Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.3b Pretoria Mar. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i3b.16850
RESEARCH
Prevalence of group A streptococcal carriage in school children from Cape Town: A cross-sectional study and systematic review
M E EngelI, *; H A MoloiII, III, *; L AbdullahiIV; S NkepuV; B MuhamedVI; D D BarthVII; A WhitelawVIII; J B DaleIX; B M MayosiX, XI, †
IMPH, PhD; Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
IIMPH; Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
IIIMPH; Health Systems Research Unit, South African Medical Research Council, Cape Town, South Africa
IVPhD; Vaccines for Africa Initiative, Faculty of Health Sciences, University of Cape Town, South Africa
VDepartment of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
VIPhD; Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
VIIPhD; Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
VIIIMB ChB, MMed; Vaccines for Africa Initiative, Faculty of Health Sciences, University of Cape Town, South Africa
IXMD; Department of Microbiology, National Health Laboratory Service, Tygerberg Hospital and Stellenbosch University, Cape Town, South Africa
XMB ChB, DPhil; Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
XIMB ChB, DPhil; Department of Medicine, University of Tennessee Health Science Center, Memphis, Tennessee. USA
XIIThe Deanery, Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Asymptomatic children can be a major reservoir of pharyngeal group A streptococcus (GAS). The role of GAS carriage causing subsequent infections resulting in the manifestation of clinical symptoms, or being associated with transmission to uninfected individuals, is not entirely clear. Furthermore, data on GAS carriage from countries in Africa remain scant with only a few studies reporting carriage
OBJECTIVES: We performed a cross-sectional study to determine the prevalence of asymptomatic pharyngeal carriage of group A streptococci in school children in Cape Town. We considered our results in the context of a meta-analysis of data of GAS carriage in Africa
METHODS: We conducted a school-based cross-sectional study from 2009 to 2011 in two Cape Town peri-urban communities, enrolling 950 healthy learners. Pharyngeal swabs were obtained from learners and processed at the National Health Laboratory Service (NHLS) microbiology laboratory at Groote Schuur Hospital, Cape Town. Thereafter, we conducted a systematic review through a comprehensive literature search among several sources. Prevalence estimates with 95% confidence intervals (CIs) were determined using a random-effects meta-analysis model
RESULTS: GAS was isolated from 31 participants corresponding to a carrier rate of 3% (95% CI 2% - 4%). Combining our results with 18 other studies revealed a pooled prevalence of 9% (95% CI 6% - 11%). Regional pooled rates were similar across southern, eastern and northern Africa, of between 9% (95% CI 6% - 11%) and 11% (95% CI 4% - 21%) while countries within Central Africa had a pooled estimate of 7% (95% CI 5% - 9%). Western Africa had the lowest pooled estimate of 2% (95% CI 1% - 2%
CONCLUSION: There was a relatively low rate of carriage of GAS in asymptomatic school children residing in South Africa. Pooled prevalence rates revealed regional differences across the African continent as regards the rate of GAS carriage, with the western and northern African regions having rates of GAS carriage that were lower and higher respectively than those of East, Central and southern African countries, which demonstrated similar rates of carriage
Streptococcus pyogenes, also known as group A streptococcus (GAS), is a major cause of infections worldwide, commonly causing pharyngitis in children aged 5-15 years.[1] Of concern, repeated untreated or inappropriately treated episodes of GAS pharyngitis may result in autoimmune diseases such as acute rheumatic fever (ARF), which may develop into rheumatic heart disease (RHD). The global burden of RHD is estimated to be 33 million prevalent cases, 9 million disability-adjusted life years lost and 275 000 deaths each year.[2-4]
GAS can asymptomatically colonise the upper respiratory tract of children, referred to as GAS carriers; penicillin has been shown not to be effective as the first-line treatment for eradicating GAS carriage.[5] The role of GAS carriage causing subsequent infections resulting in the manifestation of clinical symptoms, or being associated with transmission to uninfected individuals, is not entirely clear.[5-8] Therefore, it is of interest to understand the background prevalence of GAS carriage in communities in comparison with prevalence of GAS in symptomatic pharyngitis in order to interpret the findings of diagnostic tests of true infection appropriately[9] Finally, knowledge of the emm types of GAS recovered from asymptomatic carriers may be useful in the light of recent advances towards the development of multivalent M protein-based streptococcal vaccines.[10]
There is a dearth of recent studies on GAS carriage rates in school-aged children; in South Africa (SA), only three studies exist, conducted more than 25 years ago.[11-13] Among these, carriage among the black population was reported to be 16.8% in an urban setting,[13] 5.2% in lower-socioeconomic households in peri-urban Soweto[11] and 1.62% in participants from a remote traditional community.[13] A study involving mostly grade 3 learners of either mixed or Indian ancestry reported GAS carriage rates >20% in summer and <5% in spring.[12] The remaining study comprising urban white participants had a carriage prevalence of only 3.4%.[13]
Furthermore, data on GAS carriage from countries in Africa remain scant with only a few studies reporting carriage. An earlier systematic review by Shaikh et al.[9] reported a pooled prevalence of GAS carriage of 12% (95% Confidence interval (CI) 9% - 14%) in healthy children < 18 years old residing in low- to middle-income countries. This review, unfortunately, did not include African data available at the time, including studies from SA, Ethiopia and Tunisia.[11-21]
We conducted a cross-sectional study to describe the rate of GAS carriage among school children living in Cape Town, SA. Thereafter we analysed our findings in the context of published data through systematic review and meta-analysis of literature on the prevalence of GAS carriage in children aged 5-15 years, residing in African countries,. Our review is essential, given the high burden of acute and chronic complications caused by GAS infections in Africa,[22] and is intended to update the findings of the previous reviews on GAS carriage.
Methods
Cross-sectional study
This study was nested within a larger cross-sectional school-based screening study for asymptomatic RHD among healthy learners attending primary and secondary schools in Bonteheuwel and Langa.[23] These two communities are considered to be of lower socioeconomic status and comprise mainly black African (Langa) and mixed ancestry (Bonteheuwel) populations. They are plagued with social problems such as poverty, unemployment, crime, drug and alcohol abuse, poor housing and overcrowding.
The study, performed as part of a larger RHD screening project (Engel et al.), recruited participants by convenience sampling over a 3-year period from February 2009 to November 2011. The sample size, set at a minimum of 450 participants per community, was based on published studies in similar SA communities[11,12] for estimation of at least 5% difference of GAS carriage across the two communities within a precision of 5%; additionally, we assumed a 5% non-response rate. School-going children, whose parents provided informed consent, were eligible for participation in the study. We excluded children who had taken antibiotics in the 3 months prior to the study.
A throat swab sample was taken by swabbing the tonsillar and posterior pharyngeal areas; samples were transferred in transport media for processing by the Microbiology Laboratory of the National Health Laboratory Service located at Groote Schuur Hospital. Swabs were inoculated onto 4% sheep blood agar plates according to a standard protocol, inverted and incubated anaerobically at 35°C for 24 - 48 hours. All cultures of beta-haemolytic colonies were further identified by Gram stain and catalase. Statistical analyses were performed using STATA version 14.0 (Stata Statistical Software: Release 14. StataCorp, USA). Comparisons were made using the χ2 test or Fisher's exact test. A p-value <0.05 was considered indicative of a statistically significant difference. The study was performed with the approval of the University of Cape Town Faculty of Health Sciences Research Ethics Committee, and informed consent was obtained in writing from a parent or legal guardian of each participant. Forms were provided in the local languages of Afrikaans, English and isiXhosa. In addition, children aged >8 years were required to provide assent.
Systematic review
For the systematic review, we employed rigorous methods drawn from the scientific techniques and guidelines offered by the Cochrane Collaboration;[24] (PROSPERO registration number: CRD 42015019589). We considered observational studies such as population-based, cross-sectional, and longitudinal studies. Published and unpublished studies with a population inclusive of healthy children residing in geographic regions confined to the African continent were eligible for inclusion. Studies had to have reported on prevalence of GAS carriage; a participant was deemed a GAS carrier if he/she tested positive for GAS bacteria through a positive rapid antigen detection test (RADT) or positive laboratory throat culture, but demonstrated no clinical signs and symptoms. We excluded publications lacking primary data and/or an explicit description of the methods. Where an eligible study was published in duplicate, the most recent complete version was included.
We developed a comprehensive search strategy that incorporated a combination of free term text items, including carriage, asymptomatic, etc., and medical subject headings (MESH) such as Streptococcus pyogenes. In addition, to maximise the likelihood of finding articles from Africa, we applied an African search filter described previously by Pienaar and colleagues.'251 The search was conducted independently by two reviewers (HM, DB) among the following electronic databases: EbscoHost, PubMed and Scopus. Also, we searched the American Society for Microbiology (http://www.asm.org/) websites for additional sources. Furthermore, we searched the proceedings from the ΧΓΧ Lancefield International Symposium on Streptococci and Streptococcal Disease (http://www.lancefield2014.com). Lastly, searches in Google Scholar complemented the searches, including articles among grey literature. The search strategy was appropriately modified to suit the vocabulary of individual databases. Publication date and language restriction were not applied to searches. To obtain additional publications, we scanned the reference lists of all potential articles retrieved from electronic searches. Also, relevant authors and experts in the field were contacted for additional data. The last manual internet search was conducted on 15 March 2019.
Search results from individual databases, reference searches and unpublished articles were managed with Mendeley referencing software (Mendeley Ltd, UK). Applying the predefined inclusion criteria, we reviewed the titles and abstracts of the full list of potential articles, resolving differences by discussion where necessary. Full text copies of potentially eligible studies were retrieved for detailed evaluation. We extracted relevant data onto a predefined form. We evaluated studies for risk of bias related to internal validity, external validity and generalisability of the study results. An assessment of risk of bias informed the evaluation of heterogeneity in the pooled analysis. We employed the quality assessment tool for evaluating prevalence studies as suggested by Hoy et αl.[26] and adapted by Werfalli et al.,[27] which allows for a composite score to assist with relative comparison between the studies, thereby reducing reviewers' subjectivity. The scoring system tool categorises high-risk studies as those with an overall score of 0 - 5 points, moderate risk as 6 - 8 and low risk >8 points.
To calculate the unadjusted prevalence estimates of streptococcus carriage of children within the age groups of 5 - 15 years, for each study we individually recalculated the reported prevalence and confirmed the numerators and denominators as reported by the authors in 11 studies.[13,16 19-21,28-33] For one study, data pertinent to our target age group were extracted from studies incorporating adults and children.[34] Where data for our target age were reported as part of a wider age range, we included all the data within that category, provided the age did not exceed 25 years,[13,19] the upper end of age-at-risk for rheumatic fever.[35]
Secondly, where sample populations included both symptomatic GAS children and asymptomatic GAS children, we only extracted information from healthy children/children without any signs or symptoms of disease.[17,36] Thirdly, the earliest full set of results were used where studies reported follow-up data of children over a period of months[12] and years.[14] This was to avoid an over- and underestimation of GAS carriage, that would arise by taking an average of all follow-up data of an individual study.
Using Stata (version 13.1), the Freeman-Tukey double arcsine transformation metaprop routine was used to calculate the combined prevalence estimate and standard error across the unadjusted estimates. The Freeman-Tukey stabilises the variance of study-specific prevalence, minimising the influence from studies with extremely small prevalence or extremely large prevalence estimates.[37]
We stratified the aggregated prevalence by region in order to assess similarities and difference within the Africa continent. Our hypothesis was that GAS carriage would differ regionally because of settings and climatic differences. We also evaluated prevalence according to study design in order to assess methodological influences on overall estimates. Our hypothesis was that GAS carriage would not be statistically different across study designs. Furthermore, we evaluated the effect of sample size on the pooled prevalence estimate; estimates reported in two recent large studies by Abdissa et al.[28] and Braito et al.[21] demonstrated an estimated sample size of 796 as being adequate.[39] Sub-analysis of GAS colonisation risk factors (gender, crowding and seasonality) could not be performed owing to data limitations; these factors are therefore presented as qualitative data. For each study, none of the missing data was relevant to warrant contacting the corresponding authors to request the missing information. We assessed heterogeneity across studies to determine the extent of variation between studies included in the meta-analysis. Heterogeneity was assessed by inspecting the extent of overlap within the forest plots, through the Cochran's χ' test (using 10% level of significance), and the I2 statistic (where 50% or higher values indicate substantial heterogeneity).[40] CIs around heterogeneity estimates were calculated using the heterogi command in Stata. Where heterogeneity was statistically significant, we conducted a sensitivity analysis to assess the influence of various study characteristics such as the quality of the study, age of participants and season(s) of participation.
Results
Cross-sectional study
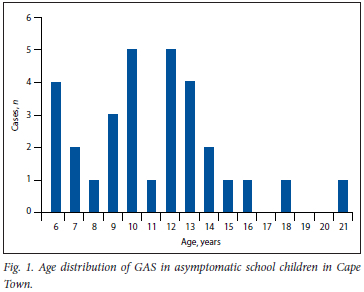
Nine-hundred and fifty healthy learners attending schools within the two communities were enrolled into our study over a 3-year period (2009 - 2011). The median age of the participants was 11 years (range 3-24 years); males constituted 43% of the study participants (Fig. 1).
Group A streptococci isolation
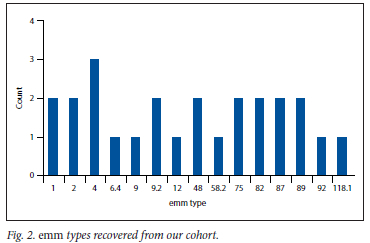
GAS was isolated from 31 participants corresponding to a carrier rate of 3.3% (95% CI 2% - 4%) among healthy school-aged learners (Table 1). GAS was recovered from almost all ages of learners (mean (standard deviation) age was 11.09 (3.6) years) with no association between GAS status and age (p=0.628). GAS isolation was not associated with seasonality (p>0.05) or gender (p>0.05). There was a statistically significant difference in the isolation rates of GAS by community, with pupils from Langa having increased odds of having isolated positive culture (odds ratio (OR) 3.13, 95% CI 1.38 - 7.09). Fig. 2 details the emm types recovered from our cohort.
Systematic review
The literature search results are reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement.[41] (Appendix, supplementary Fig. 1 details the search results.) We retrieved 1 647 records from electronic database searches which, together with 20 additional references identified through hand searching of relevant reference lists, rendered a total of 1 667 articles. After removal of duplicates, 681 references were assessed for eligibility. Of these, 647 articles were excluded based on title and abstract, leaving 34 articles requiring further evaluation as full-text articles. Finally, 19 studies met our inclusion criteria. Reasons for exclusion were duplicated publication (n=2), irrelevant populations (n=5), objectives (n=5) or outcomes (n=3).
Studies included in the analysis (n=19) comprised 18 peer-reviewed journal articles,[11-21,28-31,33,34,36] together with our school-based cross-sectional study (Table 2). From these studies, 16 were cross-sectional studies, while 3 were longitudinal studies. The study populations ranged from a minimum age of 2 months to a maximum age of 25 years. GAS was diagnosed by microbiological culture of throat swab specimens in all studies except one,[36] which utilised a rapid strep diagnostic method as a means of identifying GAS in the pharynx. Studies were conducted in schools (12 studies[11,12,14,18,20,21,28-33]), clinics/ outpatient departments (4 studies[15,17,19,36]), and homes (1 study[34]). Two studies did not state the setting of their sample population.[13,16]
All studies were conducted within a range of African regions: southern Africa (6 studies[11-13,15,19,32]), northern Africa (5 studies[14,17,20,29,30]), eastern Africa (5 studies[18,21,28,31,36]), western Africa (2 studies[16,33]) and central Africa (1 study[34]). Studies included in the analysis were conducted in both urban and rural areas or exclusively in urban areas; no studies were conducted exclusively in a rural area.
Supplementary Fig. 2 (Appendix) depicts risk of bias as assessed according to the Hoy criteria as modified by Werfalli et al. [26,27] Five studies had a low risk of bias,[18,28,30,32,34] while the rest of the studies had a moderate risk of bias. No study was deemed as having a high risk of bias.
This review found an overall prevalence of 9% (95% CI 6% - 11%) for GAS carriage in school children residing in African countries; test for heterogeneity, I2=97.9%, p<0.0001; 19 studies, n=30 842 (Fig. 3).
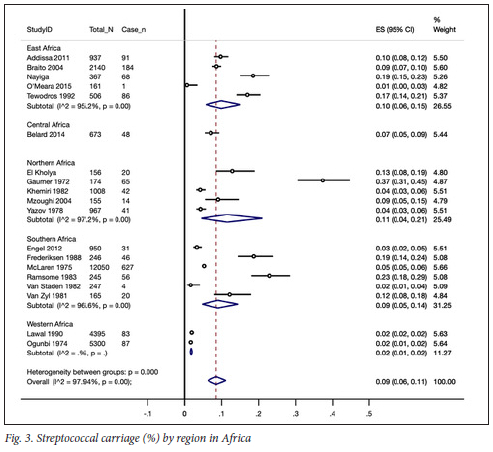
The regional pooled rates ranged between 2% and 11% across the five regions of the African continent. Pooled rates were similar across eastern, northern and southern Africa, ranging between 9% and 11% (71=16 studies). There was only one study within Central Africa with a pooled estimate of 7% (95% CI 5% - 9%), while in western Africa (n=2 studies), a lower pooled estimate of 2% (95% CI, 1% - 2%) was observed. The 95% CIs of the pooled estimates between western, eastern, northern and southern Africa combined did not overlap, thus indicating a statistically significant difference (Fig. 4).
Data for estimating the prevalence of GAS carriage by cross-sectional and longitudinal study design were provided by 16 and 3 studies, respectively. There was no difference between the pooled prevalence estimates for cross-sectional studies (8% (95% CI 6% - 11%)) and longitudinal studies (9% (95% CI 1% - 26%)); test for heterogeneity between subgroups, p=0.90 (Appendix, supplementary Fig. 3).
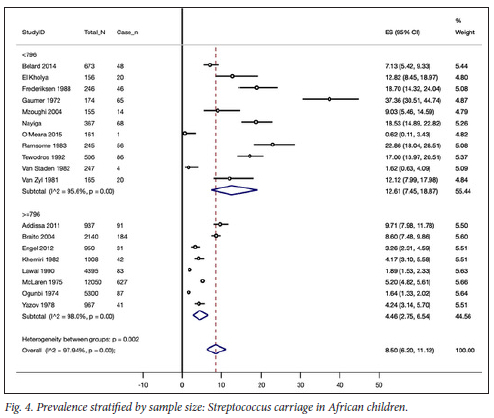
Eight studies had sample sizes of 796 or greater that together gave a combined prevalence of 4% (95% CI 3% - 7%). This contrasted with the combined estimate of 13% (95% CI 7% - 19%) rendered by inadequately powered studies n=11 studies). This difference was not statistically significant (test for heterogeneity between subgroups, p=0.1) (Fig. 4).
A limited number of studies reported on risk factors having a possible association with GAS colonisation in children. However, owing to a lack of reporting of the breakdown of prevalence according to possible risk factors, it was not possible to conduct a pooled meta-analysis, and therefore we discuss the findings in a narrative. Two studies, conducted in household residences, found the number living in the household not to be an associated risk factor for GAS carriage.'12341 Another study, conducted in a school environment, found GAS rates to be significantly higher in children coming from suburbs characterised by lower socioeconomic conditions, including a higher household density.[32]
In 3 studies, no significant difference was found between gender of participants,[28,32,34] while 1 study reported a higher GAS carrier rate in girls compared with boys.[29] Seasonality showed contrasting associations as regards peak GAS carriage. Two studies reported higher GAS carrier rates during the winter months,[14,17] whereas 2 others found a peak prevalence during summer months.[11,12] However, 1 study reported no seasonal variations in carriage rates.[32]
Given the considerable heterogeneity (I2=98% (95% CI 98% - 99%)), we conducted sensitivity analyses in respect of study quality and age ranges of participants. When quality assessment was considered, studies considered as having a low risk of bias ()i=5 studies) had a pooled prevalence of 9% (95% CI 5% - 14%) while studies with moderate risk of bias ()1=14 studies) had a pooled prevalence of 8% (95% CI 6% - 11%); this difference was not statistically significant (test for heterogeneity between subgroups, p=0.67). (Appendix, supplementary Fig. 5)
Six studies had a limited age range among their participants,[12,15-18,21] where the age ranges were not inclusive of the whole age range stipulated in our inclusion criteria; therefore we tested the potential contribution to heterogeneity within our meta-analysis, of studies having incomplete age ranges of participants. The sensitivity analysis revealed a pooled prevalence estimate of 7% (95% CI 5% - 10%) within studies that had a complete age range and 12% (95% CI 5% - 21%) in studies having only some of the age ranges within our inclusion criteria. This difference was not statistically significant (test for heterogeneity between subgroups, p=0.21). (Appendix, supplementary Fig. 6)
There were not enough data to analyse the possible influence of seasonality on the heterogeneity.
Discussion
Carriage of GAS in the pharynx of asymptomatic individuals is an important factor in the pathogenesis of these infections and, most importantly, in maintaining the organisms in the population. GAS are human-specific pathogens and other hosts or vectors do not play a major role in the maintenance or spread of the organisms. Therefore, persistence of the organisms on the pharyngeal mucosa or skin is considered a critical determinant for their survival in the human population. It is not surprising that carriage rates >10% have been reported in school settings in both industrialised and developing countries.[42-44] In a longitudinal study[5] of carriage in 100 schoolchildren over a 4-year period, it was observed that throughout each of the 4 years, 27 - 32% of the cohort were GAS carriers and the mean prevalence of carriage was 15.9%. In this study, subjects carried a single emm type for a mean of 10.8 weeks with a range of 3-34 weeks. In a more recent longitudinal study[45] of school-age subjects with new pharyngeal acquisitions of GAS, 20% of the subjects had persistent carriage of the same emm type for 12 weeks or longer, with an average of 23 weeks and a range of 12 - 53 weeks. The latter study was designed to detect changes in serum antibody responses against a panel of GAS antigens following new pharyngeal GAS acquisitions.
Of particular interest was the observation that of the subjects who acquired a new emm type, 63% were asymptomatic yet mounted an immune response to at least one streptococcal antigen. These episodes most likely represented asymptomatic new infections that previously may have simply been considered carriage of GAS and of little importance in relation to post-streptococcal immune sequelae. Thus, GAS carriage not only reflects the reservoir of circulating strains, but may also be a sampling of organisms that have caused asymptomatic true infections in the majority of the subjects found to have positive cultures.
We conducted this cross-sectional study to investigate GAS carriage rates in a population of SA schoolchildren. Our present rate of 3.3% GAS carriage differs from those previously reported in SA settings: 16.8% (urban),[13] 5.2% (lower-socioeconomic peri-urban households)[11] and 1.62% (remote traditional).[13]
Furthermore, we did not observe a seasonal variation between winter and summer isolation rates, which may be related in part to the climatic differences between Cape Town and the other regions in SA where the respective studies were conducted.[12] Prevalence rates of GAS carriage showed no significant difference between male and female participants.
GAS carriage was significantly associated with the Langa school district, having more than three times the odds of GAS carriage (p=0.038) compared with Bonteheuwel. Given that Langa rates poorly in several key indicators of socioeconomic status, the statistically significant higher prevalence observed in Langa may be explained by socioeconomic factors such as overcrowding and lack of suitable housing. This finding corroborates those of earlier studies conducted in SA among urban Black communities,[11,13] historically known to have resided in overcrowded poverty-stricken conditions. Of interest, in our study, carriage of GAS in communities with apparently high rates of rheumatic fever and RHD is remarkably low (overall 3.3%). A similar low carriage rate (3.7%) of GAS was reported in a study from the Northern Territory of Australia.[46]
We analysed our cross-sectional findings in the context of published African data through a meta-analysis. The pooled GAS carriage estimate of 9% (95% CI 6% - 11%) among African schoolchildren was lower than the 12% (95% CI 9% - 14%) previously reported in a systematic review comprising studies in low-, middle- and high-income countries.[9] The low pooled estimate of 2% (95% CI 1% -2%) in western Africa contrasted significantly with pooled prevalence rates in northern (11%), eastern (10%), southern (9%) and central Africa (7%).
We further considered the impact of sample size on the overall prevalence estimate using n=796 as the cut-off, based on recommended parameters.[39] Adequately powered studies rendered a pooled prevalence estimate of only 4% GAS carriage (n=8 studies), considerably lower than the combined 12% estimate among smaller inadequately powered studies. Given that studies of pharyngitis report GAS prevalence of >20%,[47] our findings emphasise the association between GAS and pharyngitis, given the low background rate of GAS carriage found within our review.
This meta-analysis presents the first comprehensive synthesis of the prevalence of GAS in children aged 5 -25 residing in Africa, and reveals two important findings: (i) there is a relatively low rate of carriage of GAS in asymptomatic schoolchildren residing in Africa; and (ii) there are regional differences across the African continent as regards the rate of GAS carriage, with the western and northern African regions having rates of GAS carriage that are lower and higher respectively than those of eastern, central and southern African countries, which demonstrated similar rates of carriage. The data from this review had contradicting results for risk factors, such as gender, seasons and crowding, thought to be associated with GAS colonisation.
In comparison with single studies among schoolchildren in other low-income countries, our pooled rate of GAS carriage was similar to the 10% found in Nepal,[48] which is more than the prevalence of 8.4% found in Chennai, India,[49] but less than the 15% observed in Iraq.[50] Our results were robust regarding study design, with cross-sectional studies and longitudinal studies having similar rates of 8% and 9% respectively, which agree with the overall prevalence. Additionally, considering an assessment of the quality of included studies, they revealed similar rates with 9% for low-risk-of-bias studies and 8% for studies with a moderate risk of bias.
One of the main strengths of this review is the comprehensive search of multiple databases using an African search filter for the first time. We systematically and vigorously assessed all the data available with no language exclusions, using the most recently published standard quality assessment tools for prevalence studies. Furthermore, for the first time in GAS prevalence reviews, double arcsine transformation was used to stabilise the variance of primary studies before pooling, thus limiting the impact of studies with either small or large prevalence on the overall pooled estimates, as well as across major subgroups.
Significant heterogeneity in the prevalence estimates is regarded as a limitation to this systematic review. Attempts to completely explain the heterogeneity were unsuccessful in terms of subgroup analyses. It is difficult to make comparisons between populations unless every effort is made to use standard epidemiological and laboratory efforts in each survey. Therefore, caution must be applied in trying to draw too many conclusions about differences in prevalence rates that are in the same range. Also, four articles were translated into English[13,19,29,30] which might have led to information loss, thus reducing the quality of data extracted.
Conclusions
The findings of this review and primary study provide important baseline information for healthcare workers regarding the carriage of GAS among asymptomatic children in Africa. Given the low rate of GAS carriage, it is highly likely that GAS isolated during pharyngitis plays a pathogenic role.
Declaration. None.
Acknowledgements. None.
Author contributions. MEE conceived of the study. MEE designed the primary study and together with HM and LA, designed the secondary study. JD, AW and BMM gave conceptual advice, interpreted the data and edited the manuscript. MEE implemented and managed the primary study, conducted the analysis with BMM, interpreted the data and wrote the manuscript; HM implemented and managed the secondary study. SN recruited participants and together with LA and DDB collected and managed the data and assisted with the analysis. All authors read and approved the final draft of the manuscript.
Funding. This work is based on the research supported in part by the National Research Foundation of South Africa (grant no. 116287) and National Institutes of Health (UO1AI060592).
Conflicts of interest. None.
References
1. Bisno AL, Gerber MA, Gwaltney JM, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 2002;35(2):113-125. https://doi.org/10.1086/340949 [ Links ]
2. Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010. A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197-2223. https://doi.org/10.1016/S0140-6736(12)61689-4 [ Links ]
3. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013. A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386(9995):743-800. https://doi.org/10.1016/S0140-6736(15)60692-4 [ Links ]
4. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013. A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385(9963):117-171. https://doi.org/10.1016/s0140-6736(14)61682-2 [ Links ]
5. Martin J, Green M, Barbadora K, Wald E. Group A streptococci among school-aged children. Clinical characteristics and the carrier state. Pediatrics 2004;114(5):1212-1219. https://doi.org/10.1542/peds.2004-0133 [ Links ]
6. Kaplan E, Gastanaduy A, Huwe B. The role of the carrier in treatment failures after antibiotic therapy for group A streptococci in the upper respiratory tract. J Lab Clin Med 1981;98(3):326-335. [ Links ]
7. Cockerill F III, MacDonald K, Thompson R, et al. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA 1997;277(1):38-43. [ Links ]
8. Mazón A, Gil-Setas A, Sota de la Gándara L, Vindel A, Sáez-Nieto J. Transmission of Streptococcus pyogens causing successive infections in a family Clin Microbiol Infect 2003;9(6):554-559. https://doi.org/10.1046/j.1469-0691.2003.00567.x [ Links ]
9. Shaikh N, Leonard E, Martin J. Prevalence of streptococcal pharyngitis and streptococcal carriage in children. A meta-analysis. Pediatrics 2010;126(3):e557-564. https://doi.org/10.1542/peds.2009-2648 [ Links ]
10. Dale J, PenfoundT, Chiang E, Walton W New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 2011;29(46):8175-8178. https://doi.org/10.1016/j.vaccine.2011.09.005 [ Links ]
11. McLaren MJ, Hawkins DM, Koornhof HJ, et al. Epidemiology of rheumatic heart disease in black shcoolchildren of Soweto, Johannesburg. BMJ 1975;3(5981):474-478. https://doi.org/10.1136/bmj.3.5981.474 [ Links ]
12. Ransome OJ, Roode H, Spector I, Reinach SG. Pharyngeal carriage of group A beta-haemolytic streptococci in coloured and Indian schoolchildren. S Afr Med J 1983;64(20):779-781. [ Links ]
13. Van Staden D, Nel W, van Zyl M. Group A β-haemolytic streptococci in a traditional Black community. S Afr Med J 1982;2(16):569-570. [ Links ]
14. El Kholya A, Sorourh H, Houser B, et al. A three-year prospective study of streptococcal infections in a population of rural Egyptian school children. J Med Microbiol 1973;6(1):101-110. https://doi.org/10.1099/00222615-6-1-101 [ Links ]
15. Frederiksen B, Henrichsen J. Throat carriage of Streptococcus pneumoniae and Streptococci!' pyogenes among infants and children in Zambia. J Trop Pediatr 1988;34(3):114-117. https://doi.org/10.1093/tropej/34.3.114 [ Links ]
16. Ogunbi LO, Lawal A, Lasi Q, Ogunbi O. Streptococcal pyoderma in a Lagos school population. Niger Med J 1974;4:178-180. [ Links ]
17. Mzoughi R, Bouallegue O, Selmi H, Ben Said H, Essoussi AS, Jeddi M. Group A streptococci in children with acute pharyngitis in Sousse, Tunisia. East Mediterr Health J Egypt 2004;10(4-5):488-493. [ Links ]
18. Tewodros W, Muhe L, Daniel E, Schalen C, Kronvall G. A one-year study of streptococcal infections and their complications among Ethiopian children. Epidemiol Infect 1992;109(2):211-225. https://doi.org/10.1017/s0950268800050172 [ Links ]
19. Van Zyl M, van Staden D, Potgieter M. [Beta-haemolytic streptococci as a cause of sore throat in the Pretoria area]. S Afr Med J 1981;59(22):783-784. [ Links ]
20. Yazov, L, Petros WG, Stump E. Epidemiological studies on rheumatic heart disease and streptococcal carriers among school-children in Addis-Ababa, Ethiopia. Z Rheumatol 1978;37(9-10):304-308. [ Links ]
21. Braito A, Galgani I, Mohammed M, et al. Epidemiology of streptococcus group A in school aged children in Pemba. East Afr Med J 2004;81(6):307-312. https://doi.org/10.4314/eamj.v81i6.9180 [ Links ]
22. Carapetis J, Steer A, Mulholland E, Weber M. The global burden of group A streptococcus disease. Lancet Infect Dis 2005;5(11):685-694. https://doi.org/10.1016/s1473-3099(05)70267-x [ Links ]
23. Engel ME, Haileamlak A, Zühlke L, et al. Prevalence of rheumatic heart disease in 4720 asymptomatic scholars from South Africa and Ethiopia. Heart 2015;101(17):1389-1394. https://doi.org/10.1136/heartjnl-2015-307444 [ Links ]
24. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.C updated March 2011]. 2011. https://handbook-5-1.cochrane.org (accessed 18 November 2022). [ Links ]
25. Pienaar E, Grobler L, Busgeeth K, Eisinga A, Siegfried N. Developing a geographic search filter tc identify randomised controlled trials in Africa. Finding the optimal balance between sensitivity and precision. Health Info Libr J 2011;28(3):210-215. https://doi.org/10.1111/j.1471-1842.2011.00936.x [ Links ]
26. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies. Modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65(9):934-939. https://doi.org/10.1016/j.jclinepi.2011.11.014 [ Links ]
27. Werfalli M, Musekiwa A, Engel ME, Ross I, Kengne AP, Levitt NS. The prevalence of type 2 diabetes mellitus among older people in Africa. A systematic review study protocol. BMJ Open 2014;(6):4:e004747. https://doi.org/10.1136/bmjopen-2013-004747 [ Links ]
28. Abdissa A, Asrat D, Kronvall G, et al. Throat carriage rate and antimicrobial susceptibility pattern of group A streptococci (GAS) in healthy Ethiopian school children. Ethiop Med J 2011;49(2):125-130. [ Links ]
29. Gaumer B, Dudek. [Survey of healthy carriers of beta Streptococcus haemolyticus in the school population at Sousse (Tunisia)]. Tunis Med 1972;50(3):181-185. [ Links ]
30. Khemiri F, Brari M. [Beta-hemolytic streptococci in the schoolchildren of the Tunis governorship]. Arch Inst Pasteur Tunis 1982;59(2-3):243-250. [ Links ]
31. Nayiga I, Okelio E, Lwabi P, Ndeezi G. Prevalence of group A streptococcus pharyngeal carriage and clinical manifestations in school children aged 5-15 yrs in Wakiso District, Uganda. BMC Infect Dis 2017;17(1):248. https://doi.org/10.1186/sl2879-017-2353-5. [ Links ]
32. Engel ME. A study of determinants and prevalence of Rheumatic Heart Disease in Cape Town. Thesis]. University of Cape Town, Faculty of Health Sciences ,Department of Medicine, 2012 (accessed 1 December 2022). Available from. http://hdl.handle.net/11427/3381 [ Links ]
33. Lawal SF, Odugbemi T, Coker AO, Solanke EO. Persistent occurrence of beta-haemolytic streptococci in a population of Lagos school children. J Trop Med Hyg 1990;93(6):417-418. [ Links ]
34. Bélard S, Toepfner N, Arnold B, Aiabi AS, Berner R. β-hemolytic streptococcal throat carriage and tonsillopharyngitis. A cross-sectional prevalence study in Gabon, Central Africa. Infection 2014;43(2):177-183. https://doi.org/10.1007/sl5010-014-0709-y [ Links ]
35. Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet 2005;366(9480):155-168. https://doi.org/10.1016/s0140-6736(05)66874-2 [ Links ]
36. O'Meara WP, Mort JA, Laktabai J, et al. Etiology of pediatric fever in western Kenya. A case-control study of falciparum malaria, respiratory viruses, and streptococcal pharyngitis. Am J Trop Med Hyg 2015;92(5):1030-1037. https://doi.org/10.4269/ajtmh.l4-0560 [ Links ]
37. Nyaga V, Arbyn M, Aerts M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch Pub Health 2014;72(1):39. https://doi.org/10.1186/2049-3258-72-39 [ Links ]
38. Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci. Systematic review and implications for vaccine development. Lancet Infect Dis 2009;9(10):611-616. https://doi.org/10.1016/s1473-3099(09)70178-1 [ Links ]
39. Daniel W. Bio statistics. A Foundation for Analysis in the Health Sciences. New Jersey. John Wiley & Sons, 1999. [ Links ]
40. Hozo S, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. https://doi.org/10.1186/1471-2288-5-13 [ Links ]
41. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Met a-Analyses. The PRISMA Statement (reprinted from Annals of Internal Medicine). Phys Ther 2009;89(9):873-880. [ Links ]
42. Gunnarsson RK, Holm SE, Soderstrom M. The prevalence of beta-haemolytic streptococci in throat specimens from healthy children and adults. Implications for the clinical value of throat cultures. Scand J Prim Health Care 1997;15(3):149-155. https://doi.org/10.3109/02813439709018506 [ Links ]
43. Jasir A, Noorani A, Mirsalehian A, Schalen C. Isolation rates of Streptococcus pyogenes in patients with acute pharyngotonsillitis and among healthy school children in Iran. Epidemiol Infect 2000;124(1):47-51. https://doi.org/10.1017/s0950268899003088 [ Links ]
44. Kim S, Lee NY. Epidemiology and antibiotic resistance of group A streptococci isolated from healthy schoolchildren in Korea. J Antimicrob Chemother 2004;447-450. https://doi.org/10.1093/jac/dkh363 [ Links ]
45. Hysmith ND, Kaplan EL, Cleary PP, et al. Prospective longitudinal analysis of immune responses in pediatric subjects after pharyngeal acquisition of group A streptococci. J Pediatric Infect Dis Soc 2017 6(2);187-196. https://doi.org/10.1093/jpids/piw070 [ Links ]
46. McDonald M, Towers R, Fagan P, et al. Recovering streptococci from the throat, a practical alternative to direct plating in remote tropical communities. J Clin Microbiol 2006;44(2):547-552. https://doi.org/10.1128/jcm.44.2.547-552.2006 [ Links ]
47. Rimoin AW, Walker CLF, Hamza HS, et al. The utility of rapid antigen detection testing for the diagnosis of streptococcal pharyngitis in low-resource settings. Int J Infect Dis 2010;14(12):e1048-e1053. https://doi.org/10.1016/j.ijid.2010.02.2269 [ Links ]
48. Dumre SP, Sapkota K, Adhikari N, et al. Asymptomatic throat carriage rate and antimicrobial resistance pattern of Streptococcus pyogenes in Nepalese school children. Kathmandu Univ Med J 2009;7(28):392-396. https://doi.org/10.3126/kumj.v7i4.2760 [ Links ]
49. Lloyd C, Jacob S, Menon T. Pharyngeal carriage of group A streptococci in school children in Chennai. Indian J Med Res 2006;124(2):195-198. [ Links ]
50. Mohammed M, Saieh S. Carriage state of GAβHS among Yemeni school children and the upper limit of normal for ASO in different population groups. Iraqi J Sci 2010;51:63-70. [ Links ]
 Correspondence:
Correspondence:
M E Engel
mark.engel@uct.ac.za
Accepted 24 October 2022
* These authors contributed equally to this work.
† Deceased.














