://doi.org/10.1007/s11096-018-0657-1 [ Links ]
13. Haque A, Daniel S, Maxwell T, et al. Postmarketing surveillance studies: An industry perspective on changing global requirements and implications. Clin Ther 2017;39(4):675-685. https://doi.org/10.1016/j.clinthera.2017.03.011 [ Links ]
14. Brewer T, Colditz GA. Post-marketing surveillance and adverse drug reactions: Current perspectives and future needs. JAMA 1999;281(9):824-829. https://doi.org/10.1001/jama.281.9.824 [ Links ]
15. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: A review of recent observational studies. Drug Saf 2015;38(5):437-453. https://doi.org/10.1007/s40264-015-0281-0 [ Links ]
16. Arnaud M, Bégaud B, Thurin N, et al. Methods for safety signal detection in healthcare databases: A literature review. Expert Opin Drug Saf 2017;16(6):721-732. https://doi.org/10.1080/14740338.2017.1325463 [ Links ]
17. Kumar A, Khan H. Signal detection and their assessment in pharmacovigilance. Open Pharma Sci J 2015;2:66-73. https://doi.org/10.2174/1874844901502010066 [ Links ]
18. Palleria C, Leporini C, Chimirri S, et al. Limitations and obstacles of the spontaneous adverse drugs reactions reporting: Two 'challenging' case reports. J Pharmacol Pharmacother 2013;4(Suppl 1):S66-S72. https://doi.org/10.4103/0976-500X.120955 [ Links ]
19. Lester J, Neyarapally GA, Lipowski E, et al Evaluation of FDA safety-related drug label changes in 2010. Pharmacoepidemiol Drug Saf 2013;22(3):302-305. https://doi.org/10.1002/pds.3395 [ Links ]
20. Mehta U, Kalk E, Boulle A, et al. Pharmacovigilance: A public health priority for South Africa. S Afr Health Rev 2017;2017:125-133. [ Links ]
21. Lindquist M. VigiBase, the WHO Global ICSR database system: Basic facts. Drug Inf J 2008;42(5):409-419. https://doi.org/10.1177/009286150804200501 [ Links ]
22. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting E2D. https://database.ich.org/sites/default/files/E2D_Guideline.pdf (accessed 15 December 2019). [ Links ]
23. South African Health Products Regulatory Authority. Adverse drug reactions & quality problem reporting form. Pretoria: SAHPRA, 2020. https://www.sahpra.org.za/wp-content/uploads/2020/01/6.04_ARF1_v5.1_27Jan2020.pdf (accessed 5 June 2022). [ Links ]
24. South African Health Products Regulatory Authority. Adverse drug reaction and product quality reporting. Pretoria: SAHPRA, 2022. https://primaryreporting.who-umc.org/ZA (accessed 5 June 2022). [ Links ]
25. South African Health Products Regulatory Authority. MedSafety app. Pretoria: SAHPRA, 2022. https://medsafety.sahpra.org.za/ (accessed 5 June 2022). [ Links ]
26. Uppsala Monitoring Centre. Uppsala, UMC, 2022. A mine of information on potential safety risks. https://who-umc.org/pv-products/vigiflow/#:~:text=VigiFlow%20is%20a%20web%2Dbased,triage%20and%20assessment%20of%20cases (accessed 5 June 2022). [ Links ]
27. Alshammari TM, Al-Kathiri WaH, Louet HL, et al Completeness of adverse drug reactions reports of the Saudi adverse event reporting system. Saudi Med J 2015;36(7):821-828. https://doi.org/10.15537/smj.2015.7.11751 [ Links ]
28. Awodele O, Aliu R, Ali I, et al. Patterns of adverse drug reaction signals in NAFDAC pharmacovigilance activities from January to June 2015: Safety of drug use in Nigeria. Pharmacol Res Perspect 2018;6(5):e00427. https://doi.org/10.1002/prp2.427 [ Links ]
29. Aagaard L, Strandell J, Melskens L, et al. Global patterns of adverse drug reactions over a decade. Drug Saf 2012;35(12):1171-1182. https://doi.org/10.1007/BF03262002 [ Links ]
30. Thiessard F, Roux E, Miremont-Salamé G, et al Trends in spontaneous adverse drug reaction reports to the French pharmacovigilance system (1986 - 2001). Drug Saf 2005;28(8):731-740. https://doi.org/10.2165/00002018-200528080-00007 [ Links ]
31. Kiguba R, Ndagije HB, Nambasa V, et al. Adverse drug reaction onsets in Uganda's VigiBase: Delayed international visibility, data quality and illustrative signal detection analyses. Pharmaceut Med 2018;32(6):413-427. https://doi.org/10.1007/s40290-018-0253-7 [ Links ]
32. Masuka JT, Khoza S. An analysis of the trends, characteristics, scope, and performance of the Zimbabwean pharmacovigilance reporting scheme. Pharmacol Res Perspect 2020;8(5):e00657. https://doi.org/10.1002/prp2.657 [ Links ]
33. Bergvall T, Norén GN, Lindquist M. vigiGrade: A tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf 2014;37(1):65-77. https://doi.org/10.1007/s40264-013-0131-x [ Links ]
34. Lagerlund O, Strese S, Fladvad M, et al. WHODrug: A global, validated and updated dictionary for medicinal information. Thera Innov Reg Sci 2020^4(5):1116-1122. https://doi.org/10.1007/s43441-020-00130-6 [ Links ]
35. WHO Collaborating Centre for Drug Statistics Methodology. ATC Structure and Principles. Geneva: WHO, 2018. https://www.whocc.no/atc/structure_and_principles/ (accessed 1 December 2020). [ Links ]
36. MedDRA (Medical Dictionary for Regulatory Activities). MedDRA Hierarchy. 2020. https://www.meddra.org/how-to-use/basics/hierarchy (accessed 21 November 2020). [ Links ]
37. Council for International Organizations of Medical Sciences. Reporting adverse drug reactions; definitions of terms and criteria for their use. CIOMS, 1999. https://cioms.ch/publications/product/reporting-adverse-drug-reactions-definitions-of-terms-and-criteria-for-their-use/ (accessed 20 November 2020). [ Links ]
38. Agu KA, Oparah AC. Adverse drug reactions to antiretroviral therapy: Results from spontaneous reporting system in Nigeria. Perspect Clin Res 2013;4(2):117-124. https://doi.org/10.4103/2229-3485.111784 [ Links ]
39. Agu KA, Isah MA, Oqua D, et al. Incidence of adverse drug reactions in patients on antiretroviral therapy: A study of pharmaceutical care in HIV interventions in Nigeria. West African J Pharm 2013;24:30-42. [ Links ]
40. Ampadu HH, Hoekman J, de Bruin ML, et al. Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: Analyses of spontaneous reports in VigiBase. Drug Saf 2016;39(4):335-345. https://doi.org/10.1007/s40264-015-0387-4 [ Links ]
41. Anebo ZG, Abacioglu N. Patterns of adverse drug reaction reporting in Ethiopia: A database analysis of spontaneous reports from 2013 to 2018. Asian Pac J Trop Med 2022;15(2):56-62. https://doi.org/10.4103/1995-7645.338436 [ Links ]
42. Watson S, Caster O, Rochon PA, et al. Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. E Clin Med 2019;17:e100188. https://doi.org/10.1016/j.eclinm.2019.10.001 [ Links ]
43. Hadi MA, Neoh CF, Zin RM, et al. Pharmacovigüance: Pharmacists' perspective on spontaneous adverse drug reaction reporting. Integrated Pharm Res Pract 2017;6:91-98. https://doi.org/10.2147/IPRP.S105881 [ Links ]
44. Jordaan PH. Pharmacists' perception towards pharmacovigilance and the reporting of adverse drug reactions in South Africa. Master's dissertation. Potchefstroom: North-West University, 2020. https://repository.nwu.ac.za/handle/10394/34958 (accessed 18 April 2023). [ Links ]
45. Haines HM, Meyer JC, Summers RS, et al. Knowledge, attitudes and practices of health care professionals towards adverse drug reaction reporting in public sector primary health care facilities in a South African district. Eur J Clin Pharmacol 2020;76(7):991-1001. https://doi.org/10.1007/s00228-020-02862-8 [ Links ]
46. Terblanche A. Pharmacovigilance and the reporting of adverse drug reactions. SA Pharm J 2018(6): 238245. https://doi.org/10.1080/21548331.2017.1381013 [ Links ]
47. Sienkiewicz K, Burzynska M, Rydlewska-Liszkowska I, et al. The importance of direct patient reporting of adverse drug reactions in the safety monitoring process. Int J Environ Res Public Health 2021;19(1):413. https://doi.org/10.3390%2Fijerph19010413 [ Links ]
48. Health Action International. Direct patient reporting in the European Union: A snapshot of reporting systems in seven member states. Amsterdam: HAI, 2015. https://haiweb.org/wp-content/uploads/2015/09/Direct-Patient-Reporting-in-the-EU.pdf (accessed 22 November 2020). [ Links ]
49. Pillay S, Mulubwa M, Viljoen M. Parental reporting of adverse drug reactions in South Africa: An online survey. Afr J Prim Health Care Fam Med 2021;13(1):e1-e8. https://doi.org/10.4102%2Fphcfm.v13i1.2880 [ Links ]
50. Alsbou M, Abdeen G, Batarseh A, et al. Analysis of the national pharmacovigilance database in Jordan (2010 - 2014). Biomed Pharmacol J 2017;10:319-328. https://doi.org/10.13005/bpj/1112 [ Links ]
51. Ozcan G, Aykac E, Kasap Y, et al. Adverse drug reaction reporting pattern in Turkey: Analysis of the national database in the context of the first pharmacovigilance legislation. Drugs Real World Outcomes 2016;3(1):33-43. https://doi.org/10.1007/s40801-015-0054-1 [ Links ]
52. Mouton JP, Njuguna C, Kramer N, et al. Adverse drug reactions causing admission to medical wards: A cross-sectional survey at 4 hospitals in South Africa. Medicine 2016;95(19):e3437. https://doi.org/10.1097/MD.0000000000003437 [ Links ]
53. Rosli R, Ming LC, Abd Aziz N, et al. A retrospective analysis of spontaneous adverse drug reactions reports relating to paediatric patients. PLoS ONE 2016;11(6):e0155385. https://doi.org/10.1371/journal.pone.0155385 [ Links ]
54. Avert. HIV and AIDS in South Africa. 3 July, 2020. https://www.avert.org/about-hiv-aids (accessed 27 November 2020). [ Links ]
55. South African Health Products Regulatory Authority. Post-marketing reporting of adverse drug reactions to human medicines in South Africa. Pretoria: SAHPRA, 30 January 2020. https://www.sahpra.org.za/wp-content/uploads/2020/03/2.33_ADR_reporting_postmarketing_v6_Jan2020.pdf (accessed 5 November 2020). [ Links ]
 Correspondence:
Correspondence:
H Steyn
hanlie.steyn@nwu.ac.za
Accepted 23 March 2023














