Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.4 Pretoria abr. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i4.875
CME
Autoimmune encephalitis: Epidemiology, pathophysiology and clinical spectrum (part 2)
J HiesgenI; C M SchutteII
IDr med; Department of Neurology, Faculty of Health Sciences, University of Pretoria, South Africa
IIMB ChB, MD; Department of Neurology, Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
Autoimmune encephalitis (AE) represents a growing number of severe autoimmune-inflammatory diseases affecting both the white and grey matter of the brain. In part 1 of this series, we focused on the epidemiology, pathophysiology and clinical presentation of this condition, with two illustrative cases. In this part, we introduce the clinical criteria for AE, particularly for the diagnosis of anti-N-methyl-D-aspartate (NMDA) receptor encephalitis, which were developed to facilitate immune treatment in suspected cases before antibody results are available. We subsequently discuss the work-up, differential diagnosis and treatment options for patients with this disease.
Autoimmune encephalitis (AE) represents a growing number of severe autoimmune-inflammatory diseases affecting both the white and grey matter of the brain.
Diagnostic criteria
General diagnostic criteria for possible AE
Previous diagnostic criteria for any form of encephalitis required a clinical picture of an encephalopathy characterised by an altered mental state, and evidence of inflammation. In the absence of tissue to demonstrate inflammation, clinical features such as fever or seizures at onset, cerebrospinal fluid (CSF) abnormalities, imaging and/or electroencephalogram (EEG) findings are used to ascertain an inflammatory pathology.[1]
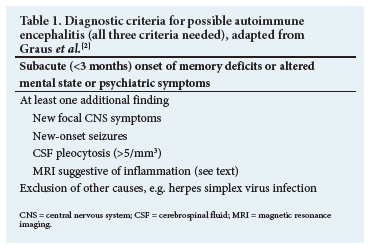
However, the clinical presentation of an AE does not always correspond to these criteria and patients might present without a reduced level of consciousness, fever or abnormal CSF results, and neuroimaging may be normal or nonspecific. In addition, the results of antibody testing are typically only available after several days or even weeks. To assist in the diagnosis of AE, clinical criteria not relying on antibody testing were developed. These criteria can guide clinicians in suspected cases to establish a diagnosis and, after reasonable exclusion of other causes, start immunotherapy early. In Table 1, the diagnostic criteria for possible AE are summarised.[2]
Specific diagnostic criteria for anti-NMDA receptor encephalitis
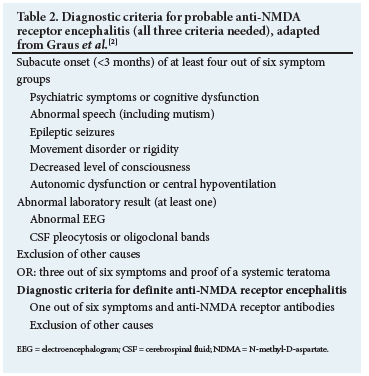
As discussed in part 1, anti-anti-N-methyl-D-aspartate (NMDA) receptor encephalitis is the most frequent AE and often manifests with a typical sequence of symptoms. After nonspecific prodromal symptoms (headache/fatigue), psychiatric symptoms (apathy, anxiety, depression, insomnia, paranoid psychosis, catatonia) typically occur. These are followed by neurological features such as movement disorders and/or seizures and, if the full clinical spectrum is seen, autonomic dysregulation, hypoventilation and a decrease in the level of consciousness will develop. The disease predominantly affects younger patients, and ~80% are female.3 Antibodies, mostly against the GLUN1 subunit of the NMDA receptor, are found, and tumours (predominantly ovarian teratoma) are discovered in >50% of females.[4] With these specifics in mind, separate diagnostic criteria for NMDA receptor encephalitis were devised. They are summarised in Table 2.
Special investigations
Neuroimaging
All patients presenting with an encephalitis picture should undergo neuroimaging, initially mainly to exclude other causes, such as hydrocephalus or brain tumours. A computed tomography (CT) scan of the brain will usually be the first modality to achieve this.
Although magnetic resonance imaging (MRI) has a much higher sensitivity compared with CT, it may be normal in 50% - 66% of patients with AE.[3,5] However, features suggestive of inflammation or demyelination, such as hyperintensities in the medial temporal lobes on T2-weighted imaging or FLAIR (fluid attenuated inversion recovery), e.g. in limbic encephalitis, or multifocal cortical and subcortical lesions, may be seen.[6] It is important to be aware that imaging alone cannot distinguish between infectious and non-infectious inflammation. Rarely, lesions are also found in the basal ganglia,[7] brainstem or spinal cord.[8]
EEG
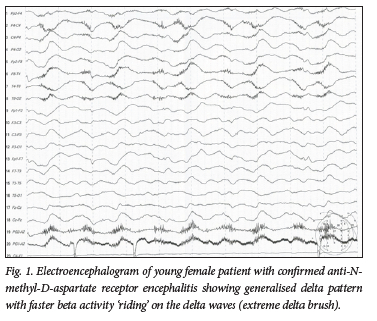
Electroencephalography is abnormal in >90% of patients with AE. Findings include focal and generalised epileptiform and non-epileptiform abnormalities, occurring during ictal events as well as interictally.[9,10] In addition, a generalised cerebral dysfunction with diffuse slowing of the background activity is a common finding. A new, characteristic EEG pattern, termed 'extreme delta brush', was described in 2012;[11] it has been reported in 30% of patients with AE and seizures. Extreme delta brush is unique in adults with anti-NMDA receptor encephalitis and is associated with status epilepticus, intensive care unit (ICU) admission and prolonged hospital stay.[12] Fig. 1 shows an example of extreme delta brush in a comatose patient with anti-NMDA receptor encephalitis. The occurrence of a prolonged status epilepticus refractory to second-line treatment in a patient not known with epilepsy, any other preexisting neurological disease or acute structural, toxic, or metabolic cause is called new-onset refractory status epilepticus (NORSE). While the cause of NORSE might remain cryptogenic, the most common identified cause is autoimmune encephalitis in 19% of those cases.[13] Therefore, NORSE should prompt thorough workup and treatment as possible AE.
CSF
In patients with encephalitis, a lumbar puncture is essential to exclude an active central nervous system (CNS) infection. Here, the focus should be on viral infections, with herpes simplex virus 1 (HSV1), varicella-zoster virus and other viruses from the herpes virus family being common organisms.[14] Bacterial infections, such as neurosyphilis, tuberculosis, listeriosis or fungal infections should also be excluded.
Up to 80% of patients with AE have abnormal CSF results. Abnormalities can include pleocytosis (white blood cells in CSF >5/mm3), with or without mildly increased protein.[15] Oligoclonal bands in the CSF indicating intrathecal immunoglobulin synthesis were reported to be positive in 40% - 60%.[5,16]
Specific antibody testing
The presence of specific antibodies associated with AE (e.g. anti-NMDA receptor antibodies) in the CSF is diagnostic[2] and, if a specific subtype of AE is highly suspected, testing for a single antibody can be requested. However, currently most laboratories offer antibody panels with several distinct antibodies, depending on the clinical syndrome. The National Health Laboratory Service, and
ll private laboratories in South Africa (SA), offer an AE panel with antibodies against neuronal surface antigens including anti-NMDA receptors, a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor 1 and 2, y-aminobutyric acid (GABA) receptor B, contactin-associated protein-like 2 (CASPR-2) and leucine-rich glioma inactivated protein 1 (LGI1). Antibody testing against the rarer antibodies such as immunoglobulin-like cell-adhesion molecule 5 (IGLON5), dipeptidyl-peptidase-like protein 6 (DPPX) and glycine are available at a few private laboratories. A separate panel for antibodies against intracellular antigens (classic paraneoplastic encephalitis) is also available, including antibodies against Hu, Ri, Yo, Ma/Ta, CV2 and amphiphysin. CSF analysis for antibodies is preferable since this shows a much higher sensitivity when compared with serum serology.[15] While antibodies are frequently detected both in serum and CSF, a negative serum serology has been observed in up to 15% of patients.[17]
Differential diagnosis
Because the spectrum of neurological and psychiatric symptoms at presentation is wide, several alternative conditions should be considered. Of these, infectious causes of encephalitis are the most important. Missing a CNS infection can be fatal, and most patients are treated with acyclovir for HSV1 encephalitis, until negative CSF results are obtained. In this context, it is important to note that anti-NMDA receptor encephalitis can occur as an epiphenomenon after herpes encephalitis, as has been briefly described in part 1.[18,19]
One medical condition that should specifically be mentioned is neuroleptic malignant syndrome. The condition itself may present similarly to AE, but - as described above - patients with AE very frequently present with psychiatric symptoms and may be given antipsychotic medication to control symptoms. If they then develop confusion, changes in the level of consciousness and rigidity with autonomic symptoms, the condition can easily be misinterpreted as neuroleptic malignant syndrome. Therefore, psychiatric patients who develop intolerance to antipsychotic medication should raise suspicion of an underlying AE and ought to be worked up accordingly.[20]
Other medical conditions, such as symptomatic epilepsies, metabolic and toxic encephalopathies, rheumatological disorders, tumours and cerebrovascular diseases, may give rise to a similar clinical picture as seen in AE.
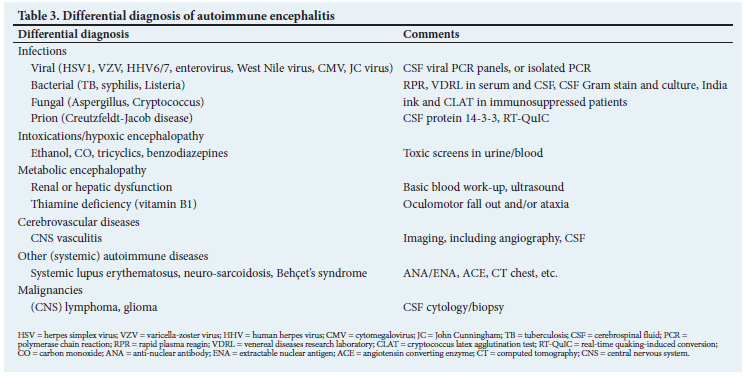
Table 3 summarises the most important conditions to consider in the differential diagnosis of AE.
Management
It has been shown that early recognition and treatment of AE is associated with better outcome. The recent (2021) proposed best practice recommendations for AE state that delay of therapy while awaiting antibody results is impractical, potentially hazardous and should be avoided.[21]
Currently, there are no randomised controlled studies regarding treatment of AE, and therefore, recommendations are based on case series and expert opinions. In principle, treatment consists of suppression of brain inflammation, antibody removal, prevention of further antibody production and, if present, tumour removal.
Immunotherapy
Initial immunotherapy usually consists of intravenous corticosteroids (1 g methylprednisolone daily for 3 - 5 days with slow oral taper), plasmapheresis (5 - 10 sessions every other day), or intravenous immunoglobulin (IVIG 2 g/kg over 2 - 5 days), which can be combined in severe cases. The use of steroids may be challenging in patients with diabetes mellitus and hypertension, and theoretically, could also worsen psychiatric symptoms. Plasma exchange needs an intensive care setting and may be associated with an increased bleeding risk and volume shifts. IVIG, however, is easy to administer, but may be associated with an increased thromboembolic risk and, rarely, occurrence of anaphylaxis and renal insufficiency.
In non-responding patients (no meaningful clinical/radiological response after 2-4 weeks), a transition to second-line therapy is warranted. Rituximab, an effective second-line agent with an acceptable side-effect profile, is used most often.[4,22] Cyclophosphamide, mycophenolate and azathioprine are other alternatives.[23] Patients who are refractory to first- and second-line therapies are challenging to manage, and the evidence for third-line therapies is anecdotal, with small case series or individual patient reports. In developed health systems, tocilizumab (an interleukin 6 receptor antagonist) and bortezomib (a proteasome inhibitor) are options in refractory cases.[24]
Supportive and symptomatic therapy
Patients with AE may experience behavioural and mood disturbances, seizures and movement disorders, as well as pain, sleep disturbances and autonomic dysfunction. With the overlapping clinical presentation of neuroleptic malignant syndrome, there may be reason to anticipate a hypersensitivity to antipsychotic medication, and low-dose olanzapine is suggested if necessary. Benzodiazepines may also be helpful. Interestingly, seizures often respond better to immunotherapy than to anti-epileptic medication alone. Severe autonomic instability and uncontrollable seizures may necessitate admission and management in an ICU.
As mentioned before, a thorough tumour search is important, and if proven, the surgical removal of the tumour should be performed as soon as the patient's condition is stable enough.
Prognosis
Prognosis varies depending on AE subtype, associated antibodies, possible paraneoplastic origin, patient age and severity of disease.[25] While spontaneous clinical improvement is rare, with prompt and effective therapy, the potential for a good recovery is high. More than 80% of patients with anti-NMDA receptor encephalitis have a good outcome and are able to function independently after 2 years.[3]
As with many other autoimmune disorders, relapses of AE may occur. The frequency of relapses depends on the subtype of AE, and ranges from 12% to 35%.[4] Relapses were found to be more common in patients without a tumour, after delayed immunotherapy as well as high antibody titres.[26]
While a large proportion of patients recovers completely, some long-term sequelae may occur. Seizures are very frequent in the acute phase of AE, but only ~3% of patients experienced persistent seizures and required ongoing anti-epileptic medications during long-term follow-up.[27] Unfortunately, a minority of patients may have permanent disabilities, including neurocognitive deficits such as antero- and retrograde amnesia.[28]
The importance of early recognition and initiation of therapy for AE must be emphasised. In patients with anti-NMDA receptor encephalitis, for example, treatment delay of >4 weeks resulted in poor functional outcomes at 1 year.[29] This point is also demonstrated in patients with LGI1-antibody encephalitis and faciobrachial dystonic seizures. While only 10% respond to anti-seizure treatment alone, 51% of patients show cessation of seizures after immunotherapy. On follow-up, the patients with prolonged seizure activity have been shown to develop cognitive impairment after 90 days, while those with early immunotherapy have had normal cognition and less disability.[30]
Mortality due to AE is low in most cohorts, and often associated with prolonged admission to ICU and complications arising from mechanical ventilation, sepsis and status epilepticus.[31]
Key messages
• AE is the most common form of encephalitis.
• AE is eminently treatable and has a good prognosis.
• Many forms of AE have characteristic clinical pictures.
• Anti-NMDA receptor encephalitis shows psychiatric symptoms, followed by neurological findings such as movement disorders, and autonomic symptoms, and is often associated with ovarian teratomas.
• Immunotherapy for AE may be curative, especially if instituted early.
Declaration. None.
Acknowledgements. We want to thank all clinicians, especially our colleagues from ICU, gynaecology and internal medicine who are involved in the management of our patients. We are grateful to Prof. F Suleman from our Department of Radiology for providing the MRI images (part 1) and to Prof. A Mochan for critical review of the manuscript.
Author contributions. Equal contributions.
Funding. None.
Conflicts of interest. None.
References
1. Venkatesan A, Tunkel AR, Bloch KC, et al. International Encephalitis Consortium. Case definitions, diagnostic algorithms, and priorities in encephalitis: Consensus statement of the International Encephalitis Consortium. Clin Infect Dis 2013;57(8):1114-1128. https://doi.org/10.1093/cid/cit458 [ Links ]
2. Graus F, Titulaer MJ, Balu R, et al A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15(4):391-404. https://doi.org/10.1016/S1474-4422(15)00401-9 [ Links ]
3. Titulaer MJ, McCracken L, Gabilondo I, et al Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: An observational cohort study. Lancet Neurol. 2013;12(2):157-165. https://doi.org/10.1016/S1474-4422(12)70310-1 [ Links ]
4. Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med 2018;378:840-851. https://doi.org/10.1056/NEJMra1708712 [ Links ]
5. Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10(1):63-74. https://doi.org/10.1016/S1474-4422(10)70253-2 [ Links ]
6. Kelley BP, Patel SC, Marin HL, et al. Autoimmune encephalitis: Pathophysiology and imaging review of an overlooked diagnosis. Am J Neuroradiol 2017;38(6):1070-1078. https://doi.org/10.3174/ajnr.A5086 [ Links ]
7. Son DK, Cho SM, Ryu HU, et al. Anti-NMDAR encephalitis with bilateral basal ganglia MRI lesions at a distance of time: A case report. BMC Neurol 2022;22:121. https://doi.org/10.1186/s12883-022-02652-y [ Links ]
8. Heine J, Prüss H, Bartsch T, et al. Imaging of autoimmune encephalitis - relevance for clinical practice and hippocampal function. Neuroscience 2015;19(309):68-83. https://doi.org/10.1016/j.neuroscience.2015.05.037 [ Links ]
9. Kovac S, Alferink J, Ahmetspahic D, et al. Update Anti-N-Methyl-D-Aspartat-Rezeptor-Enzephalitis [Update on anti-N-methyl-D-aspartate receptor encephalitis-. Nervenarzt 2018;89(1):99-112. https://doi.org/10.1007/s00115-017-0405-0 [ Links ]
10. Veciana M, Becerra JL, Fossas P, et al. EEG extreme delta brush: An ictal pattern in patients with anti-NMDA receptor encephalitis. Epilepsy Behav 2015;49:280-285. https://doi.org/10.1016/j.yebeh.2015.04.032 [ Links ]
11. Schmitt SE, Pargeon K, Frechette ES, et al. Extreme delta brush: A unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology 2012;11;79(11):1094-1100. https://doi.org/10.1212/WNL.0b013e3182698cd8 [ Links ]
12. Parwani J, Ortiz JF, Alli A, et al Understanding seizures and prognosis of the extreme delta brush pattern in anti-N-methyl-D-aspartate (NMDA) receptor encephalitis: A systematic review. Cureus 2021;13(9):e18154. https://doi.org/10.7759/cureus.18154 [ Links ]
13. Gaspard N, Foreman BP, Alvarez V, et al. New-onset refractory status epilepticus: Etiology, clinical features, and outcome. Neurology 2015;85(18):1604-1613. https://doi.org/10.1212/WNL.0000000000001940 [ Links ]
14. Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect Dis 2010;10(12):835-844. https://doi.org/10.1016/s1473-3099(10)70222-x [ Links ]
15. Wang R, Guan HZ, Ren HT, et al CSF findings in patients with anti-N-methyl-D-aspartate receptor-encephalitis. Seizure 2015;29:137-142. https://doi.org/10.1016/j.seizure.2015.04.005 [ Links ]
16. Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: Temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010;133(6):1655-1667. https://doi.org/10.1093/brain/awq113 [ Links ]
17. Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: A retrospective study. Lancet Neurol 2014;13(2):167-177. https://doi.org/10.1016/S1474-4422(13)70282-5 [ Links ]
18. Prüss H, Finke C, Höltje M, et al N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012;72(6):902-911. https://doi.org/10.1002/ana.23689 [ Links ]
19. Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: Case report. Neurology 2013;81(18):1637-1639. https://doi.org/10.1212/WNL.0b013e3182a9f531 [ Links ]
20. Lejuste F, Thomas L, Picard G, et al. Neuroleptic intolerance in patients with anti-NMDAR encephalitis. Neurol Neuroimmunol Neuroinflamm 2016;3(5):e280. https://doi.org/10.1212/NXI.0000000000000280 [ Links ]
21. Abboud H, Probasco JC, Irani S, et al Autoimmune encephalitis: Proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatr 2021;92:757-768. https://doi.org/10.1136/jnnp-2021-326096 [ Links ]
22. Nepal G, Shing YK, Yadav JK, et al. Efficacy and safety of rituximab in autoimmune encephalitis: A meta-analysis. Acta Neurol Scand 2020;142(5):449-459. https://doi.org/10.1111/ane.13291 [ Links ]
23. Uy CE, Binks S, Irani SR. Autoimmune encephalitis: Clinical spectrum and management. Pract Neurol 2021;21(5):412-423. https://doi.org/10.1136/practneurol-2020-002567 [ Links ]
24. Dinoto A, Ferrari S, Mariottto S. Treatment options in refractory autoimmune encephalitis. CNS Drugs 2022;2. https://doi.org/10.1007/s40263-022-00943-z [ Links ]
25. Broadley J, Seneviratne U, Beech P, et al. Prognosticating autoimmune encephalitis: A systematic review. J Autoimmun 2019;96:24-34. https://doi.org/10.1016/j.jaut.2018.10.014 [ Links ]
26. Zhong R, Chen Q, Zhang X. Relapses of anti-NMDAR, anti-GABABR and anti-LGI1 encephalitis: A retrospective cohort study. Front Immunol 2022;9(13):918396. https://doi.org/10.3389/fimmu.2022.918396 [ Links ]
27. Liu X, Guo K, Lin J, et al. Long-term seizure outcomes in patients with autoimmune encephalitis: A prospective observational registry study update. Epilepsia 2022;63(7):1812-1821. https://doi.org/10.1111/epi.17245 [ Links ]
28. Hébert J, Day GS, Steriade C, et al. Long-term cognitive outcomes in patients with autoimmune encephalitis. Can J Neurol Sci 2018;45(5):540-544. https://doi.org/10.1017/cjn.2018.33 [ Links ]
29. Balu R, McCracken L, Lancaster E. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology 2019;92(3):e244-e252. https://doi.org/10.1212/WNL.0000000000006783 [ Links ]
30. Thompson J, Bi M, Murchison AG, et al Faciobrachial Dystonic Seizures Study Group. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 2018;141(2):348-356. https://doi.org/10.1093/brain/awx323 [ Links ]
31. Schubert J, Brâmer D, Huttner HB, GENERATE and IGNITE network. Management and prognostic markers in patients with autoimmune encephalitis requiring ICU treatment. Neurol Neuroimmunol Neuroinflamm 2018;6(1):e514. https://doi.org/10.1212/NXI.0000000000000514 [ Links ]
 Correspondence:
Correspondence:
J Hiesgen
juliane.hiesgen@up.ac.za
Accepted 3 February 2023














