Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.4 Pretoria abr. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i4.16772
RESEARCH
Placental histopathology, maternal characteristics and neonatal outcome in cases of preterm birth in a high-risk population in South Africa
K E SprongI; C A WrightII, III; M MabengeIV; S GovenderV
IBSc, PhD; Department of Biochemistry and Microbiology, Faculty of Science, Nelson Mandela University, Gqeberha, South Africa
IIMB BCh, PhD; Lancet Laboratories, Johannesburg, South Africa
IIIMB BCh, PhD; Division of Anatomical Pathology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IVMB ChB, MMed (O&G); Department of Obstetrics and Gynaecology, Dora Nginza Hospital, Walter Sisulu University, Gqeberha, South Africa
VBSc, PhD; Department of Biochemistry and Microbiology, Faculty of Science, Nelson Mandela University, Gqeberha, South Africa
ABSTRACT
BACKGROUND: Preterm birth remains a global health concern and is one of the most common pregnancy complications associated with perinatal morbidity and mortality
OBJECTIVE: To investigate placental pathology and its associations with obstetric, maternal and neonatal outcomes in the Eastern Cape region of South Africa (SA) in order to help understand its associations with preterm birth in that region
METHODS: In this prospective study, placentas were collected consecutively from patients attending a public tertiary referral hospital in SA, delivering preterm (n=100; 28 - 34 weeks gestational age) and term (n=20; >36 weeks gestational age). Placentas were submitted for histopathology, and comparisons with maternal characteristics and neonatal outcomes in preterm birth were undertaken
RESULTS: Histological analysis revealed pathology in all preterm placentas (100%), with maternal vascular malperfusion (47%) and abruptio placentae (41%) most commonly identified. Acute chorioamnionitis (21%) was associated with term births (p=0.002). Maternal characteristics and neonatal outcomes significantly associated with preterm birth included pre-eclampsia (p=0.006), neonatal respiratory distress syndrome (p=0.004) and neonatal jaundice (p=0.003). Intrauterine demise (p=0.004) and alcohol abuse (p<0.005) were significantly associated with term delivery. The number of mothers delivering preterm who were HIV-positive was high (41%
CONCLUSION: The pathology identified in all preterm placentas supports the need to update institutional policies for submission of placentas from all preterm births for histopathology, particularly in countries with a high burden of preterm birth
Preterm birth (PTB) is defined as birth before 37 weeks' gestation, with subcategories of extremely preterm (<28 weeks), very preterm (28 - 32 weeks), moderate preterm (32 - 34 weeks) or late preterm (34 - 37 weeks), according to gestational age.[1] PTB is one of the most common pregnancy complications and is the leading cause of perinatal morbidity and mortality.[2] Latest global PTB estimates, published in 2019, indicate that there were an estimated 14.84 million live PTBs in 2014, with Asia and sub-Saharan Africa accounting for 12 million of these.[3] The global PTB rate is highest in low- to middle-income countries, as the global rate was estimated at 9.8% (confidence interval (CI) 8.3 - 10.9) in 2000, and 10.6% (CI 9.0 - 12.0) in 2014, compared with South Africa ( SA) at 10.04% (CI 7.3 - 13.29) in 2000, and 12.43% (CI 8.63 - 17.13) in 2014.[3]
PTB is often multifactorial in nature and can be divided into indicated or spontaneous PTB.[4,5] Risk factors associated with indicated PTB include pre-eclampsia, abruptio placentae, fetal distress and intrauterine growth restriction. Spontaneous PTB, along with preterm labour and preterm premature rupture of membranes (PPROM), is regarded as a syndrome and has multiple aetiologies such as infection, inflammation, uterine overdistension and maternal and fetal/placental vascular disease.'61 Maternal risk factors for PTB include previous PTB, high blood pressure, diabetes, periodontal disease, multiple gestations, race, weight, stress, substance abuse and intrauterine infections.[4,6-8]
The placenta is the principal organ of pregnancy responsible for metabolic, endocrine and respiratory processes and provides barrier protection for the fetus against infection and maternal environmental exposures throughout gestation.'91 Therefore, the placenta plays a crucial role in the development of the fetus and in fetal or neonatal morbidity and mortality. When placental function is compromised, the fetus is subjected to an adverse intrauterine environment that can trigger several pregnancy complications, including PTB.[9]
Changes in placental microanatomy and inflammation detected by histopathological examination can be classified into acute or chronic inflammatory responses of the placenta, with a maternal inflammatory response (acute chorioamnionitis, ACAM) and/or fetal inflammatory response (umbilical vasculitis/funisitis), with varying neonatal outcomes.[10,11] Brink et al.[12] observed an 'abnormal' accelerated villous maturation pattern in SPTB and preterm deaths, which suggested an inability of the placenta to adapt, and may be a trigger for SPTB. Funisitis was the only inflammatory response significant for SPTB.
Investigation of placental pathology following PTB is a powerful tool for elucidating the underlying aetiology of the delivery, and may help identify neonates at risk of adverse outcomes.[13,14] There are vital benefits to examination of the placenta, including immediate diagnosis of treatable conditions in mother and neonate, estimating the risk for recurrent PTB and guiding the management of future pregnancies.[15]
This study explored the association between placental histology, maternal and neonatal outcomes and clinical presentation of PTB in a tertiary hospital setting in SA.
Methods
This prospective hospital-based study included patients who delivered in the labour ward at a tertiary referral hospital in the Eastern Cape Province, SA, from March 2016 to November 2017. This hospital services a large area and only patients who present with complicated deliveries are eligible for admission to the maternity unit. Ethics approval was granted from the Nelson Mandela University Research Ethics Committee (Human) (ref. no. H15-SCI-BCM-001), and permission to conduct the study was obtained from the Eastern Cape Department of Health (ref. no. EC_2015RP8_78) and from the acting clinical governance manager at the hospital. Patients were recruited and informed consent was obtained, including permission for the collection of their placenta after delivery and for their medical records to be accessed for maternal and neonatal chart review. Patients received unique and anonymous study numbers for entry into a de-identified database. Placentas (n=100) from preterm deliveries (28 - 34 weeks gestational age) were collected as the test cohort, while 20 placentas from term deliveries (>38 weeks gestational age) were collected as the control cohort. Placentas were collected consecutively according to confirmation of inclusion criteria of gestational age and whether the mother was able and willing to give informed consent after delivery. The gestational age range was selected as, according to the World Health Organization (WHO),[16] viability of preterm neonates reaches a 50% chance of survival at 34 weeks gestational age in low- to middle-income countries, compared with 24 weeks in high-income countries.
Formalin-fixed placentas underwent routine macroscopic examination and histology at the National Health Laboratory Services (NHLS). All cases were evaluated histologically by a single pathologist without knowledge of clinical details, using a standardised placental macroscopic evaluation protocol and histology template approved and validated by the NHLS laboratory. The macroscopic details (including placental weight, dimensions, length, insertion and hypercoiling of umbilical cord, membrane attachment and surface characteristics) for each placenta were recorded and photographed as per routine protocol. The only clinical information available was gestational age and whether live-born or stillbirth. Based on the macroscopic and microscopic data, a histological diagnosis was made according to the Amsterdam Consensus Classification System,[17] and the microscopic details and conclusion entered on the validated histology template. Hypercoiling of the umbilical cord is defined as a cord coil index of >0.3. Fetal distress, defined as progressive fetal hypoxia and/or acidaemia secondary to inadequate fetal oxygenation. Villitis of unknown aetiology (VUE) is a destructive villous inflammatory lesion characterised by the infiltration of maternal T cells (CD8+ cytotoxic T cells) into chorionic villi. Maternal vascular malperfusion (MVM) consists of placental pathological findings seen in the maternal decidual vessels, reflecting abnormal spiral artery remodelling, as well as in the villous parenchyma, reflecting abnormalities in oxygenation and flow dynamics in the intervillous space.
A maternal chart review collated demographic and obstetric characteristics such as maternal race, age, parity, smoking status, diabetes, pre-eclampsia, HIV status and the mode of delivery (caesarean section (CS) or normal vertex delivery (NVD)). Certain fetal/neonatal characteristics that were available shortly after delivery were recorded in the maternal chart before a neonatal chart was opened. Demographic information was required to investigate patterns around different disease conditions in the study population group.[18] The histopathology data were compared with various maternal characteristics, obstetric diagnoses and neonatal outcomes in order to identify potential correlations.
A neonatal chart review was performed for each neonate from preterm and term delivery. Separate reports for neonates from twins were analysed independently in consultation with the statistician. General characteristics such as live birth/stillbirth, neonatal gender, weight and APGAR score were recorded at birth. Neonatal charts were later reviewed for outcomes, i.e. respiratory distress syndrome, sepsis, pneumonia, neonatal jaundice and necrotising enterocolitis. Standard operating practice in SA hospitals does not include routine autopsy of the fetus in the event of a stillbirth.
Data were analysed using IBM SPSS Statistics for Windows, version 24.0. (IBM Corp, USA).
Descriptive data were used to compile means, standard deviations and frequency tables. Differences between variables were assessed using a X2 test for categorical variables or a t-test for quantitative variables with a normal distribution. Chi-squared tests of association and Pearson product moment correlations were used to establish relationships between various obstetric, maternal and neonatal characteristics. Where these associations were statistically significant, Cramers V (V>0.5 is considered a large practical significance) and correlation coefficient r (r>0.3 is considered practically significant) were used to measure the strength of the relationship. P<0.05 was considered statistically significant.
Results
Of the total preterm cohort, 100 women and 110 neonates were recruited, while the term control cohort had 20 women and 21 neonates. Of the 131 neonates, 22 were part of a twin pregnancy (11 sets of twins). Ethnicity of the cohort was determined as 87 indigenous African women and 33 women of mixed ethnicity. A number of umbilical cord abnormalities were observed in preterm placentas, such as hypercoiling (n=7), velamentous insertion (n=3), marginal insertion (n=4) and a true knot (n=1), but not in term placentas. Separate reports were generated per twin for the 11 twin pregnancies, and macroscopic examination showed that 8 were monochorionic and 3 were dichorionic placentas.
Placental pathology was present in 100% of placentas from preterm births and 70% of term placentas. Commonly observed placental lesions included acute chorioamnionitis (ACAM; 27.5%, Fig. 1), maternal vascular malperfusion (MVM; 40%, Fig. 2), abruptio placentae (AP; 39%, Fig. 3), fetal vascular malperfusion (FVM; 7.5%, Fig. 4) and villitis of unknown aetiology (VUE; 7.5%).
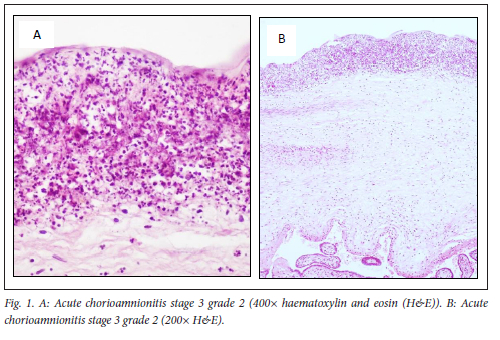
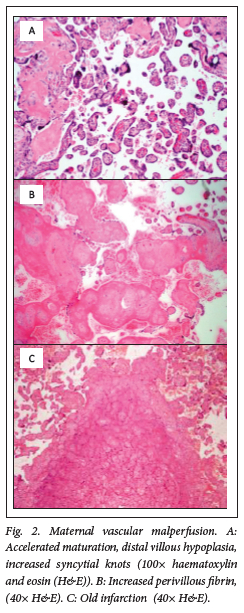
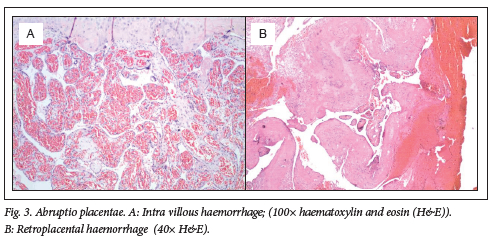
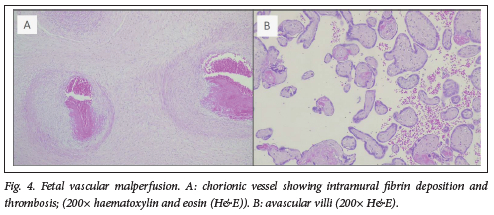
More than one pathology was present per placenta (Table 1). ACAM was detected more frequently in term placentas 11/20 (55%, P=0.002) than in preterm placentas 21/100 (21%), with a small practical significance (Cramer's V=0.29). MVM and AP were observed more frequently in preterm placentas (47% and 41%) than term placentas (40% and 35%). However, this was not statistically significant (p=0.566 and p=0.617, respectively). No other pathologies were significantly associated with preterm, rather than term, births in this cohort. Fifty-three preterm and nine term placentas showed more than one category of placental histopathology (Table 1).
The maternal and obstetric characteristics are listed in Table 2. The age of the 100 patients in the preterm group ranged from 18 - 44 years, with a median of 29 years. The age of the 20 term patients ranged from 18 - 36 years, with a median of 27 years. There was no statistically significant difference in the median age between the two groups. There was a significant difference between preterm and term groups with respect to the average parity (p=0.001), which was 1.85 (range 0 - 5) for the preterm group and 0.9 (range 0 - 3) for the term group, with a large practical significance (Cohen's d=0.82).
The study group was predominantly of African ethnicity (87/120), with the remaining being women of mixed ethnicity (33/120, p=0.784). Mode of delivery was almost equally distributed in the study group (Table 2). Substance abuse (alcohol) was reported for only 14/100 (14%) of women who delivered preterm compared with 10/20 (50%, p<0.005; Cramer's V=0.34) of women who delivered at term, with a moderate practical significance. Diagnosis of pre-eclampsia was reported for only 1/20 (5%) of women who delivered at term, compared with 36/100 (36%) of women who delivered preterm (p=0.006; Cramer's V=0.25), with a small practical significance. Pre-eclampsia was statistically significantly associated with MVM using a x2 test (p<0.001, Cramer's V=0.356) with a moderate practical significance. Smoking, absent end diastolic flow (AEDF), haemolysis elevated liver enzymes and low platelets syndrome (HELLP) and premature rupture of membranes (PROM) were not significantly associated with preterm, rather than term deliveries, in this study group. Forty-five (37.5%) patients were HIV-positive (41 in the preterm group, 4 in the term group), and 10 (8.3%) patients had diabetes in total, although not significantly associated with PTB in this study (p=0.077 and p=0.237, respectively).
Pearson x2 tests were used to determine the relationship between maternal/obstetric outcomes and placental pathologies. Statistically significant relationships were found between women who smoke and VUE (p=0.010, Cramer's V=0.226), chorangiosis (p=0.018, Cramer's V=0.207) and retroplacental haemorrhage (p=0.006, Cramer's V=0.239). Women who reported consuming alcohol during pregnancy were 3.57 times more likely to develop ACAM than those who abstained from alcohol (p=0.004, Cramer's V=0.251). Women who were diagnosed with HELLP syndrome were more likely to have placental MVM (p=0.001, Cramer's V=0.282) and FVM (p=0.004, Cramer's V=0.251) than those without HELLP. Women who experienced PROM were 5.14 times more likely to have ACAM (p=0.004, Cramer's V=0.253) and women who did not have PROM were 5.14 times more likely to have MVM (p=0.024, Cramer's V=0.198). Conversely, women who were diagnosed with pre-eclampsia were 3.11 times more likely to have MVM (p=0.004, Cramer's V=0.250), and women who did not have pre-eclampsia were 9.92 times more likely to have ACAM (p=0.0004, Cramer's V=0.310). AEDF was significantly associated with MVM (p=0.003, Cramer's V=0.260). No other relationships were statistically significant.
The adverse neonatal outcomes associated with preterm and term births are presented in Table 3. Preterm neonates weighed less (mean (standard deviation (SD)) 1 704 (571) g) than neonates born at term (mean (SD) 3 030 (642) g, p<0.0005). In total, more females were born in this study group than males (78/131 (60%) v. 53/131 (40%)), although not statistically significant p=0.993). Nine pregnancies resulted in stillborn infants, 6/110 macerated stillbirths (including a set of twins) from the preterm cohort and 3/21 macerated and 1/21 fresh stillbirth from the term cohort (p=0.004, Cramers V=0.27). In total, 21/121 (16%) neonates died in the neonatal period (within 28 days) and 8/121 (6%) neonates were born with congenital abnormalities, although not statistically significant (p=0.747 and p=0.743, respectively). Fetal distress was reported in 28/110 (25%) of preterm pregnancies and only 2/21 (10%) of term pregnancies, although not statistically significant (p=0.09).
Neonatal complications that were significantly associated with PTB were respiratory distress (RDS) (p=0.004; Cramer's V=0.26) and neonatal jaundice (NNJ) (p=0.003; Cramer's V=0.27), both with small practical significance. Sepsis, which was diagnosed clinically in 28/131 neonates (p=0.122), was most commonly caused by Klebsiella pneumoniae and Acinetobacter baumanii; 17 neonates were also diagnosed with pneumonia. Intrauterine growth restriction (IUGR), transient tachypnoea of the newborn (TTN), hyaline membrane disease (HMD), and patent ductus arteriosus (PDA) were not significantly associated with preterm, rather than term neonates, in this study group.
A Pearson product-moment correlation was run to determine the relationship between placental pathology and neonatal outcomes (Table 4). For this analysis, a correlation coefficient r is statistically significant at the 0.05 level for n=131 if r>0.179 and practically significant, regardless of the sample size, if r>0.300, therefore significant (both statistically and practically) if r>0.300. There was a strong positive correlation between gestational age and ACAM (r=0.258) between neonatal weight and ACAM (r=0.236) and between neonatal demise and FVM (r=0.202), which was statistically significant. There was a strong, negative correlation between HMD and ACAM (r=-0.181) and between neonatal weight and MVM (r=-0.225), which was statistically significant. A strong, statistically and practically significant positive correlation was determined between fetal distress and MVM (r=0.319).
Discussion
SA has a higher rate of PTB than developed countries, in that the PTB rate increased from 11.39% in 2010 to 12.43% in 2014 compared with 9.56% in the USA in 2014.[3,19] PTB is a multifactorial syndrome with a variety of risk factors and long-term health consequences for the child. Placental pathology provides important diagnostic information to ascertain the associations of PTB.[20]
Macroscopic examination of placentas from this study group showed a number of umbilical cord abnormalities, including hypercoiling (n=7), velamentous insertion (n=3), marginal insertion (n=4) and a true knot (n=1), which can cause diminished blood flow predisposing the placenta to FVM and be associated with fetal distress, intrauterine growth restriction and perinatal mortality.[21] In this study, FVM was significantly associated with neonatal demise (r=0.202). In contrast, a prospective observational study by Kulkarni et al.[22] found that MVM was more commonly associated with fetal demise than FVM (58.4% v. 19%).
A study by Tantbirojn et al.[23] analysed cord abnormalities in association with placental histology and adverse perinatal outcome. They found that FVM was significantly more prevalent (p<0.001) in cases of gross cord abnormalities (29/102; 28.4%) than in matched controls, with adverse neonatal outcome (5/84; 6%). Adverse neonatal outcomes including stillbirth (p<0.001), IUGR (p=0.009) and meconium staining as a marker for fetal distress (p<0.001) were also significantly associated with cord abnormalities compared with controls.[23] In the present study, 7/15 (27%) cord abnormalities were associated with ACAM with a fetal inflammatory response, and of the 7 neonates from those births, 4 neonates had severe congenital deformities, 2 neonates were diagnosed with sepsis and 1 neonate was jaundiced. It is recommended that placentas with gross cord abnormalities be sent for histopathology to look for the presence of FVM, which may predict adverse perinatal outcome. The co-existence of gross cord abnormalities and ACAM with fetal inflammatory response (FIR) warrants further investigation.
In this study, ACAM was present in 21/100 (21%) preterm placentas and significantly associated with 11/20 (55%) term placentas (p=0.002). Microbial infection of the amniotic cavity is often polymicrobial in nature. However, the human Ureaplasma spp. are the micro-organisms most frequently associated with ACAM and PTB.'24,251 The host immune response to intra-amniotic infection is typically stronger in preterm than term gestations. This is due to infection at term having a shorter duration, possibly only initiated during parturition, and routine monitoring of cervical dilation and having a low inoculum size, which only elicits a mild inflammatory response rarely leading to fetal infection. On the other hand, in preterm gestation, microbial infection is established prior to parturition, leading to an increased duration of infection, increased microbial biomass and increased likelihood of fetal infection.[26] Curtin et al.[27] performed a retrospective study using standardised pathological guidelines and found that histological ACAM was present in 367/641 (57%) term parturients, which were frequently asymptomatic. As in this study, their study was also predicated on submission of placentas for histological examination, which may be subject to bias, as clinicians are more likely to request examination in cases of clinical indications. A study by Palmsten et al.[28] that also included spontaneous and medically indicated preterm births, similar to this study, found that neither subclinical nor clinical ACAM was associated with PTB in their cohort of complicated high-risk pregnancies. They attribute the lack of association between ACAM and PTB to the inclusion of indicated PTB and women with risk factors for indicated PTB such as hypertension, which other studies exclude.'28,291 The risk factor relates to fetal vasculitis or the FIR, which is associated with a higher risk of neonatal morbidity than ACAM alone. In this study, ACAM with FIR was present in preterm placentas (9%), but not in any term placentas (0%), although not statistically significant (p=0.163). In preterm gestations ACAM may precipitate delivery with its sequelae of preterm complications. In term gestations the FIR is extremely significant in predicting neonatal outcome, as adverse outcomes are related to release of cytokines/chemokines by neutrophils of placenta and/or fetal origin.[30] In this study there was frequently more than one category of placental histopathology: ACAM with a FIR in 9 preterm and ACAM + AP in 3 preterm and 3 term placentas. These combinations are not surprising. In the Amsterdam consensus classification, in ACAM the FIR indicates the fetus is responding to the infection - a poor prognostic indicator - and ACAM may weaken the vessels in the maternal decidua, causing a retroplacental haemorrhage (inflammatory abruptio).[26]
A total of 55/120 (46%) of placentas showed MVM, which results from decreased perfusion of the placenta due to defective remodelling of the maternal spiral artery. Clinical manifestations include pre-eclampsia and HELLP syndrome.'311 MVM was significantly associated with fetal distress (r=0.319) in this study. Pre-eclampsia was present in 24/55 (44%; p<0.001) cases of MVM, while the remainder may have been subclinical or not associated with pre-eclampsia. Chisholm et al.[32] found evidence of MVM in 82/109 (75%) of PTB <34 weeks, which is higher than the incidence in this study of 47/100 (47%). It was the most common pathology from their cohort of preterm births, as was the outcome in this study.
Abruptio placentae was present in preterm (41%) and term (35%) placentas. Abruptio placentae (41%), neonatal jaundice (42%), pre-eclampsia (36%), fetal distress (25%), neonatal sepsis (24%) and IUGR (23%) are the most common conditions accompanying medically indicated PTB.'331 A very large retrospective study in the USA analysed births between 2006 and 2012 (n=27 796 465) for the presence of abruptio placentae. The overall prevalence rate of abruptio placentae was 9.6 per 1 000 (0.96%) births.[34] The large discrepancy between prevalence rates is influenced by the cohort selection criteria, as selection of a high-risk cohort may bias the prevalence rates determined. In the present study, the term group was selected based on pre-existing conditions that necessitated histological analysis according to hospital policy. However, all patients in the study group were considered high risk, compared with the retrospective analysis of all births.[34] Specific definitions, clinical criteria or histopathology for diagnosis used per study are important in critically analysing prevalence rates. A clinically meaningful diagnosis of abruptio placentae should include maternal complications as well as adverse fetal/neonatal outcomes such as IUGR and preterm delivery. The high prevalence rate of abruptio placentae in this high-risk cohort (overall 40%) highlights the importance of placental pathology and the association of abruptio placentae with adverse outcome such as intrauterine fetal demise and fetal CNS injury.[30]
A retrospective study at a tertiary academic hospital in the Western Cape Province, SA, also emphasised the importance of placental pathology in cases of adverse perinatal outcomes, specifically intrauterine demise.'351 In that study, placental histopathology was correlated with the clinical indication for submission and included review of 2 years' singleton placental histology reports. All samples were from placentas of >24 weeks' gestation. Histopathology identified in that group included ACAM, MVM, abruptio placentae, delayed villous maturation and toxoplasmosis, rubella, cytomegalovirus and herpes simplex (TORCH) infections, most commonly syphilis.[35] The results from this current study provide insight into the prevalence, type and distribution of placental pathology including ACAM, MVM and abruptio placentae from preterm births at a tertiary hospital in the Eastern Cape Province of SA. Both studies demonstrate that histopathological examination of the placenta provides valuable information and can reduce the number of cases of unknown aetiology in adverse pregnancy/perinatal outcome.
Clinical chart review for maternal and obstetric outcomes showed several significant differences between the preterm and term groups. Pre-eclampsia (p=0.006) and parity (p=0.001) were significantly associated with PTB in this study. Primiparity or grand multiparity are considered strong risk factors for PTB.[16] In this study, the mean (SD) parity was higher for women who delivered preterm (1.85 (1.20)) compared with those who delivered at term (0.9 (0.01)), with a strong practical significance (p=0.001; Cohen's d=0.82), for which the risk of recurrent PTB may be significant.
Substance abuse, specifically alcohol abuse, was significantly associated with term delivery (p<0.005) in this study. This may be due to the small sample size of the term cohort, or that alcohol use in this cohort was significantly associated with ACAM (p=0.004, Cramer's V=0.251), which was associated with term pregnancies (p=0.002). A study by Rickert et al.[36] also found that smoking and alcohol use during pregnancy were associated with ACAM (odds ratio (OR) 7.6; 95% confidence interval (CI) 2.3 - 25.8). Alcohol use during pregnancy has long been cautioned against due to the association with spontaneous abortion, stillbirth, PTB, IUGR, fetal alcohol syndrome and sudden infant death syndrome (SIDS).[37,38] SA has the highest prevalence of fetal alcohol spectrum disorder in the world, at 111.1 per 1 000 births.[39-42] This high prevalence is for certain study groups only, but not for the whole country. In a recent SA study, factors such as alcohol consumption, smoking and food security were associated with adverse pregnancy outcomes.[43]
Although not recorded in maternal charts, the use of traditional medicines during pregnancy may also have an effect on PTB in this cohort of complicated pregnancies. Herbal medicine practices are usually not supported by efficacy or safety studies, and the potential risks involved in their usage, particularly in high-risk patients such as pregnant women where teratogenicity is a concern, should be explored further.[44] In many SA cultures, it is believed that traditional medicines are integral to the cultural and spiritual beliefs of the pregnant woman and that revealing the use of traditional medicine may influence the pregnancy outcome.[45,461 The lack of scientific studies and data on the safety and efficacy of traditional medicines and their effect on the placenta requires further investigation.
More women who were HIV-positive delivered preterm (41%) than term (20%), although not statistically significant in this study (p=0.077). SA has one of the largest HIV-seropositive populations in the world, with an increase from 18.76% in 2002 to 22.71% in 2019 among women aged 15 - 49 years.[47] According to the WHO, an estimated 95% of pregnant women living with HIV in SA received antiretrovirals for preventing mother-to-child transmission.[48] According to other studies, untreated maternal HIV infection is associated with increased risk of adverse perinatal outcomes, including PTB, SGA, low birthweight and stillbirth.[49,50] In a SA study by Santosa et al.,[51] maternal HIV infection was associated with increased risk of adverse perinatal outcome (OR 1.44. 95% CI 1.03 - 2.03), NND (OR 6.15; 95% CI 1.27 - 29.88) and SGA (OR 1.55; 95% CI 1.01 - 2.37). In the present study, 10 patients had diabetes or gestational diabetes, which has previously been associated with PTB, pre-eclampsia, fetal growth disparities or fetal demise.[52]
Neonatal outcomes significantly associated with PTB in the present study included respiratory distress syndrome (p=0.004) and neonatal jaundice (p=0.003). Intrauterine demise is significantly associated with term pregnancies in this cohort (p=0.004). However, this may be a result of the selection criteria for the term control group. Six (5%) preterm pregnancies and 4/21 (19%) term pregnancies concluded in intrauterine demise, emphasising the poor outcomes associated with high-risk pregnancies in this cohort. NNJ was the most common neonatal pathology in this study cohort (48% of neonates). NNJ, or hyperbilirubinaemia, develops in ~50% of term and 80% of preterm babies, usually due to physiological underdevelopment of the liver, and if inadequately managed may progress to neurotoxic encephalopathy, seizures, hearing loss and cerebral palsy.[53,54]
A limitation of this study was that the sample size was determined by the funding available, where a larger cohort or case-controlled term cohort would have increased statistical power. Term deliveries in this cohort also represent a particularly high-risk group owing to the nature of the hospital where the study was based: a tertiary hospital, in a low socioeconomic setting, which services only complicated cases of pregnancy, while 'normal' term pregnancies are delivered in clinics in the surrounding areas. The incidence of pathology may therefore be higher than a true control group. The geographical location of the hospital also influenced the racioeconomic distribution of the study cohort of only indigenous African and mixed-race women. However, this may also be seen as a strength in this study, as the relatively high number of placentas analysed provides targeted insight into a niche group of high-risk individuals. The study group is representative of the larger population in that geographical location. In the UK and the USA, PTB is associated more with women classified as black or African-American (16 - 18%) than Caucasian women (5 - 9%).[18] Although not fully understood, maternal demographic characteristics such as low socioeconomic and educational status, single marital status and extremes of maternal age are associated with increased risk of PTB.[7,55]
Histopathological examination of the placenta may be regarded as a valuable tool in understanding the pathophysiology of adverse pregnancy and neonatal outcomes as well as guiding treatment of both mother and child. Recurrence of maternal and placental pathology in subsequent pregnancies may be managed more effectively if placental pathology is identified. The timing of placental pathological events leading to adverse outcome may also assist in the medicolegal assessment of cases.[56]
Histological analysis revealed pathology in all preterm placentas, with maternal vascular malperfusion and abruptio placentae most commonly identified. Acute chorioamnionitis was associated with term births. Maternal characteristics and neonatal outcomes significantly associated with preterm birth included pre-eclampsia, neonatal respiratory distress syndrome and neonatal jaundice. Intrauterine demise and alcohol abuse were significantly associated with term delivery.
Conclusion
The placenta may provide insight into the intrauterine conditions leading to PTB and may hold clues for risk prediction of chronic disease development in childhood or adulthood.[14,57] At present, the criteria for histological examination of placentas at the study hospital do not include routine submission of preterm placentas. Placentas are sent for histology only in cases of stillbirths, IUFD or low initial APGAR score. The placental pathology associated with 100% of preterm births in this study supports the need for amendment of hospital policies, as there is valuable information for clinicians to guide their treatment and management protocols.
Declaration. The research for this study was done in partial fulfilment of the requirements for KES's PhD degree in Microbiology at Nelson Mandela University.
Acknowledgements. We thank the patients and staff for their participation at the tertiary hospital where the study occurred.
Author contributions. KES: participated in conceptualisation of the research, collected data, participated in data analysis and write-up; SG and CAW: conceptualisation, study design, supervision, funding support, editing and review; MM: was involved in formal analysis and review of the research.
Funding. The authors acknowledge the NHLS Trust grant and National Research Foundation (NRF) development grant for Y-Rated Researchers for funding support. The opinions, findings and conclusions or recommendations expressed by the NRF-supported research are those of the authors alone, and the NRF accepts no liability whatsoever in this regard.
Conflicts of interest. None.
References
1. World Health Organization. New global estimates on preterm birth published 2018. Geneva: WHO, 2018. https://www.who.int/news-room/detail/17-11-2018-new-globalestimates-on-preterm-birth-published (accessed 3 December 2019). [ Links ]
2. Manuck TA, Rice MM, Bailit JL, et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am J Obstet Gynecol 2016;215(1):103.e1-103.e14. https://doi.org/10.1016/j.ajog.2016.01.004. [ Links ]
3. Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7(1):e37-46. http://doi.org/10.1016/s2214-109x(18)30451-0 [ Links ]
4. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371(9606):75-84. http://doi.org/10.1016/s0140-6736(08)60074-4 [ Links ]
5. Gilman-Sachs A, Dambaeva S, Salazar Garcia MD, et al. Inflammation induced preterm labor and birth. J Reproduct Immunol 2018;129:53-58. http://doi.org/10.1016/j.jri.2018.06.029 [ Links ]
6. Romero R, Dey SK, Fisher SJ. Preterm labor: One syndrome, many causes. Science 2014;345(6198):760-765. http://doi.org/10.1126/science.1251816 [ Links ]
7. Smith GCS, Shah I, Pell JP, et al. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: A retrospective cohort study. Obstetric Gynecol Survey 2007;62(5):299-300. http://doi.org/10.1097/01.ogx.0000261695.08686.5c [ Links ]
8. Di Renzo GC, Giardina I, Rosati A, et al. Maternal risk factors for preterm birth: a country-based population analysis. Eur J Obstetr Gynecol Reproduc Biol 2011;159(2):342-346. http://doi.org/10.1016/j.ejogrb.2011.09.024 [ Links ]
9. Matoba N, Mestan KK, Collins JW. Understanding racial disparities of preterm birth through the placenta. Clinical Therapeutics 2021;43(2):287-296. http://doi.org/10.1016/j.clinthera.2020.12.013 [ Links ]
10. Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: Nosology and reproducibility of placental reaction patterns. Pediatr Development Pathol 2003;6(5):435-448. http://doi.org/10.1007/s10024-003-7070-y [ Links ]
11. Min AM, Saito M, Simpson JA, et al. Placental histopathology in preterm birth with confirmed maternal infection: A systematic literature review. PLOS One 2021;16(8):e0255902. http://doi.org/10.1371/journal.pone.0255902 [ Links ]
12. Brink LT, Roberts DJ, Wright CA, et al. Placental pathology in spontaneous and iatrogenic preterm birth: Different entities with unique pathologic features. Placenta 2022;126:54-63. http://doi.org/10.1016/j.placenta.2022.06.004 [ Links ]
13. Hackney DN, Tirumala R, Salamone LJ, et al. Do placental histologic findings of chorion-decidual hemorrhage or inflammation in spontaneous preterm birth influence outcomes in the subsequent pregnancy? Placenta 2014;35(1):58-63. http://doi.org/10.1016/j.placenta.2013.11.001 [ Links ]
14. Roescher AM, Timmer A, Erwich JJHM, Bos AF. Placental pathology, perinatal death, neonatal outcome, and neurological development: A systematic review. PLoS ONE. 2014;9(2):e89419. http://doi.org/10.1371/journal.pone.0089419 [ Links ]
15. Redline RW. Classification of placental lesions. Am J Obstetr Gynecol 2015;213(4):S21-28. http://doi.org/10.1016/j.ajog.2015.05.056 [ Links ]
16. World Health Organization. Born Too Soon: Global Action Report for Preterm Birth. New York: MoD, PMNCH, Save the Children, WHO, 2012. [ Links ]
17. Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Path Laboratory Med 2016;140(7):698-713. http://doi.org/10.5858/arpa.2015-0225-cc [ Links ]
18. Goldenberg RL, Andrews WW, Goepfert AR, et al. The Alabama Preterm Birth Study: Umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstetr Gynecol 2008;198(1):43.e1-43.e5. http://doi.org/10.1016/j.ajog.2007.07.033 [ Links ]
19. World Health Organization. Global preterm birth estimates 2014. http://ptb.srhr.org/ (accessed 8 December 2019) [ Links ]
20. Morgan T. Role of the placenta in preterm birth: A review. Am J Perinatol 2016;33(03):258-266. http://doi.org/10.1055/s-0035-1570379 [ Links ]
21. Baergen R. Manual of Pathology of the Human Placenta. 2nd edition. Berlin: Springer, 2011. [ Links ]
22. Kulkarni VG, Sunilkumar KB, Nagaraj TS, et al. Maternal and fetal vascular lesions of malperfusion in the placentas associated with fetal and neonatal death: results of a prospective observational study. Am J Obstetr Gynecol 2021;225(6):660.e1-660.e12. http://doi.org/10.1016/j.ajog.2021.06.001 [ Links ]
23. Tantbirojn P, Saleemuddin A, Sirois K, et al. Gross abnormalities of the umbilical cord: Related placental histology and clinical significance. Placenta 2009;30(12):1083-1088. http://dolorg/10.1016/j.placenta.2009.09.005 [ Links ]
24. Namba F, Hasegawa T, Nakayama M, et al Placental features of chorioamnionitis colonized with ureaplasma species in preterm delivery. Pediatr Res 2010;67(2):166-172. http://doi.org/10.1203/pdr.0b013e3181c6e58e [ Links ]
25. Redline RW. Inflammatory response in acute chorioamnionitis. Sem Fetal Neonatal Med 2012;17(1):20-25. http://doi.org/10.1016/j.siny.2011.08.003 [ Links ]
26. Kim CJ, Romero R, Chaemsaithong P, Kim J-S. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstetr Gynecol 2015;213(4):S53-69. http://doi.org/10.1016/j.ajog.2015.08.041 [ Links ]
27. Curtin W, Katzman P, Florescue H, Metlay L. Accuracy of signs of clinical chorioamnionitis in the term parturient. J Perinatol 2013;33:422-428. http://doi.org/10.1038/jp.2012.135 [ Links ]
28. Palmsten K, Nelson KK, Laurent LC, et al. Subclinical and clinical chorioamnionitis, fetal vasculitis, and risk for preterm birth: A cohort study. Placenta 2018;67:54-60. http://doi.org/10.1016/j.placenta.2018.06.001 [ Links ]
29. Hillier S, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. Int J Gynecol Obstetr 1989;29(1):96-97. http://doi.org/10.1016/0020-7292(89)90145-8 [ Links ]
30. Redline RW. The clinical implications of placental diagnoses. Sem Perinatol 2015;39(1):2-8. http://doi.org/10.1053/j.semperi.2014.10.002 [ Links ]
31. Ravishankar S, Redline RW. The placenta. Neonatal Neurol 2019;57-66. http://doi.org/10.1016/b978-0-444-64029-1.00003-5 [ Links ]
32. Chisholm KM, Norton ME, Penn AA, Heerema-McKenney A. Classification of preterm birth with placental correlates. Pediatr Dev Pathol 2018;21(6):548-560. http://doi.org/10.1177/1093526618775958 [ Links ]
33. Ananth CV, Getahun D, Peltier MR, Smulian JC. Placental abruption in term and preterm gestations. Obstetr Gynecol 2006;107(4):785-792. http://doi.org/10.1097/01.aog.0000207560.41604.19 [ Links ]
34. Ananth CV, Lavery JA, Vintzileos AM, et al. Severe placental abruption: Clinical definition and associations with maternal complications. Am J Obstetr Gynecol 2016;214(2):272.e1-272.e9. http://doi.org/10.1016/j.ajog.2015.09.069 [ Links ]
35. Malusi Z, Schubert PT, Theron GB, Wright CA. The value of histopathology of the placenta in a tertiary referral hospital in South Africa. S Afr J Obstetr Gynaecol 2019;25(2):64. http://doi.org/10.7196/sajog.1434 [ Links ]
36. Rickert V. Prevalence and risk factors of chorioamnionitis among adolescents. Obstetr Gynecol 1998;92(2):254-257. http://doi.org/10.1016/s0029-7844(98)00135-5 [ Links ]
37. Hannigan JH, Armant DR. Alcohol in pregnancy and neonatal outcome. Sem Neonatol 2000;5(3):243-254. http://doi.org/10.1053/siny.2000.0027 [ Links ]
38. Dukes KA, Burd L, Elliott AJ, et al. The Safe Passage Study: Design, methods, recruitment, and follow-up approach. Paediatr Perinatal Epidemiol 2014;28(5):455-465. http://doi.org/10.1111/ppe.12136 [ Links ]
39. May PA, Baete A, Russo J, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 2014;134(5):855-866. http://doi.org/10.1542/peds.2013-3319 [ Links ]
40. Roozen S, Peters G-JY, Kok G, et al. Worldwide prevalence of fetal alcohol spectrum disorders: A systematic literature review including meta-analysis. Alcoholism Clin Experimental Res 2016;40(1):18-32. http://doi.org/10.1111/acer.12939 [ Links ]
41. Lange S, Probst C, Gmel G, et al Global prevalence of fetal alcohol spectrum disorder among children and youth: A systematic review and meta-analysis. Obstet Gynecol Survey 2018;73(4):189-191. http://doi.org/10.1097/01.ogx.0000532194.88210.00 [ Links ]
42. Maxwell JR, Yellowhair TR, Davies S, et al. Prenatal alcohol exposure and chorioamnionitis results in microstructural brain injury in a preclinical investigation. Ann Pediatric Res 2020;4(1):1031. [ Links ]
43. Zar HJ, Pellowski JA, Cohen S, et al. Maternal health and birth outcomes in a South African birth cohort study. Hill B, editor. PLOS ONE. 2019;14(11):e0222399. http://doi.org/10.1371/journalpone.0222399 [ Links ]
44. Bernstein N, Akram M, Yaniv-Bachrach Z, Daniyal M. Is it safe to consume traditional medicinal plants during pregnancy? Phytotherapy Res 2020;35(4):1908-1924. http://doi.org/10.1002/ptr.6935 [ Links ]
45. Peltzer K, Phaswana-Mafuya N, Treger L. Use of traditional and complementary health practices in prenatal, delivery and postnatal care in the context of HIV transmission from mother to child (PMTCT) in the Eastern Cape, South Africa. Afr J Trad Complementary Alternative Med 2010;6(2). http://doi.org/10.4314/ajtcam.v6i2.57087 [ Links ]
46. Maputle SM, Mothiba TM, Maliwichi L. Traditional medicine and pregnancy management: Perceptions of traditional health practitioners in Capricorn District, Limpopo Province. Stud Ethno-Med 2015;9(1):67-75. http://doi.org/10.1080/09735070.2015.11905422 [ Links ]
47. Satoh S, Boyer E. HIV in South Africa. Lancet 2019;394(10197):467. http://doi.org/10.1016/s0140-6736(19)31634-4 [ Links ]
48. World Health Organization. Mother-to-child transmission of HIV 2019. https://www.who.int/gho/hiv/epidemic_response/PMTCT_text/en/ (accessed 15 December 2019). [ Links ]
49. Wedi COO, Kirtley S, Hopewell S, et al. Perinatal outcomes associated with maternal HIV infection: A systematic review and meta-analysis. Lancet HIV 2016;3(1):e33-e48. http://doi.org/10.1016/s2352-3018(15)00207-6 [ Links ]
50. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000 - 15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388(10063):3027-3035. http://doi.org/10.1016/s0140-6736(16)31593-8 [ Links ]
51. Santosa WB, Staines-Urias E, Tshivuila-Matala COO, Norris SA, Hemelaar J. Perinatal outcomes associated with maternal HIV and antiretroviral therapy in pregnancies with accurate gestational age in South Africa. AIDS 2019;33(10):1623-1633. http://doi.org/10.1097/qad.0000000000002222 [ Links ]
52. Kapur A, McIntyre HD, Hod M. Type 2 diabetes in pregnancy. Endocrinol Metab Clin N Am 2019;48(3):511-531. http://doi.org/10.1016/j.ecl.2019.05.009 [ Links ]
53. Woodgate P, Jardine LA. Neonatal jaundice. BMJ Clin Evid 2011;2011;0319. [ Links ]
54. Ullah S, Rahman K, Hedayati M. Hyperbilirubinemia in neonates: Types, causes, clinical examinations, preventive measures and treatments: A narrative review article. Iranian J Pub Health 2016;45(5):558-568. [ Links ]
55. Thompson JMD, Irgens LM, Rasmussen S, Daltveit AK. Secular trends in socio-economic status and the implications for preterm birth. Paediatt Perinatal Epidemiol 2006;20(3):182-187. http://doi.org/10.1111/j.1365-3016.2006.00711.x [ Links ]
56. Wright C. The placenta - a Cinderella story. S Afr Fam Pract 2007;49(7):4-8. http://doi.org/10.1080/20786204.2007.10873588 [ Links ]
57. Faye-Petersen OM. The placenta in preterm birth. J Clin Pathol 2008;61(12):1261-1275. http://doi.org/10.1136/jcp.2008.055244 [ Links ]
 Correspondence:
Correspondence:
S Govender
sharlene.govender@mandela.ac.za
Accepted 9 February 2023














