Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.3 Pretoria Mar. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i3.16783
RESEARCH
Acute kidney injury after major non-cardiac surgery: Incidence and risk factors
E RossouwI; S ChettyII
IMB ChB, FCA (SA); Department of Anesthesiology and Critical Care, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIFCA (SA), PhD; Department of Anesthesiology and Critical Care, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND: Acute kidney injury (AKI) is a major post-surgical complication that contributes to morbidity and mortality. AKI is well documented after cardiac surgery. However, less is known regarding the incidence and risk factors following major non-cardiac surgery. Globally the incidence of AKI post major surgery has been evaluated; however, there are no data available for South Africa (SA
OBJECTIVES: To ascertain the incidence of AKI after major non-cardiac surgery at a tertiary academic SA hospital. Secondary outcomes were to identify perioperative risk factors that are associated with an elevated risk to develop AKI in the postoperative period
METHODS: The study was conducted at Tygerberg Hospital, a single tertiary centre in Cape Town, SA. Perioperative records of adults who underwent major non-cardiac surgery were retrospectively collected. Variables pertaining to potential risk factors for AKI were captured, and serum creatinine levels were recorded up to 7 days postoperatively and compared with baseline measurements to determine whether AKI had developed. Descriptive statistics along with logistic regression analysis were used to interpret results
RESULTS: The overall incidence of AKI was 11.2% (95% confidence interval (CI) 9.8 - 12.6). Based on surgical discipline, trauma surgery (19%), followed by abdominal (18.5%) and vascular surgery (17%) had the highest incidence. Independent AKI risk factors were identified after multivariate analysis. These were: chronic obstructive pulmonary disease (odds ratio (OR) 2.19; 95% CI 1.09 - 4.37; p=0.005), trauma surgery (OR 3.00; 95% CI 1.59 - 5.64; p=0.001), abdominal surgery (OR 2.14; 95% CI 1.33 - 3.45; p=0.002), vascular surgery (OR 2.42; 95% CI 1.31 - 4.45; p=0.004), urology procedures (OR 2.45; 95% CI 1.31 - 4.45; p=0.005), red blood cell transfusion (OR 1.81; 95% CI 1.21 - 2.70; p=0.004), emergency surgery (OR 1.74; 95% CI 1.15 - 2.65; p=0.009) and inotrope use (OR 2.77; 95% CI 1.80 - 4.26;p<0.001
CONCLUSION: The results of our study are in keeping with international literature regarding the incidence of AKI after major non-cardiac surgery. The risk factor profile, however, is in several regards different from what has been found elsewhere
Acute kidney injury (AKI) is a frequent complication in hospitalised patients worldwide.[1-3] AKI negatively impacts patient outcomes and contributes to prolonged length of hospital stay and mortality[4] A delay in diagnosis of hospital-acquired AKI was observed in 43% of patients, as reported in the 2009 National Confidential Enquiry into Patient Outcomes and Death (NCEPOD) in the UK. The NCEPOD also stated that 20% of hospital-acquired AKI cases are preventable.[5] Cost of care for AKI patients is higher than non-AKI patients, which is related to the increased need for renal replacement therapy (RRT) and intensive care unit (ICU) admission.[6]
AKI risk factors, incidence and outcomes vary between medical and surgical patient populations. In surgical patients, postoperative AKI has been studied at length in cardiac and vascular procedures.[7] In recent years, an increasing number of studies has focused on postoperative AKI in other surgical groups, such as major abdominal procedures.[8-10] However, limited literature is available from sub-Saharan Africa on AKI in the non-cardiac, nonvascular surgical population.[11] South African (SA) studies on AKI have predominantly focused on medical patients. Local literature includes studies on hospitalised patients (predominantly medical),[3] HIV-positive patients,[12] COVID-19 patients,[13] post-cardiac surgery patients,[7] medically and surgically managed trauma patients[14] and critically ill patients. [15,16] However, there is a paucity of local literature on non-cardiac post-surgical AKI studies.
Developing countries, such as SA, often have different patient characteristics compared with patients studied in the developed world. Our patients' median age is younger, a higher ratio of trauma and urgent procedures are performed and a fifth of the adult population is potentially immune-compromised due to HIV infection.[17] Awareness of the local incidence and risk profile would assist in risk-stratifying, mitigating perioperative risk factors and anticipating the need for postoperative surveillance. Considering the dire consequences of AKI, it is important to quantify AKI incidence in SA post-surgical patients. This study's primary aim was to ascertain the AKI incidence after major non-cardiac surgery at a tertiary academic hospital. Secondary aims were to identify perioperative risk factors that are associated with an increased risk to develop AKI postoperatively.
Methods
Study setting
This retrospective descriptive cohort study was conducted at Tygerberg Hospital. Tygerberg Hospital is an academic public sector hospital servicing an estimated population of 3.4 million individuals in the Western Cape Province, SA. The hospital's theatre complex performs approximately 30 784 surgeries per year, including major surgeries in various disciplines.[18] This research is presented according to the STROBE checklist for cohort studies.[19] Ethical clearance was granted by the Health Research Ethics Committee of Stellenbosch University (ref. no. S21/10/193).
Study population
Expecting an AKI incidence of 13% after major non-cardiac surgery,[10] the sample size needed to indicate the true incidence of AKI with 95% confidence interval (CI) was calculated at 680. To accurately identify risk factors, up to threefold the calculated sample size for incidence was needed, thus making the final estimated sample size 2 040.
To calculate the incidence of AKI after major non-cardiac surgery we identified qualifying study participants from postoperative registers and accessed information on the variables required from two electronic databases: Tygerberg Hospital electronic records (Enterprise Content Management (ECM)) and the National Health Laboratory Service (NHLS).
Study criteria
Eligible participants included any adult (> 18 years) who underwent major non-cardiac surgery. Major surgery was defined as any procedure that required hospital admission postoperatively associated with significant fluid shifts or expected blood loss of >500 mL. It includes: open resection of organs; thoracic surgery; spinal and intracranial surgery; vascular surgery; large joint replacements; mastectomy with reconstruction; or laparoscopic surgery (except cholecystectomy or tubal ligation). [20] Exclusion criteria included individuals with end-stage kidney disease, defined by estimated glomerular filtration rate (eGFR) <15 mL/min/1.73m2,[21] previous RRT, cardiac surgery or caesarean section.
Data collection
The starting date for study participant enrolment was 31 August 2021, working retrospectively. Data were collected for a 12-month period. The required participant data were accessed, using participant hospital numbers, from the two electronic databases.
Data points collected from perioperative notes included: participant hospital number; surgery performed; date of procedure; age, gender; surgical discipline; American Society of Anesthesiology physical status (ASA);[22] comorbidities; elective v. emergency surgery; inotrope use intraoperatively; red blood cell (RBC) transfusion received intraoperatively; postoperative transfer: ward, post-anaesthesia high care unit (PAHCU) or ICU. Five data points were collected from the NHLS: pre-operative haemoglobin level; serum creatinine (sCr); eGFR; and worst sCr within 48 hours and 7 days postoperatively. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was used to calculate the eGFR.[23]
AKI incidence was determined by comparing the pre-operative serum creatinine (sCr) with the postoperative serum creatinine up to 7 days post procedure using the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 definition of AKI.[21] Risk factors for AKI were determined by univariate and multivariate logistic regression analysis of perioperative variables.
Statistical analysis
Collected data were stored in an Excel (Microsoft, USA) spreadsheet. The data were analysed using statistical software STATA version 17 (StataCorp, USA). Categorical variables are presented as number and percentage and continuous variables are presented as mean and standard deviation (SD) for normally distributed data. The two-sample f-test was used to compare study characteristics and risk factors with continuous variables, based on normal distribution. The X2 test or Fishers Exact test was used to compare categorical variables. Univariate and multivariate logistic regression analysis were used to evaluate pre-operative and intraoperative risk factors. Risk factors are expressed as odds ratio (OR), 95% CI andp-value using a significance level of 5%.
Results
Baseline demographic findings
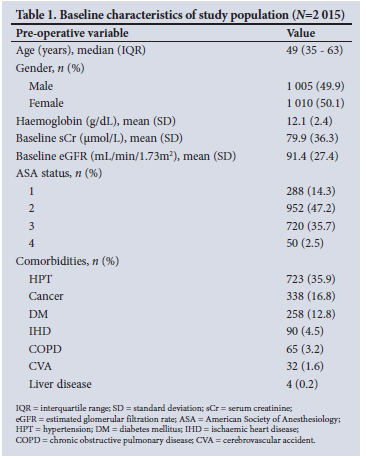
A total of 2 015 participant records were analysed (Fig. 1.). Table 1 displays several baseline participant characteristics. Nearly half of the participants presented to major surgery anaemic (49.3%, n=994/2 015). Twelve percent (n=246/2 015) of participants had existing renal impairment (eGFR <60 mL/min/1.73m2) prior to surgery. The most prevalent comorbidity was hypertension (35.9%, n=723/2 015). The study participants were from various surgical disciplines, with the most common being abdominal surgery (Table 2).


Incidence of AKI
The incidence of AKI post major non-cardiac surgery was 11.2% (n=225/2 015; 95% CI 9.8 - 12.6). Of the 225 AKI cases, 109 (5.4%; 95% CI 4.5 - 6.5) had stage 1 AKI, 57 (2.8%; 95% CI 2.1 - 3.6) stage 2 AKI, and 59 (2.9%; 95% CI 2.2 - 3.7) stage 3 AKI. Fig. 2. illustrates the AKI incidence and staging in the three most common surgical disciplines, and Table 2 displays AKI incidence for each surgical discipline as well as emergency v. elective procedures. Participant with pre-operative renal impairment represented 29.3% (n=66/225) of AKI cases. Of all AKI cases, n=18/225 (8%) had incomplete data sets available. Incomplete data was predominantly intraoperative information regarding blood transfusion and inotrope use.

Fifty-four percent of study participants (n-1090/2 015) had postoperative biochemistry available for comparison. Participants who had no biochemistry in the first 7 days postoperatively were assigned to the 'no AKF group. AKI incidence for participants who had both pre- and postoperative sCr measurements available was 20.6% (n=225/1 090).
There were n=139/1 005 (13.8%) males who developed AKI postoperatively compared with n=86/1 010 (8.5%) females (p<0.001 ). Ten percent (n=75/739) of patients aged <40 years developed AKI compared with 11.8% (n=82/690) patients in the 41 -60-year age group and 11.6% (n=68/586) in the >60-year age group (p>0.5). The AKI incidence related to patient variables (pre-,intra- and postoperative) is listed in Table 3.

Identifying risk factors: Univariate regression analysis
Several dependant risk factors were identified by univariate logistic regression analysis. The majority of AKI cases (69.8%, n=157/225) were aged <60 years, and age had no correlation to AKI (Spearman correlation 0.02). Male gender (OR 1.7; 95% CI 1.2 - 2.2; p<0.001) was found to be associated with AKI.
Seven comorbidities were assessed and three of these, namely hypertension (HPT) (OR 1.4; 95% CI 1.0 - 1.8; p=0.017), diabetes mellitus (DM) (OR 1.7; 95% CI 1.2 - 2.5; p=0.001) and chronic obstructive pulmonary disease (COPD) (OR 3.4; 95% CI 2.0 - 6.0; p<0.001) were significantly associated with AKI. Two ASA physical status classes reached statistical significance: ASA 2 (p=0.002) and ASA 4 (p<0.001). ASA 2 was identified as a protective indicator (OR 0.4; 95% CI 0.3 - 0.7) while ASA 4 (OR 3.2; 95% CI 1.5 - 6.4) was identified as an AKI risk factor.
Three surgical disciplines were identified as AKI risk factors: abdominal (OR 2.2; 95% CI 1.7 - 3.0; p<0.001), trauma (OR 2.1; 95% CI 1.5 - 2.9; p<0.001) and vascular surgery (OR 1.7; 95% CI 1.1 - 2.6; p=0.015). Four other surgical disciplines (orthopaedic surgery (OR 0.3; 95% CI 0.2 - 0.6; p<0.001), gynaecology (OR 0.4; 95% CI 0.2 - 0.6; p<0.001), ear, nose and throat (OR 0.2; 95% CI 0.06 - 1; p=0.036) and neurosurgery (OR 0.06; 95% CI 0.02 - 0.5; p<0.001)) were also significantly associated with AKI; however these were protective indicators.
Emergency procedures (OR 3.1; 95% CI 2.3 - 4.2; p<0.001), inotropic agent use intraoperatively (OR 12.8; 95% CI 9.2 - 17.8; p<0.001), RBC transfusion (OR 3.9; 95% CI 2.9 - 5.3; p<0.001) and postoperative ICU admission (OR 3.8; 95% CI 2.6 - 5.4; p<0.001) were also associated with an increased risk for AKI. Both baseline sCr and eGFR were weakly correlated (Spearman correlations 0.17 and -0.17, respectively) with postoperative AKI.
Some risk factors were more strongly associated with postoperative AKI than others, with inotrope use, blood transfusion and need for ICU admission after surgery having the strongest association.
Identifying independent risk factors: Adjusted multivariate regression
Multivariate logistic regression analysis (Fig. 3) revealed further information to identify independent risk factors. Multiple imputations were performed by chained equations to account for missing data points. Age (OR 1.0; 95% CI 0.99 - 1.02; p=0.521) was not associated with AKI. Male gender (OR 0.87; 95% CI 0.6 - 1.26; p=0.476) was not an independent risk factor, in contrast with the initial association after univariate analysis.

Of the three comorbidities initially associated with AKI (HPT, DM and COPD), only COPD was identified as an independent risk factor for AKI (OR 2.19; 95% CI 1.09 - 4.37; p=0.026). A low baseline haemoglobin was not a risk factor for AKI (OR 1.09; 95% CI 0.78 - 1.53; p=0.591). No ASA physical status class was associated with AKI as independent risk factor, in contrast to univariate analysis. ASA 2 (OR 0.95; 95% CI 0.63 - 1.70; p=0.885), ASA 3 (OR 1.73; 95% CI 0.86 - 3.47; p=0.121) and ASA 4 (OR 2.12; 95% CI 0.79 - 5.68; p=0.135), as displayed in Fig. 3, all cross the non-significance line of OR = 1.
Four surgical disciplines were identified as independent risk factors for AKI: abdominal (OR 2.14; 95% CI 1.33 - 3.45; p=0.002), trauma (OR 3.00; 95% CI 1.59 - 5.64, p=0.001), urology (OR 2.45; 95% CI 1.31 -4.59; p=0.005) and vascular (OR 2.42; 95% CI 1.31 - 4.45; p=0.004) surgery.
Other surgical and intraoperative factors that remained risk factors for AKI developing included undergoing an emergency procedure (OR 1.74; 95% CI 1.15 - 2.65; p=0.009), receiving a RBC transfusion intraoperatively (OR 1.81; 95% CI 1.21 - 2.70; p=0.004) and inotropic agent use intraoperatively (OR 2.77; 95%CI 1.8-4.26;p<0.001).
Postoperative transfer to a general ward (OR 0.43; 95% CI 0.26 - 0.71; p=0.001) was the only variable that was associated with lowered risk for AKI, and ICU admission was no longer identified as a risk factor (OR 1.51; 95% CI 0.97 - 2.34; p=0.062).
In summary, one preoperative patient factor was independently associated with AKI: COPD. Surgical and intraoperative factors were abdominal, trauma, vascular and urological procedures, emergency surgery, blood transfusion and inotrope use. Postoperative general ward admission was the only protective indicator significantly associated with AKI.
Discussion
Perioperative AKI is a well-documented complication in the surgical patient. In this single-centre retrospective cohort of major non-cardiac surgeries, the AKI incidence was 11.2%. The incidence of AKI for each surgical discipline was also determined, with the highest three risks being trauma (19%), abdominal (18.6%) and vascular surgery (17%). Several independent risk factors for postoperative AKI were identified. COPD was the only pre-operative risk factor associated with AKI. Surgical and intraoperative risk factors identified were abdominal, trauma, urological, vascular procedures, emergency surgery, RBC transfusion and inotropic agent use intraoperatively.
Baseline characteristics indicated that the mean age (49 years) of our study participants was lower than in comparative studies (62 -64 years).[8-10] This difference may be due to the high local trauma burden as well as SAs lower life expectancy compared with high-income countries. HPT (35.9%) was the most
prevalent comorbidity in our cohort. This is significant since HPT is described as a risk factor for AKI by some authors. [8,10,24] However, this was not found in our cohort after adjusted regression analysis. Similar studies reported a wide range of HPT prevalence at 12%,[8] 50%[9] and 75%.[10] The Grams et al.[10] cohort was unique since they analysed records from retired military personnel who were older, with more frequent and severe stages of comorbid disease.
The incidence of AKI we found is similar to that found in another large retrospective study (11.8%).[10] However, their cohort included cardiac surgery. In contrast to our findings, a Nigerian study quoted a postoperative AKI incidence double that of our study (22.5%).[11] Two key differences between our cohort and the Nigerian study are that their sample size was considerably smaller, and included urine output measurements for diagnosing AKI. Other authors[8,5] agree that using urine output as part of defining AKI could overestimate the incidence and may be a contributing factor in the high incidence seen in the Nigerian study. Reasons proposed for this relate to a physiological decrease in urine output during surgical conditions due to effects of anaesthesia, periods of hypovolaemia and increased vasopressin and aldosterone release.
We calculated AKI incidence for each surgical discipline, identifying trauma surgeries ( 19%) to provide the highest incidence. Therefore, preoperative resuscitation measures performed in the trauma unit should be emphasised. Davies et al.[26] demonstrated this by implementing improved fluid balance monitoring in their trauma centre, which led to a reduction of AKI incidence by 33%. Their reduced AKI rates were achieved by inexpensive tools such as implementing new fluid balance charts, training and education. The authors emphasised multidisciplinary team input, laboratory-based AKI alerts along with protocol-guided management and motivating nursing staff and junior doctors.
Most publications on perioperative AKI in non-cardiac patients focused solely on abdominal surgery.[8,9,11,25,27-29] Not surprisingly, in our cohort, abdominal procedures were the most frequent type of surgery (21.9%), with an AKI incidence of 18.6%. This compared favourably with the Teixeira et al.[9] study, which quoted an AKI incidence of 22.4%. Conversely, studies by O'Connor et al.[27] and Long et al.[8] were much lower, at 13.4% and 6.7%, respectively. However, these studies cannot be used as direct comparison with our cohort, since Teixeira et al. included more high-risk patients and Long et al. included minor surgeries in their cohort.
We found one pre-operative characteristic, COPD, to be an independent risk factor for postoperative AKI. Initial univariate analysis also identified male gender, HPT, DM, ASA class 4 physical status and existing renal impairment as associated with AKI. Our finding differs from other publications that identified numerous pre-operative risk factors for postoperative AKI.[8,10,11] Some of these included the following comorbidities: HPT, DM, chronic lung disease, congestive cardiac failure, coronary artery disease, liver disease, cerebrovascular disease, peripheral artery disease and malignancies. Other factors quoted by the authors ranged from either male or female gender, older age, obesity, ASA class 4 and 5, revised cardiac risk index scores, chronic angiotensin receptor blocker (ARB) or angiotensin converting enzyme (ACE) inhibitor use, pre-existing chronic kidney disease, baseline anaemia and eGFR <60 mL/min/1.73m2. We found a weak negative correlation (-0.17) between eGFR and AKI, in keeping with similar reports.[8,9]
Strikingly, nearly half (49.3%) of our participants presented for major surgery anaemic. Although anaemia was not associated with AKI in our study, pre-operative anaemia is an established risk factor for AKI in cardiac patients undergoing coronary bypass surgery[30] (hazard ratio 1.23) as well as after orthoptic liver transplantation (OR 1.72; 95% CI 1.11 - 2.67).[31] A large retrospective study in non-cardiac surgical patients by Wash et al [32] demonstrated that 29% of patients presented to surgery anaemic. Compared with non-anaemic counterparts, patients with a Hb 10 - 12 g/dL had double the odds of postoperative AKI, and those with Hb <8 g/dL had a 3.7-fold risk.[32]
Most independent risk factors we identified were related to surgical as opposed to patient factors. Emergency surgery as a risk factor for AKI is logical because of the associated events involved in these types of procedures, namely haemodynamic instability, major fluid shifts, higher probability of receiving blood transfusion, inotropic agents and increased likelihood of ICU admission. Despite these factors, we found that emergency surgery was still an independent risk factor (OR 1.74; 95% CI 1.15 - 2.65) after adjusting for the measured confounding variables.
Another non-modifiable risk factor we found relates to the surgical discipline involved in performing the operation. We found major trauma, abdominal, urological or vascular procedures are independent risk factors for AKI. Vascular surgery is occasionally grouped together with cardiac patients, since they often share similar characteristics and surgical techniques.[33] Urology procedures are omitted by some researchers when evaluating AKI owing to the closely related interplay between surgical manipulation of the kidneys and renal function. We included urology for two reasons: firstly, the discipline is not limited to the kidneys - major bladder and prostate operations would have been excluded. Secondly, knowing the incidence and severity of AKI in nephrectomy patients is a valuable indicator of long-term outcomes.
Blood transfusion is not consistently shown as a risk factor for AKI. However, we found an OR 1.8 (95% CI 1.21 -2.7) comparable to Teixeira et al[9] (OR 2.2 95% CI 1.4 - 3.5). Others with comparable findings to ours included Raji et al.,[11] Gameiro et al.[25] and Iyigun et al.[24]
We concluded that the use of inotropic agents was a high risk factor for AKI (OR 2.8 95% CI 1.8 - 4.26). This is in agreement with data published by Hou et al. [34] In contrast, Wu et al.[29] stated that the use of vasopressor agents resulting in AKI was unknown.
Our findings add new information to the existing body of knowledge on perioperative AKI. To our knowledge, only the African Surgical Outcomes Study has quantified postoperative AKI that includes non-cardiac surgical patients in SA.[35] Half their study recruits were from SA hospitals. They found an AKI incidence of 13 per 1 000; however, they included minor procedures, cardiac and obstetric surgeries in their cohort. Another value of our findings advocates for increased postoperative surveillance of renal function in at-risk patients.
A potential bias of our study is that the assessed period overlapped with the COVID-19 pandemic. Several elective surgical lists were cancelled for periods during the review. This could have led to a disproportionate number of emergency surgeries included in our cohort, and may have skewed the true incidence.
The retrospective nature of this study limited us to existing records, and a sizable percentage of the variables assessed were incomplete. This was compensatedfor in the size of our cohort as well as by implementing multiple imputation statistical models. The results thus reflect a realistic representation of perioperative AKI, as occurring in the usual course of clinical care. Additionally, monitoring postoperative serum creatinine is not standard practice. In our cohort, 54% of participants had both pre- and postoperative serum creatine measurements available for comparison. A number as low as 33.8% was found elsewhere.[8] Had we omitted unmonitored cases in our analysis, it would likely have overestimated the true incidence of postoperative AKI.
We relied on information from hard-copy registers and handwritten perioperative anaesthetic charts. Perioperative charts are scanned into an electronic database, which was missing in around one in 10 eligible study participants. High-income countries have nationalised electronic records, with access to complete patient history and current medications. We did not include potential AK1 risk factors such as participant race, HIV status, COVID-19 results, ARBs, ACE inhibitors and statin use.[10] Our study reported limited information regarding intraoperative details such as individual procedure performed, type and dose of inotrope used, as well as type and amount of fluid infused, including number of blood transfusions, and did not state whether contrast media were used. We were also affected by the inability to accurately assess intraoperative blood pressure measurement. This limitation is of importance as Wu et al[29] demonstrated that a mean arterial pressure (MAP) targeted between 80 and 95 mmHg led to the lowest incidence of postoperative AKI compared with both lower and higher MAP targets. Lastly, a limitation of retrospective studies is that causal effect cannot be determined.
The definition and diagnoses of AKI may soon change once again. Renal biomarkers are increasingly used to define renal injury. Recent studies are evaluating the urine biomarkers neutrophil gelatinase-associated lipocalin[36] and insulin-like growth factor binding protein 7.[37] These markers are being used to define and prevent AKI, and to predict renal recovery, adverse outcomes or progression to CKD. The findings of this study can aid in rational and cost-effective use of expensive diagnostic biomarkers,
Conclusion
AKI after non-cardiac surgery is common, leads to adverse patient outcomes and adds to the burden of limited resources. Our findings, with postoperative AKI incidence at 11.2%, concur with international data. Additionally, we found mostly non-modifiable risk factors for AKI (COPD, emergency surgery, type of surgery) and two modifiable variables (RBC transfusion and intraoperative inotrope use). Future studies may provide more insight into perioperative AKI where a prospective design is used that includes haemodynamic and fluid balance parameters. Renal biomarkers are an exciting possibility that may prove useful in diagnosing AKI earlier and guiding treatment, preventing complications and improving patient outcomes.
Declaration. The research for this study was done in partial fulfilment of the requirements for ER's MMed (Anaes) degree at Stellenbosch University.
Acknowledgements. We acknowledge Dr Carl Lombard for his contribution as bio statistician.
Author contributions. ER was the principal investigator of the study and was responsible for writing the manuscript. SC was the study supervisor, and reviewed and edited the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI. A meta-analysis. Clin J Am Soc Nephrol 2013;8(9):1482-1493. https://doi.org/10.2215/cjn.00710113 [ Links ]
2. Nash K, Hafeez A, Hou S. Hospital-acquire d renal insufficiency. Am J Kidney Dis 2002;39(5):930-936. https://doi.org/10.1053/ajkd.2002.32766 [ Links ]
3. Fenna K, Erasmus RT, Zemlin AE. Hospital-acquired acute kidney injury prevalence in in adults at a South African tertiary hospital. Afr Health Sci 2019;19(2):2189-2197. https://doi.org/10.4314/ahs.vl9i2.44 [ Links ]
4. Hoste EAJ, Kelium JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018;14(10):607-625. https://doi.org/10.12788/jhm.2683 [ Links ]
5. Stewart J, Findlay G, Smith N, Kelly K, Mason M. Adding insult to injury. A review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure). A report by the National Confidential Enquiry into Patient Outcome and Death 2009. https://www.ncepod.org.uk/2009aki.html (accessed 12 August 2021). [ Links ]
6. Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalised patients. J Hosp Med 2017;12(2):70-76. https://doi.org/10.12788/jhm.2683 [ Links ]
7. Leballo G, Moutlana HJ, Muteba MK, Chakane PM. Factors associated with acute kidney injury and mortality during cardiac surgery. Cardiovasc J Afr 2021;32(6):22-27. https://doi.org/10.5830/cvja-2020-063 [ Links ]
8. Long TE, Helgason D, Helgadottir S, et al. Acute kidney injury after abdominal surgery. Incidence; risk factors, and outcome. Anesth Analg 2016;122(6):1912-1920. https://doi.org/10.1213/ane.0000000000001323 [ Links ]
9. Teixeira C, Rosa R, Rodrigues N, et aL Acute kidney injury after major abdominal surgery. A retrospective cohort analysis. Crit Care Res Pract 2014;1:1-8. https://doi.org/10.1155/2014/132175 [ Links ]
10. Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery. A retrospective analysis of veterans health administration data. Am J Kidney Dis 2016;67(6):872-880. https://doi.org/10.1053/j.ajkd.2015.07.022 [ Links ]
11. Raji YR, Ajayi SO, Ademola AF, et al. Acute kidney injury among adult patients undergoing major surgery in a tertiary hospital in Nigeria. Clin Kidney J 2018;11(4):443-449. https://doi.org/10.1093/ckj/sfxl44 [ Links ]
12. Chothia MY, Ramsunder N. In-hospital mortality of HIV-positive patients with acute kidney injury a decade after the roll-out of anti-retroviral therapy in Cape Town, South Africa. African J Nephrol 2019;22(1):46-53. https://doi.org/10.21804/22-1-3423 [ Links ]
13. Diana NE, Kalla IS, Wearne N, et al. Acute kidney injury during the COVID-19 pandemic -experience from two tertiary centres in South Africa. Wits J Clin Med 2020,2(3).189-198. https://doi.org/10.18772/26180197.2020.v2n3a2 [ Links ]
14. Skinner DL, Kong VY, de Vasconcellos K, et al. Acute kidney injury on presentation to a major trauma service is associated with poor outcomes. J Surg Res 2018;232:376-382. https://doi.org/10.1016/j.jss.2018.06.069 [ Links ]
15. Khuweldi M, Skinner D, De Vasconcellos K. The incidence and outcomes of patients with acute kidney injury in a multidisciplinary intensive care unit in Durban, South Africa. South African J Crit Care 2020;36(2).80. https://doi.org/10.7196/SAJCC.2020.v36i2.426 [ Links ]
16. Aylward RE, van der Merwe E, Pazi S, et al. Risk factors and outcomes of acute kidney injury in South African critically ill adults. A prospective cohort study. BMC Nephrol 2019,20(460).1-11. https://doi.org/10.1186/sl2882-019-1620-7 [ Links ]
17. Maluleke R. Mid-year population estimates 2018. Statistics South Africa, 2018. https://www.statssa.gov.za/publications/P0302/P03022018.pdf [ Links ]
18. Western Cape Department of Health. Tygerberg Hospital. WCDoH, 2016. https://www.westerncape.gov.za/assets/departments/health/tygerberg_hospital_information_pamphlet_-_2016.pdf (accessed 17 September 2021). [ Links ]
19. Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement. Guidelines for reporting observational studies. PLoS Med 2007,4(10).e296. https://doi.org/10.1371/journal.pmed.0040296 [ Links ]
20. Logix MD. Routine preoperative lab test guidelines. LogixMD, 2016. https://professionals.wrha.mb.ca/old/professionals/primary-care-providers/files/PreopTestAlgorithum.pdf (accessed 17 September 2021). [ Links ]
21. Kelium JA, Lameire N, Acute Kidney Injury Work Group. KDIGO clinicai practice guideline for acute kidney injury. Kidney Int 2012,2(1).1-138. https://kdigo.org/guidelines/acute-kidney-injury/ (accessed 23 July 2021). [ Links ]
22. American Society of Anesthesiology. ASA physical status classification. ASA, 2020. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (accessed 04 August 2021). [ Links ]
23. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604-612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 [ Links ]
24. Iyigun M, Aykut G, Tosun M, et al. Perioperative risk factors of acute kidney injury after non-cardiac surgery. A multicenter, prospective, observational study in patients with low-grade American Society of Anesthesiologists physical status. Am J Surg 2019;218(3):457-461. https://doi.org/10.1016/j.amjsurg.2019.01.031 [ Links ]
25. Gameiro J, Fonseca JA, Neves M, Jorge S, Lopes JA. Acute kidney injury in major abdominal surgery. Incidence, risk factors, pathogenesis and outcomes. Ann Intens Care 2018;8(22):1-10. https://doi.org/10.1186/sl3613-018-0369-7 [ Links ]
26. Davies A, Srivastava S, Seligman W, et al. Prevention of acute kidney injury through accurate fluid balance monitoring. BMJ Open Qual 2017;6(2):e000006. https://doi.org/10.1136/bmjoq-2017-000006 [ Links ]
27. O'Connor ME, Kirwan CJ, Pearse RM, Prowle JR. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med 2016;42(4):521-530. https://doi.org/10.1007/S00134-015-4157-7 [ Links ]
28. Kee YK, Kim H, Jhee JH, et al. Incidence of and risk factors for delayed acute kidney injury in patients undergoing colorectal surgery. Am J Surg 2019;218(5):907-912. https://doi.org/10.1016/j.amjsurg.2019.03.027 [ Links ]
29. Wu X, Jiang Z, Ying J, Han Y, Chen Z. Optimal blood pressure decreases acute kidney injury after gastrointestinal surgery in elderly hypertensive patients. A randomised study. Optimal blood pressure reduces acute kidney injury. J Clin Anesth 2017;43:77-83. https://doi.org/10.1016/j.jclinane.2017.09.004 [ Links ]
30. Oprea AD, Del Rio JM, Cooter M, et al. Pre- and postoperative anemia, acute kidney injury, and mortality after coronary artery bypass grafting surgery. A retrospective observational study. Can J Anesth 2018;65(1):46-59. https://doi.org.10.1007/S12630-017-0991-0 [ Links ]
31. Lichtenegger P, Schiefer J, Graf A, et al. The association of pre-operative anaemia with survival after orthotopic liver transplantation. Anaesthesia 2020;75:472-428. https://doi.org/10.111l/anae.14918 [ Links ]
32. Walsh M, Garg AX, Devereaux PJ, et al. The association between perioperative hemoglobin and acute kidney injury in patients having non-cardiac surgery. Anesth Analg 2013;117(4):924-931. [ Links ]
33. Nadim MK, Forni LG, Bihorac A, et al. Cardiac and vascular surgery-associated acute kidney injury. The 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) Group special report. J Am Hear Assoc 2018;7(11):1-27. https://doi.org/10.1161/jaha.118.008834 [ Links ]
34. Hou K, Chen Q, Zhu X, et al. Correlation between vasoactive-inotropic score and postoperative acute kidney injury after cardiovascular surgery. Hear Surg Forum 2021;24(2):282-292. https://doi.org/10.1532/hsf.3537 [ Links ]
35. Biccard BM, Madiba TE, Kluyts HL, et al. Perioperative patient outcomes in the African Surgical Outcomes Study. A 7-day prospective observational cohort study. Lancet 2018;391(10130):1589-1598. https://doi.org/10.1016/S0140-6736(18)30001-1 [ Links ]
36. Macdonaid N, Pearse RM, Murray PT, et al. The role of goai-directed therapy in the prevention of acute kidney injury after major gastrointestinal surgery. Substudy of the OPTIMISE trial. Eur J AnaesthesioJ 2019;36(12):924-932. https://doi.org/10.1097/eja.0000000000001104 [ Links ]
37. Kim MG, Cho Y, Lim SY, et al. Urinary tissue inhibitor of metalioproteinase-2 and insulin-like growth factor-binding protein 7 as biomarkers of patients with established acute kidney injury. Korean J Intern Med 2020;35(3):662-671. https://doi.org/10.3904/kjim.2018.266 [ Links ]
 Correspondence:
Correspondence:
E Rossouw
1454u70@sun.ac.za
Accepted 25 October 2022














