Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 n.6 Pretoria Jun. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i6.624
IN PRACTICE
Ivermectin drug-induced liver injury
M W SonderupI; W MudiniII; C W N SpearmanIII
IMMed (Med), FCP (SA); Division of Hepatology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
IIMMed (Anat Path), FCPath (SA) Anat; Department of Anatomical Pathology, University of Cape Town and National Health Laboratory Service, Groote Schuur Hospital, Cape Town, South Africa
IIIFCP (SA), PhD; Division of Hepatology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
ABSTRACT
Ivermectin remains a popular, albeit unproven, therapy used in both the prevention and treatment of COVID-19. We discuss a patient who developed jaundice and a liver injury 3 weeks after initiating ivermectin for COVID-19 prevention. Liver histology demonstrated a pattern of injury that was both portal and lobular, with a bile ductulitis with marked cholestasis. She was managed with low-dose corticosteroids, later tapered, and withdrawn. She remains well a year after presenting.
Ivermectin has been widely touted as being both preventive and curative for COVID-19. The evidence to support these claims has not been forthcoming.[1,2] In South Africa (SA), official public health policy from the SA Health Products Regulatory Authority (SAHPRA), early during the COVID-19 pandemic and later again confirmed, despite major legal challenges, advised against the use of ivermectin and its claimed medicinal benefits.[3] Despite this, the use of ivermectin remained widespread. Ivermectin toxicity, including seizures and gastrointestinal side-effects, has been reported.[4] Ivermectin drug-induced liver injury (DILI) has been described, albeit only once in 2006 in a patient using the medication for the treatment of Loa Loa infection.[5] We report a patient who used ivermectin for COVID-19 prophylaxis and developed a DILI.
A 70-year-old woman presented with jaundice and cholestatic hepatitis. Her medical background included hypertension, managed for almost 10 years with perindopril. She experienced occasional migraines, for which she used a combination of paracetamol and codeine with infrequent use of sublingual rizatriptan. She had not had a migraine for more than 8 months prior to presentation. She was a non-smoker and took no alcohol.
She was not vaccinated with any of the available COVID vaccines and had never been diagnosed with confirmed SARS-CoV-2 infection. During the third wave of COVID-19 in SA, dominated by the Delta variant of SARS-CoV-2, she chose to use oral ivermectin as prophylaxis against COVID. Her primary care doctor had prescribed the ivermectin, and she used a product obtained from a pharmacy that was dispensing ivermectin for COVID-19 prophylaxis and treatment. She administered it daily as a single 12 mg oral dose.
Approximately 3 weeks after starting the ivermectin, she noted darkening of her urine, followed by jaundice. She presented to her family practitioner. Her nasopharyngeal COVID-19 polymerase chain reaction (PCR) swab was negative. He initiated several investigations that revealed the following (normal range in brackets): total bilirubin 129 μmol/L;[5,6] conjugated bilirubin 114 μmol/L;[5,6] alkaline phosphatase 463 (40 - 160) U/L; gamma glutamyl transpeptidase 740 (<40) U/L; alanine transaminase 1 098 (<40) U/L; aspartate transaminase 811 (<40) U/L. Her international normalised ratio was 1.3. Ultrasound of the liver was normal and all initial viral markers, including hepatitis A IgM, hepatitis B surface antigen and core IgM, and hepatitis C antibody, were negative. She was referred to us for evaluation.
We performed additional investigations that included a negative hepatitis E PCR and normal immunoglobulin levels, and autoantibodies associated with autoimmune liver disease (anti-nuclear factor, anti-smooth muscle antibody, anti-liver kidney microsome type 1 and antimitochondrial antibody) were all negative.
Given the absence of other causality, a preliminary diagnosis of ivermectin DILI was considered. We advised that she stop ivermectin, and a liver biopsy was performed.
Liver biopsy (Figs 1 and 2) demonstrated portal inflammation and interface activity. Portal triaditis with interface hepatitis comprising lymphocytes, plasma cells, histiocytes and eosinophils was evident. Mild ductular reaction with secondary ductulitis was also noted. Scattered lobular necroinflammatory foci were evident with prominent zone 3 cholestasis. The liver biopsy injury pattern was compatible with a DILI. The R-ratio value was calculated at 8.6, with >5 suggesting a more hepatocellular type of injury. Her RUCAM score (Roussel Uclaf causality assessment method) of 8 supported a highly probable association between ivermectin and a DILI.
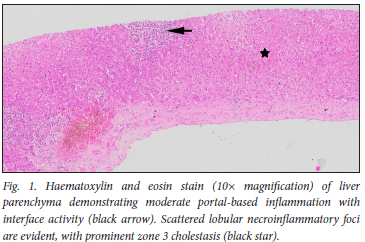
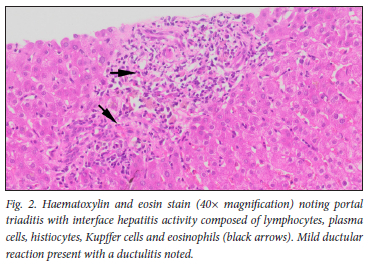
Given the necroinflammation observed on biopsy and her liver profile, we initiated prednisone at 0.5 mg/kg. Liver enzymes declined consistently over an 8-week period, and eventually normalised after 3 months. Prednisone was tapered and withdrawn over a 3-month period. One year after prednisone withdrawal, the patient's liver profile remains normal.
Discussion
Ivermectin associated severe DILI has only been previously reported once.[5] The described case was in the setting of treatment for loiasis, and liver biopsy demonstrated lobular inflammation, confluent necrosis and apoptosis. The patient was anicteric and recovered uneventfully. Our patient, however, presented with jaundice and histologically demonstrated a more moderate to severe interface hepatitis with a mixed inflammatory infiltrate. Bile duct targeting with a ductulitis was conspicuous, with notable zone 3 cholestasis evident on biopsy. Although there were no systemic symptoms of an immune-allergic nature, the elevated transaminases and moderate portal and lobular inflammation persuaded us to initiate low-dose corticosteroids.
We were able to taper her prednisone, with no rebound elevation of enzymes noted over 1 year of follow-up. The mechanism of this DILI is not known, but the case highlights the potential risks associated with the use of medicinal products for unproven indications. Herbal and dietary supplement use is prevalent, and increasing, and now accounts for 20% of cases of hepatotoxicity in the USA.[6] We observed several patients with such injuries during COVID-19 admitted to our unit, most notably related to the use of African wormwood or Artemesia afra (colloquially called umhlonyane or wilde als locally) as a COVID-19 preventive therapy.
Clinically, the patient reinforces the ever-present requirement to obtain a thorough history of all medicine and supplement use in patients presenting with a liver injury and unexplained jaundice. Drug- or toxin-induced liver injuries should be considered as differential diagnoses in a clinical setting, as demonstrated in this patient. A complete causality assessment, as shown, should provide a reliable answer as to the likelihood of a DILI. The use of corticosteroids is not universally advocated in DILIs, rather guided by the histological picture. Where necroinflammation and interface hepatitis are present, corticosteroid use can be considered, at low dose preferably, and weaned when resolution occurs. Liver biopsy is therefore crucial in this decision-making, and should always be considered, if possible and safe, and if histopathological expertise is available. Our patient's histology had striking inflammatory activity, with a mixed population of inflammatory cells, and responded well to low-dose corticosteroids. Off steroids, she remains well.
Declaration. None.
Acknowledgements. The patient who kindly consented to the report being published.
Author contributions. MS drafted the initial manuscript with WM. All authors contributed to the development of the final manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Reis G, Silva E, Silva DCM, et al. Effect of early treatment with ivermectin among patients with Covid-19. N Engl J Med 2022;386(18):1721-1731. https://doi.org/10.1056/nejmoa2115869 [ Links ]
2. Popp M, Reis S, Schießer S, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev 2022;6(6):CD015017. https://doi.org/10.1002%2F14651858.CD015017.pub3 [ Links ]
3. South African Health Products Regulatory Authority. SAHPRA statement - latest development on ivermectin court case. Pretoria: SAHPRA, 2020. https://www.sahpra.org.za/news-and-updates/sahpra-statement-latest-development-on-ivermectin-court-case/ (accessed 16 May 2023). [ Links ]
4. Temple C, Hoang R, Hendrickson RG. Toxic effects from ivermectin use associated with prevention and treatment of Covid-19. N Engl J Med 2021;385(23):2197-2198. https://doi.org/10.1056/nejmc2114907 [ Links ]
5. Veit O, Beck B, Steuerwald M, Hatz C. First case of ivermectin-induced severe hepatitis. Trans R Soc Trop Med Hyg 2006;100(8):795-797. https://doi.org/10.1016/j.trstmh.2006.02.003 [ Links ]
6. Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology 2017;65(1):363-373. https://doi.org/10.1002/hep.28813 [ Links ]
 Correspondence:
Correspondence:
M W Sonderup
msonderup@samedical.co.za
Accepted 31 January 2023














