Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.3 Pretoria Mar. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i3.16812
RESEARCH
Impact of interventions for tuberculosis prevention and care in South Africa - a systematic review of mathematical modelling studies
L R BrownI; C van SchalkwykII; A K de VilliersIII, IV; F M MarxV, VI, VII
IMSc; South African DSI-NRF Centre of Excellence in Epidemiological Modelling and Analysis (SACEMA), Stellenbosch University, Cape Town, South Africa
IIPhD; South African DSI-NRF Centre of Excellence in Epidemiological Modelling and Analysis (SACEMA), Stellenbosch University, Cape Town, South Africa
IIIMSc; South African DSI-NRF Centre of Excellence in Epidemiological Modelling and Analysis (SACEMA), Stellenbosch University, Cape Town, South Africa
IVMSc; Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
VPhD; South African DSI-NRF Centre of Excellence in Epidemiological Modelling and Analysis (SACEMA), Stellenbosch University, Cape Town, South Africa
VIPhD; Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
VIIPhD; Division of Infectious Disease and Tropical Medicine, Center for Infectious Diseases, Heidelberg University Hospital, Heidelberg, Germany
ABSTRACT
BACKGROUND: Substantial additional efforts are needed to prevent, find and successfully treat tuberculosis (TB) in South Africa (SA). In the past decade, an increasing body of mathematical modelling research has investigated the population-level impact of TB prevention and care interventions. To date, this evidence has not been assessed in the SA context
OBJECTIVE: To systematically review mathematical modelling studies that estimated the impact of interventions towards the World Health Organization's End TB Strategy targets for TB incidence, TB deaths and catastrophic costs due to TB in SA
METHODS: We searched the PubMed, Web of Science and Scopus databases for studies that used transmission-dynamic models of TB in SA and reported on at least one of the End TB Strategy targets at population level. We described study populations, type of interventions and their target groups, and estimates of impact and other key findings. For studies of country-level interventions, we estimated average annual percentage declines (AAPDs) in TB incidence and mortality attributable to the intervention
RESULTS: We identified 29 studies that met our inclusion criteria, of which 7 modelled TB preventive interventions (vaccination, antiretroviral treatment (ART) for HIV, TB preventive treatment (TPT)), 12 considered interventions along the care cascade for TB (screening/case finding, reducing initial loss to follow-up, diagnostic and treatment interventions), and 10 modelled combinations of preventive and care-cascade interventions. Only one study focused on reducing catastrophic costs due to TB. The highest impact of a single intervention was estimated in studies of TB vaccination, TPT among people living with HIV, and scale-up of ART. For preventive interventions, AAPDs for TB incidence varied between 0.06% and 7.07%, and for care-cascade interventions between 0.05% and 3.27%
CONCLUSION: We describe a body of mathematical modelling research with a focus on TB prevention and care in SA. We found higher estimates of impact reported in studies of preventive interventions, highlighting the need to invest in TB prevention in SA. However, study heterogeneity and inconsistent baseline scenarios limit the ability to compare impact estimates between studies. Combinations, rather than single interventions, are likely needed to reach the End TB Strategy targets in SA
South Africa (SA) remains one of the countries with the highest tuberculosis (TB) burden in the world.[1] In 2021, an estimated 304 000 people developed TB, and 55 000 died from the disease;[1] TB therefore remains the leading infectious disease cause of death in the country.[2] Recent measures to contain the spread of SARS-CoV-2[3] have led to considerable declines in individuals accessing healthcare services, TB testing and individuals diagnosed with TB,[4] resulting in an expected increase in TB prevalence and mortality. These developments have also seriously affected SA's progress towards the milestones and targets set for the World Health Organization (WHO)'s End TB Strategy[5] that aims to reduce the number of TB deaths by 95%, the TB incidence rate by 90% (relative to 2015) and the percentage of TB-affected families facing catastrophic costs due to TB to zero by 2035.
To mitigate these adverse consequences on TB epidemiology and to restore progress towards the End TB Strategy targets, substantial additional efforts are needed to prevent, find and successfully treat TB in SA. A comprehensive consultation process, co-ordinated by the TB Think Tank of the National Department of Health, has been initiated to define additional interventions to be implemented as part of SA's upcoming 2023 - 2028 National Tuberculosis Programme (NTP) Strategic Plan.
For policy-makers to identify and implement strategies for optimal outcomes towards the End TB Strategy targets, evidence must be collected to inform decisions.[6] Generating this evidence directly through empirical research poses considerable challenges. Cluster-randomised trials to estimate the population-level impact of interventions on TB incidence, mortality and catastrophic costs demand considerable resources and time. They often focus on a limited set (e.g. of one or two) interventions, yielding limited insights into how these interventions will compare with alternatives.[7]
Mathematical models for infectious diseases are valuable tools for evaluating the effect of intervention strategies and assisting policymakers in making informed decisions.[8,9] Transmission-dynamic models of TB are increasingly used[10-12] to estimate the impact of interventions on population-level outcomes in high-burden countries, including SA. To date, the evidence from mathematical modelling research on interventions to reduce TB incidence, mortality and catastrophic costs in SA has not been systematically assessed.
In an effort to support decision-making for TB in SA, we reviewed mathematical modelling studies that estimated the population-level impact of interventions towards the End TB Strategy targets for TB incidence and mortality, and catastrophic costs associated with TB. We aimed to describe the types of interventions, intervention designs and target populations considered, and the impact estimated through modelling. We also aimed to highlight gaps in TB modelling research that could be addressed in future research to inform TB policy-making in the country.
Methods
We employed the PICOS (Population, Intervention, Control, Outcomes and Study design) tool[13] to define the research question and the design of this systematic review. The review protocol is registered with the international prospective register for systematic reviews (PROSPERO; CRD42021276526). We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for 2020.[14] The review design and PRISMA checklist (Table S1 - S3) are available in the appendix (https://www.samedical.org/file/1985).
Search strategy and selection criteria
We conducted a systematic search of the published literature using the PubMed, Web of Science, and Scopus databases. Search strings are provided in Table S2 in the appendix. Additionally, we searched the TB Modelling and Analysis Consortium (TB MAC)'s list of mathematical and economic TB modelling studies,[15] Global Index Medicus, African Index Medicus and reference lists of eligible studies. We also consulted four leading global experts in TB modelling to identify additional publications not included in the initial search from their personal databases. The search was conducted up to 28 September 2021. We included articles that used population-based transmission dynamic models of TB in SA at country or sub-country level, and reported population-level reductions in TB incidence, TB mortality and/or catastrophic costs associated with TB. We excluded articles that reported statistical models of empirical data or cohort models that were not transmission dynamic. We further excluded reviews of modelling studies and articles describing mathematical models that did not refer to the SA population (or a population in SA).
Data extraction and analysis
Titles and/or abstracts of articles identified during the initial search were screened by two reviewers. The full texts of these studies were then retrieved and independently assessed for eligibility. Data extracted from eligible studies included the type of model, study population, intervention details, key study outcomes and model projections. For studies that modelled multiple scenarios of the same intervention, we extracted the scenario that resulted in the greatest impact. We described modelling results by type of intervention and target population with respect to estimated gains towards the End TB strategy targets. In addition, for studies describing country-level TB models, we compared average annual percentage declines (AAPDs) in TB incidence and mortality estimated for different interventions relative to base-case (no intervention). For articles reporting percentage declines over the entire model time horizon, we calculated AAPD using the formula:
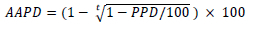
where t denotes the time horizon of the model, and PPD the period percentage decline attributable to the intervention investigated (i.e. the percentage difference between the baseline scenario and intervention scenario at the end of the time horizon) reported in a study. An example of calculating AAPDs is illustrated in Fig. S1 in the appendix.
Risk of bias assessment
An adapted risk-of-bias tool[16-18] was used to assess the methodological quality of eligible modelling studies. The tool uses a set of questions regarding the study aims and objectives, model structure, setting and population, methods of fitting, assumptions and others. An overall score consideration of 0 for each criterion was given if no required information was provided, 1 if some aspects of the study were incomplete, and 2 if the necessary information was clear and appropriate for the research question. A full description of the questions and score considerations is available in the appendix (Table S5). A risk-of-bias score (0 - 28) was given to each study by adding itemised scores for each criterion. The quality of eligible studies was deemed very high (>22), high (19 - 22), medium (14 - 18) or low (<14) according to the risk of bias score.
Results
Search process and selection of articles
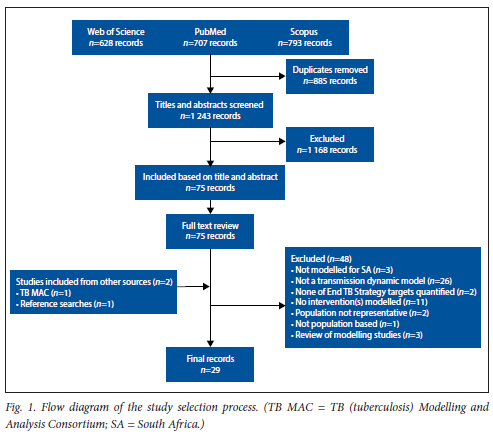
Our initial search yielded 2 128 records, of which 1 243 were unique. The majority of articles that were excluded at title and abstract screening (n=1 168) described statistical models, descriptive analyses or static cost-effectiveness models. Following full-text review of 75 articles, we identified a total of 29 that met the inclusion criteria. A full breakdown of articles identified for this review is shown in Fig. 1. Detailed reasons for exclusion at full-text review are provided in the appendix (Table S9.1 - 9.2).
Risk-of-bias assessment
Of the 29 articles included in this review, 13 received a risk-of-bias score of 19 - 22, and were considered of high quality,[6,19-30] and 16 received a score of >22, considered very high quality.[10,31-45] A median score of 23 (out of 28) was recorded, equivalent to very high quality. Reductions in the score were due to lack of model validation, incomplete parameter descriptions, lack of justification of assumptions made, and missing information about limitations and study context. Detailed scores of the assessment are provided in the appendix (Table S6).
Characteristics of included studies
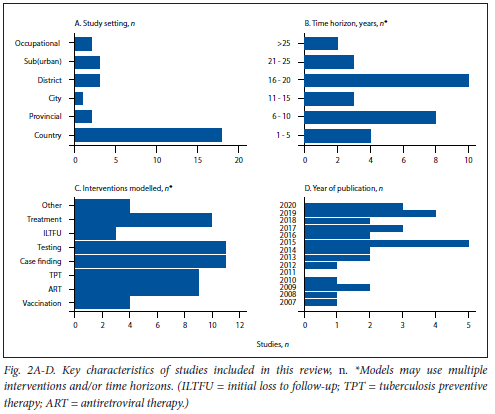
Studies varied considerably in terms of model design, time horizon over which interventions were modelled, study population, type of intervention and outcomes measured (Fig. 2; appendix Tables S4 and S7). Of the 29 articles included, 20 described deterministic compartmental models,[10,19-23,27,29-31,33-38,41-43,45] three stochastic compart-mental models'32,39-401 and three stochastic individual-based models (IBMs).'24,25,281 The remaining three studies used a combination of different types of transmission-dynamic models.[6,26,44] All but one study included stratifications by HIV status to account for the modifying effect of HIV infection on TB natural history.[29] With respect to the End TB Strategy targets, all but two studies[32,44] reported outcomes for reductions in TB incidence, 17 reported reductions in TB mortality,[10,19,20,23-26,32-34,36-41] and only one[44] considered catastrophic costs averted due to TB interventions.
Interventions modelled
Of the 29 studies identified, 22 modelled hypothetical interventions, while 7 modelled scenarios for scale-up of existing interventions.[10,22,26,30,33,38,42] Seven studies modelled preventive interventions,[22,26-28,30,36,38] 12 considered interventions along the care cascade for TB[10,19,20,23,25,29,32,37,41-44] and 10 considered a combination of both.[6,21,24,31,33-35,39,40,45] Table 1 provides an overview of key characteristics and study outcomes by type of intervention for the 18 country-level studies included in this review. Details of studies modelling the impact of interventions at sub-country level are provided in the appendix (Table S8). Estimates of impact measured in the studies are provided below.
(i) Vaccination
Three studies estimated the impact of vaccination against TB. Two studies modelled novel vaccines,[21,36] and one considered revaccination using the bacillus Calmette-Guérin (BCG) vaccine.[22] Considerable reductions in TB incidence and mortality were projected for a hypothetical novel vaccine with 70% efficacy in preventing TB infection,[21] and a different hypothetical vaccine with 100% efficacy, equally effective in people living with HIV (PLWH) and HIV-negative people, TB-infected and TB-uninfected populations.[36] The latter study considered various vaccination strategies including early-adolescent and 10-yearly mass vaccination campaigns.[36] Re-vaccinating HIV-negative adolescents in an urban high-transmission setting using the BCG vaccine (efficacy: 10 - 80%) was estimated to be of limited impact but potentially cost-effective[22] (appendix Table S8).
(ii) ART for TB prevention
Five studies estimated the impact of antiretroviral therapy (ART) scale-up for TB prevention in SA. Two[24,33] were published in 2015 and one in 2014,[26] at a time when ART eligibility in SA was limited to PLWH with a CD4 count of <500 cells per mm3,[46] and focused on expanding ART towards universal treatment (regardless of CD4 count). Reaching 80% coverage among PLWH was estimated to reduce TB incidence and mortality substantially,[26,33] while a coverage of 42% was estimated to be of lower impact.[24] Other studies focused on combinations of ART and isoniazid preventive therapy,[21] and the introduction of universal HIV testing with immediate ART following a positive test.[30]
(iii) TB preventive treatment
Six studies estimated the impact of TB preventive treatment (TPT), which all considered isoniazid monotherapy.[6,21,24,27,33,40] Target groups considered included adolescents, PLWH and people previously treated for TB. Screening adolescents attending secondary schools for latent TB infection followed by TPT for those testing positive was found to be beneficial to both the adolescent and adult populations[27] Scaling up TPT among PLWH on ART after screening for TB disease was estimated to lead to considerable reductions in population-level TB incidence and mortality in one study,[21] but was of lower impact in another.[6] Limited impact was estimated when extending TPT to HIV-negative individuals.[24] Two subsequent studies of TB in a suburban high-incidence setting concluded that TPT combined with case finding/follow-up examinations among people who previously completed TB treatment could accelerate declines in TB incidence and mortality at population level and potentially reduce costs[38,39] (appendix Table S8).
(iv) Case finding/screening
Seven studies modelled TB screening/active case finding (ACF) interventions. Target populations considered included the general population, PLWH on ART, people previously treated for TB and public health clinic attendees. Three studies considered case-finding interventions in the general population.[24,35,42] Periodic ACF reaching 60% of the general population using a hypothetical, high-sensitivity screening test was estimated to moderately reduce TB incidence with greater impact seen on mortality.[24] In a rural setting, symptom-based screening followed by Xpert, culture and/or drug susceptibility testing (DST) was estimated to simultaneously reduce incident multidrug-resistant (MDR-) and extensively drug-resistant (XDR-)TB[35] (appendix Table S8). Increasing the use of a symptom-based screening tool from 40% to 100% among people on ART was estimated to have limited impact on TB incidence.[42] Expanded access to care using outreach clinics and symptom-based screening in primary care was estimated to reduce cases of catastrophic costs due to TB substantially, with larger impact seen after 5 - 10 years.[44] One study in a high-incidence setting focused on ACF among people who had previously completed TB treatment.[39] The study showed considerable declines in TB incidence for targeted ACF, alone or in combination with TPT[39] (appendix Table S8). Two studies modelled the impact of TB screening among individuals attending public healthcare clinics.[6,23] Verbal TB symptom screening at public health clinic entrances, assuming 100% screening coverage, was estimated to reduce TB incidence countrywide.[6] In the Western Cape Province, increasing cough-based screening coverage followed by smear microscopy for those positive was estimated to have a considerable impact on TB incidence and mortality[23] (appendix Table S8).
( v) Diagnostic interventions
Five modelling studies focused on Xpert-based algorithms as the standard diagnostic test for TB in SA, prior to[10,33,34,45] and during[42] its roll-out in 2013. A diagnostic modelling study of TB in five African countries including SA suggested that the introduction and scale-up of Xpert could reduce morbidity and mortality, with less impact seen on long-term epidemiological outcomes.[10] Replacing all smear-microscopy tests with Xpert,[33] increasing the coverage of Xpert-based diagnoses from 80% in 2016 to 100% in 2035[42] and supplementing DST with Xpert[34] (appendix Table S8) were suggested to have limited impact on TB incidence and mortality. Diagnosing gold miners, an occupational group at high risk for TB in SA, with Xpert instead of radiographical screening was estimated to reduce TB incidence in mining settings substantially[45] (appendix Table S8). One study[41] estimated the impact of novel lateral flow urine lipoarabinomannan (LAM) tests for the early detection of TB in SA and found that, while future LAM tests could be important for averting TB deaths among PLWH with advanced disease, population-level impact would depend on diagnostic accuracy. All three studies that investigated the impact of DST on drug-resistant (DR)-TB concluded that, although transmission could be reduced, additional interventions would be necessary to effectively reduce the burden of DR-TB in the population.[20,29,32]
(vi) Reducing initial loss to follow-up
One study focused on interventions for reducing initial loss to follow-up (ILTFU), defined as the loss of individuals with confirmed TB from care before initiating treatment.[24] It concluded that decreasing ILTFU by 50% through higher efficiency in the diagnostic process, increased education and improved follow-up by healthcare professionals could lead to moderate reductions in TB incidence.[24]
( vii ) Treatment
Eight modelling studies focused on TB treatment-related interventions of three types: reducing poor treatment outcomes; introducing novel drugs and treatment regimens; and improving DR-TB treatment. Three studies considered reducing poor outcomes of routine TB treatment in SA.[24,33,44] Identifying treatment failure, improving cure rates[33] and increasing treatment success through improved adherence[24] were estimated to yield limited impact on TB incidence and mortality. However, another study suggested that improving treatment quality by using mobile healthcare, patient follow-up, adherence counselling and improved staffing for MDR-TB could greatly reduce catastrophic costs in TB-affected households.[44] Two studies focused on the introduction of hypothetical novel TB treatment regimens at country level[25,37] Focusing on treatment efficacy in clinical trials of novel treatment regimens, in this case a rifampicin-resistant regimen, was estimated to yield significant impact on TB incidence and mortality.[37] Rapid scale-up of a 4-month TB treatment regimen that was as effective as the standard 6-month regimen, but would reduce loss to follow-up during treatment, was estimated to be of low impact.[25] Three studies focused on treatment interventions to reduce MDR- and XDR-TB.[6,32,34] A study published in 2009, when XDR-TB treatment was only offered in tertiary hospitals in SA, estimated that early DST in combination with providing treatment at outpatient health clinics (as opposed to inpatient treatment) could substantially reduce the probability of XDR-TB epidemics.[32] Improving first-line and MDR-TB treatment success using patient monitoring and community outreach programmes[6] and MDR-TB treatment decentralisation, initialised by shortened hospitalisation and home-based treatment for individuals presenting for treatment,[34] were estimated to accelerate reductions in TB incidence and mortality (appendix Table S8).
(viii) Other interventions
Reducing delay in care-seeking among people experiencing TB-characteristic symptoms was found to have substantial impact on TB incidence and mortality.[33] Halving the annual risk of infection through a combination of interventions to enhance case management was estimated to reduce TB incidence and mortality four-fold and eight-fold, respectively.[21] One modelling study considered the use of a novel mRNA correlate-of-risk (COR) test[48] to target TPT towards high-risk HIV-negative adults. Use of this new test for effective targeting of TPT was estimated to reduce TB incidence considerably.[43]
Estimated impact by type of intervention
Fig. 3 shows AAPDs in TB incidence and mortality for different interventions, calculated from reported model outcomes and time horizons. AAPDs varied between 0.05% and 7.1% for TB incidence, and between 0.02% and 7.1% for TB mortality.
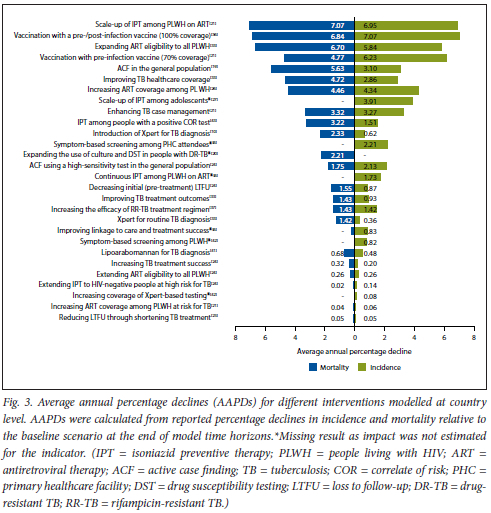
Larger impacts were estimated for preventive interventions (TB vaccination, TPT among PLWH on ART, and ART with high coverage) than for improved diagnosis and treatment. Interventions along the care cascade (e.g. case finding, diagnosis, treatment) were estimated to have greater AAPDs in TB-associated mortality than in TB incidence. AAPDs in TB incidence and mortality stratified by intervention are illustrated in Fig. S2 (appendix).
Discussion
We conducted this systematic review to synthesise the evidence for TB prevention and care in SA from studies using transmission-dynamic mathematical models.
We identified 29 eligible modelling studies, the majority of which were published in the past 6 - 7 years. Studies focused on a variety of interventions for preventing TB and strengthening the care cascade for TB. Most studies (22 of 29) investigated the impact of hypothetical novel interventions, with the remainder focusing on the scale-up of existing interventions. All but one study projected the impact of interventions on the End TB Strategy target indicators of TB incidence, TB mortality, or both. The remaining study[44] extended earlier modelling studies[9,12] to estimate the impact of interventions on the number of households experiencing TB-related catastrophic costs.
We calculated crude estimates of AAPDs in TB incidence and mortality from study outcomes of impact over different time horizons. We found that preventive interventions, including TB vaccination, TPT among PLWH and scaling up ART, were most promising to reduce TB incidence and mortality in SA. The use of novel vaccines to prevent Mycobacterium tuberculosis infection and/or TB disease was estimated to lead to substantial reductions, >5% per annum, in TB incidence at country level, highlighting the importance of vaccine research and development in the fight against TB in SA. These findings are consistent with a recent systematic review that emphasised the important role of novel vaccines towards achieving TB elimination globally.[18] Specific and data-driven strategies for delivering vaccines to key populations in SA will be important prior to the arrival of novel vaccines.[50] Varying levels of impact were projected for TPT implementation and scale-up. This variation is explained by different target populations for TPT considered and different model assumptions, including about intervention coverage and time horizons. All studies of TPT focused on isoniazid monotherapy, and none considered the impact of novel shorter regimens for TB prevention such as 3RH (a 3-month rifampicin-isoniazid course).[51] Prior to the roll-out of universal ART to PLWH in 2016, extending ART eligibility for TB prevention with high coverage was predicted to have a substantial impact on TB incidence and mortality. This is also consistent with a retrospective study conducted in 2019 that showed that recent declines in TB incidence and mortality in SA were associated with expanding access to and coverage of ART among PLWH.[52]
The majority of studies focused on interventions along the care cascade for TB. Interventions considered included screening/ active case finding, scale-up of current and introduction of novel TB diagnostic tests, reducing ILTFU and improving TB treatment. While interventions of case finding and strengthening the care cascade for TB are essential to reduce suffering from TB and improve individual-level health outcomes, their impact on reducing transmission and TB incidence may be lower compared with preventive interventions. Consistently, we found that most care-cascade interventions were estimated to have a greater effect on TB mortality than on TB incidence (Fig. 3). One exception might be interventions to reduce ILTFU, i.e. people who are bacteriologically confirmed but are lost before initiating TB treatment, a serious challenge in SA.[53] Furthermore, a large fraction of people with subclinical TB have recently been reported in SA's first national TB prevalence survey,[54] raising concerns about onward transmission from this group.[55] As people with subclinical TB are less likely to self-present for TB diagnosis, interventions to detect subclinical TB may be important in SA. Several studies in the review estimated that Xpert-based algorithms had only moderate impact on TB incidence and mortality at country level.[10,33,34,42,45] These findings align with recent studies that found that the introduction of Xpert did not result in a significant effect on TB mortality[56] and diagnostic yield.[57]
While we report impact with crude measures of annual reductions in TB incidence and mortality for single interventions, many studies considered combinations of interventions. We estimated that a 12% and 19% decline in incidence and mortality, respectively, is required between 2022 and 2035 to meet the End TB Strategy targets for SA (appendix Table S10). Of note, none of the single interventions was estimated to yield sufficient reductions over time, consistent with the idea that a combination rather than single interventions will be necessary to achieve the End TB targets.[6]
Our review identified gaps for TB modelling research in SA that, if addressed, could provide valuable additional information for decision-making. More vulnerable groups, such as people with alcohol abuse, people living with diabetes and those living in poverty, should be considered for future case-finding initiatives, as was highlighted in a recent systematic review.[58] New developments in TB diagnosis and treatment are currently underway.[59] Modelling the effect of these novel diagnostic tests and treatment regimens for active TB could assist in understanding how they should be optimally implemented in the population. Shortening the length of preventive treatment regimens is associated with higher rates of treatment success and lower loss to follow-up.[60] Modelling the impact of TPT in different target populations, such as PLWH and exposed household contacts, will be important. Additional modelling of interventions to reduce ILTFU in SA could help understand how these interventions could help reduce transmission and TB deaths in SA.[61] Beyond impact, future modelling research should also address the affordability and cost-effectiveness of interventions to inform decision-making. Only 9[10,19,22,25,29,35,40,42,44] of the 29 modelling studies identified addressed cost-effectiveness. Reducing TB-affected households facing catastrophic costs due to TB to zero represents one of the three targets of WHO's End TB Strategy. We found that only one modelling study estimated the effect of interventions on reducing households facing catastrophic costs in SA.[44] More modelling research is needed to estimate the financial impact of TB on families in SA, and to estimate the impact of TB interventions on reducing catastrophic costs. This gap is of particular relevance for SA, where over one-quarter of people face barriers such as unemployment, limited access to transport for clinic attendance and household overcrowding,[62] and where these challenges amplify TB.
This review has limitations. We restricted our analysis to modelling studies of TB in the SA population. Findings from other TB modelling studies focusing on populations outside of SA may still be relevant to the SA context, and should be taken into consideration for policy-making. While we report findings from modelling studies at different population levels, findings from studies at sub-country level might not be readily generalisable to the national level. Likewise, generalisability of country-level analyses to different local areas in SA may be limited given the considerable heterogeneity in TB burden and epidemiology in the country.[63] Our study focused on impact with respect to the End TB Strategy target indicators. We did not focus on affordability and cost-effectiveness of interventions, which are also relevant for decision-making. We intended to compare the impact of different interventions on TB incidence and mortality. Estimated AAPDs and their differences between studies have to be interpreted with caution as they are dependent on the baseline to which intervention scenarios are being compared; these baselines are not consistent between the modelling studies. Furthermore, rates of decline in TB incidence and mortality are expected to vary during the course of an intervention. Finally, heterogeneity in model structure, study design and reported outcomes further limit our ability to compare interventions with respect to their potential to generate progress towards the End TB Strategy targets.
Conclusion
We highlight an extensive body of modelling research with relevance for TB decision-making in SA. We present these findings at a time where additional guidance is urgently needed to confront recent setbacks in the fight against TB caused by health service disruptions during the COVID-19 pandemic, and to ensure progress towards the 2035 End TB Strategy targets in SA. We found that interventions focusing on prevention, including vaccination, TPT among PLWH and scaling-up ART, would have the greatest potential to reduce TB incidence and mortality. However, relating estimates of impact to the progress that would be needed in SA to achieve the End TB strategy targets revealed that single interventions will be unlikely to generate sufficient progress. Combinations of interventions rather than single interventions are therefore needed to effectively reduce TB incidence and mortality in SA. Our review discusses important knowledge gaps in modelling research, including studies of novel diagnostic tests for TB, interventions in vulnerable and high-risk populations and interventions towards reducing TB-related catastrophic costs. Closing these gaps through additional modelling research could help prioritise novel interventions and accompany already implemented interventions to better understand how they will aid progress towards TB elimination in SA.
Declaration. None.
Acknowledgements. We thank Ted Cohen, David Dowdy, Christopher Dye and Rein Houben for reviewing our search results using their personal databases and for helpful feedback.
Author contributions. Development of the protocol: LB, CvS and FM. Literature searches: LB. LB and CvS selected studies for inclusion and FM resolved disagreements in study selection. Extraction and synthesis of data: LB and CvS. Risk-of-bias assessment: LB and AdV. LB drafted the manuscript. All authors reviewed the manuscript and approved its final version for submission.
Funding. This work is supported in part by the Department of Science and Innovation and the National Research Foundation (NRF) (LB postgraduate bursary grant number: 132851). Any opinion, finding and conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard.
Conflicts of interest. None.
References
1. World Health Organization. Global Tuberculosis Report 2022. Geneva: WHO, 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed 11 November 2022). [ Links ]
2. Statistics South Africa. Mortality and causes of death in South Africa: Findings from death notification. Pretoria: Stats SA, 2017. http://www.statssa.gov.za/publications/P03093/P030932017.pdf (accessed 21 March 2022). [ Links ]
3. McQuaid CF, Vassall A, Cohen T, et al. The impact of COVID-19 on TB: A review of the data. Int J Tuberc Lung Dis 202135(6):436-446. https://doi.org/10.5588/ijtld.21.0148 [ Links ]
4. Ismail N, Moultrie H. Impact of COVID-19 intervention on TB testing in South Africa. Pretoria: National Institute for Communicable Diseases, 2020. https://www.nicd.ac.za/wp-content/uploads/2020/05/Impact-of-COVID-19-interventions-on-TB-testing-in-South-Africa-10-May-2020.pdf (accessed 2 May 2022). [ Links ]
5. Uplekar M, Weil D, Lonnroth K, et al. WHO's new End TB Strategy. Lancet 2015;385(9979):1799-1801. https://doi.org/10.1016/S0140-6736(15)60570-0 [ Links ]
6. Houben RMGJ, Menzies NA, Sumner T, et al. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: A combined analysis of 11 mathematical models. Lancet Glob Health 2016;4(11):e806-815. https://doi.org/10.1016/S2214-109X(16)30199-1 [ Links ]
7. Starks MA, Sanders GD, Coeytaux RR, et al. Assessing heterogeneity of treatment effect analyses in health-related cluster randomised trials: A systematic review. PLoS ONE 2019;14(8): e0219894. https://doi.org/10.1371/journal.pone.0219894 [ Links ]
8. Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet 2011;378(9790):515-525. https://doi.org/10.1016/S0140-6736(10)61505-X [ Links ]
9. Zwerling A, Shrestha S, Dowdy DW. Mathematical Modelling and Tuberculosis: Advances in Diagnostics and Novel Therapies. Advances in Medicine 2015;2015: 907267. https://doi.org/10.1155/2015/907267 [ Links ]
10. Menzies NA, Cohen T, Lin H-H, Murray M, Salomon JA. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: A dynamic simulation and economic evaluation. PLoS Med 2012;9(11):e1001347. https://doi.org/10.1371/journal.pmed.1001347 [ Links ]
11. Menzies NA, Gomez GB, Bozzani F, et al. Cost-effectiveness and resource implications of aggressive action in China, India and South Africa: A combined analysis of nine models. Lancet Glob Health 2016;4(11):e816-e826. https://doi.org/10.1016/S2214-109X(16)30265-0 [ Links ]
12. Houben RMJG, Lalli M, Sumner T, et al. TIME Impact - a new user-friendly tuberculosis (TB) model to inform TB policy decisions. BMC Med 2016;14(56). https://doi.org/10.1186/s12916-016-0608-4 [ Links ]
13. Saaiq M, Ashraf B. Modifying "Pico" Question into "Picos" Model for More Robust and Reproducible Presentation ofthe Methodology Employed in A Scientific Study. World J Plast Surg 2017;6(3):390-392. [ Links ]
14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev 2021;10(1):89. https://doi.org/10.1186/s13643-021-01626-4 [ Links ]
15. TB Modelling and Analysis Consortium. TB modelling literature reviews. London: TB MAC, 2017. https://tb-mac.org/tb-mac-resource/systematic-literature-review-of-existing-academic-papers-describing-mathematical-and-economic-modelling-of-tb/ (accessed 2 September 2021). [ Links ]
16. Fone D, Hollinghurst S, Temple M, et al. Systematic review of the use and value of computer simulation modelling in population health and health care delivery. J Public Health 2003;25(4):325-335. https://doi.org/10.1093/pubmed/fdg075 [ Links ]
17. Caro JJ, Eddy DM, Kan H, et al. Questionnaire to assess relevance and credibility of modeling studies for informing health care decision making: An ISPOR-AMCP-NPC good practice task force report. Value Health 2014;17(2):174-182. https://doi.org/10.1016/j.jval.2014.01.003 [ Links ]
18. Harris RC, Sumner T, Knight GM, White RG. Systematic review of mathematical models exploring the epidemiological impact of future TB vaccines. Hum Vaccines Immunotherapeutics 2016;12(11):2813-2832. https://doi.org/10.1080/21645515.2016.1205769 [ Links ]
19. Azman AS, Golub JE, Dowdy DW. How much is tuberculosis screening worth? Estimating the value of active case finding for tuberculosis in South Africa, China, and India. BMC Med 2014;12(1):216. https://doi.org/10.1186/s12916-014-0216-0 [ Links ]
20. Dowdy DW, Chaisson RE, Maartens G, Corbett EL, Dorman SE. Impact of enhanced tuberculosis diagnosis in South Africa: A mathematical model of expanded culture and drug susceptibility testing. PNAS 2008;105(32):11293-11298. https://doi.org/10.1073/pnas.0800965105 [ Links ]
21. Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health 2013;34(1):271-286. https://doi.org/10.1146/annurev-publhealth-031912-114431 [ Links ]
22. Dye C. Making wider use ofthe world's most widely used vaccine: Bacille Calmette-Guérin revaccination reconsidered. J R Soc Interface 2013;10(87):20130365. https://doi.org/10.1098/rsif.2013.0365 [ Links ]
23. Hippner P, Sumner T, Houben RMGJ, et al. Application of provincial data in mathematical modelling to inform sub-national tuberculosis program decision-making in South Africa. PLoS ONE 2019;14(1):e0209320. https://doi.org/10.1371/journal.pone.0209320 [ Links ]
24. Knight GM, Dodd PJ, Grant AD, Fielding KL, Churchyard GJ, White RG. Tuberculosis prevention in South Africa. PLoS ONE 2015;10(4):e0122514. https://doi.org/10.1371/journal.pone.0122514 [ Links ]
25. Knight GM, Gomez GB, Dodd PJ, et al. The impact and cost-effectiveness of a four-month regimen for first-line treatment of active tuberculosis in South Africa. PLoS ONE 2015;10(12):e0145796. https://doi.org/10.1371/journal.pone.0145796 [ Links ]
26. Pretorius C, Menzies NA, Chindelevitch L, et al. The potential effects of changing HIV treatment policy on tuberculosis outcomes in South Africa. AIDS 2014;28(1):S25-S34. https://doi.org/10.1097/QAD.0000000000000085 [ Links ]
27. Rhines AS, Feldman MW, Bendavid E. Modeling the implementation of population-level isoniazid preventive therapy for tuberculosis control in a high HIV-prevalence setting. AIDS 2018;32(15):2129-2140. https://doi.org/10.1097/QAD.0000000000001959 [ Links ]
28. Shrestha S, Chihota V, White RG, Grant AD, Churchyard GJ, Dowdy DW. Impact of targeted tuberculosis vaccination among a mining population in South Africa: A model-based study. Am J Epidemiol 2017;186(12):1362-1369. https://doi.org/10.1093/aje/kwx192 [ Links ]
29. Uys PW, Warren R, van Helden PD, Murray M, Victor TC. Potential of rapid diagnosis for controlling drug-susceptible and drug-resistant tuberculosis in communities where Mycobacterium tuberculosis infections are highly prevalent. J Clin Microbiol 2009;47(5):1484-1490. https://doi.org/10.1128/JCM.02289-08 [ Links ]
30. Williams BG, Granich R, de Cock KM, Glaziou P, Sharma A, Dye C. Antiretroviral therapy for tuberculosis control in nine African countries. PNAS 2010;107(45):19485-19489. https://doi.org/10.1073/pnas.1005660107 [ Links ]
31. Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: An epidemiological modelling study. Lancet 2007;370(9597):1500-1507. https://doi.org/10.1016/S0140-6736(07)61636-5 [ Links ]
32. Basu S, Friedland GH, Medlock J, et al. Averting epidemics of extensively drug-resistant tuberculosis. PNAS 2009;106(18):7672-7677. https://doi.org/10.1073/pnas.0812472106 [ Links ]
33. Chindelevitch L, Menzies NA, Pretorius C, Stover J, Salomon JA, Cohen T. Evaluating the potential impact of enhancing HIV treatment and tuberculosis control programmes on the burden of tuberculosis. J R Soc Interface 2015;12(106):20150146. https://doi.org/10.1098/rsif.2015.0146 [ Links ]
34. Gilbert JA, Long EF, Brooks RP, et al. Integrating community-based interventions to reverse the convergent TB/HIV epidemics in rural South Africa. PLoS ONE 2015;10(5):e0126267. https://doi.org/10.1371/journal.pone.0126267 [ Links ]
35. Gilbert JA, Shenoi SV, Moll AP, Friedland GH, Paltiel AD, Galvani AP. Cost-effectiveness of community-based TB/HIV screening and linkage to care in rural South Africa. PLoS ONE 2016;11(12):e0165614. https://doi.org/10.1371/journal.pone.0165614 [ Links ]
36. Harris RC, Sumner T, Knight GM, Zhang H, White RG. Potential impact of tuberculosis vaccines in China, South Africa, and India. Sci Transl Med 2020;12(564):eaax4607. https://doi.org/10.1126/scitranslmed.aax4607 [ Links ]
37. Kendall EA, Shrestha S, Cohen T, et al. Priority-setting for novel drug regimens to treat tuberculosis: An epidemiologic model. PLoS Med 2017;14(1):e1002202. https://doi.org/10.1371/journal.pmed.1002202 [ Links ]
38. Kendall EA, Azman AS, Maartens G, et al. Projected population-wide impact of antiretroviral therapy-linked isoniazid preventive therapy in a high-burden setting. AIDS 2019;33(3):525-536. https://doi.org/10.1097/QAD.0000000000002053 [ Links ]
39. Marx FM, Yaesoubi R, Menzies NA, et al. Tuberculosis control interventions targeted to previously treated people in a high-incidence setting: A modelling study. Lancet Glob Health 2018;6(4):e426-e435. https://doi.org/10.1016/S2214-109X(18)30022-6 [ Links ]
40. Marx FM, Cohen T, Menzies NA, Salomon JA, Theron G, Yaesoubi R. Cost-effectiveness of post-treatment follow-up examinations and secondary prevention of tuberculosis in a high-incidence setting: A model-based analysis. Lancet Glob Health 2020;8(9):e1223-e1233. https://doi.org/10.1016/S2214-109X(20)30227-8 [ Links ]
41. Ricks S, Denkinger CM, Schumacher SG, Hallett TB, Arinaminpathy N. The potential impact of urine-LAM diagnostics on tuberculosis incidence and mortality: A modelling analysis. PLoS Med 2020;17(12): e1003466. https://doi.org/10.1371/journal.pmed.1003466 [ Links ]
42. Sumner T, Bozzani F, Mudzengi D, et al. Estimating the impact of tuberculosis case detection in constrained health systems: An example of case-finding in South Africa. Am J Epidemiol 2019;188(6):1155-1164. https://doi.org/10.1093/aje/kwz038 [ Links ]
43. Sumner T, Scriba TJ, Penn-Nicholson A, Hatherill M, White RG. Potential population level impact on tuberculosis incidence of using an mRNA expression signature correlate-of-risk test to target tuberculosis preventive therapy. Sci Rep 2019;9(1):11126. https://doi.org/10.1038/s41598-019-47645-z [ Links ]
44. Verguet S, Riumallo-Herl C, Gomez GB, et al. Catastrophic costs potentially averted by tuberculosis control in India and South Africa: A modelling study. Lancet Glob Health 2017;5(11):e1123-e1132. https://doi.org/10.1016/S2214-109X(17)30341-8 [ Links ]
45. Vynnycky E, Sumner T, Fielding KL, et al. Tuberculosis control in South African gold mines: Mathematical modeling of a trial of community-wide isoniazid preventive therapy. Am J Epidemiol 2015;181(8):619-632. https://doi.org/10.1093/aje/kwu320 [ Links ]
46. Meyer-Rath G, Johnson LF, Pillay Y, et al. Changing the South African national antiretroviral therapy guidelines: The role of cost modelling. PLoS ONE 2017;12(10):e0186557. https://doi.org/10.1371/journal.pone.0186557 [ Links ]
47. Middelkoop K, Mathema B, Myer L, et al. Transmission of tuberculosis in a South African community with a high prevalence of HIV infection. J Infect Dis 2015;211(1):53-61. https://doi.org/10.1093/infdis/jiu403 [ Links ]
48. Scriba TJ, Fiore-Gartland A, Penn-Nicholson A, et al. Biomarker-guided tuberculosis preventive therapy (CORTIS): A randomised controlled trial. Lancet Infect Dis 2021;21(3):354-365. https://doi.org/10.1016/S1473-3099(20)30914-2 [ Links ]
49. World Health Organization. WHO TB burden estimates. Geneva: WHO, 2021. http://www.who.int/tb/country/data/download/en/ (accessed 4 November 2021). [ Links ]
50. White RG, Hanekom WA, Vekemans J, Harris RC. The way forward for tuberculosis vaccines. Lancet Respir Med 2019;7(3):204-206. https://doi.org/10.1016/S2213-2600(19)30040-2 [ Links ]
51. Schwoebel V, Koura KG, Abjobimey M, et al. Tuberculosis contact investigation and short-course preventive therapy among young children in Africa. Int J Tuberc Lung Dis 2020;24(4):452-460. https://doi.org/10.5588/ijtld.19.0712 [ Links ]
52. Dye C, Williams BG. Tuberculosis decline in populations affected by HIV: A retrospective study of 12 countries in the WHO African region. Bull World Health Organ 2019;97(6):405-414. https://doi.org/10.2471/BLT.18.228577 [ Links ]
53. Claassens MM, du Toit E, Dunbar R, et al. Tuberculosis patients in primary care do not start treatment. What role do health system delays play? Int J Tuberc Lung Dis 2013;17(5):603-607. https://doi.org/10.5588/ijtld.12.0505 [ Links ]
54. Moyo S, Ismail F, van der Walt M, et al. Prevalence of bacteriologically confirmed pulmonary tuberculosis in South Africa, 2017-19: A multistage, cluster-based, cross-sectional survey. Lancet Infect Dis 2022;22(8):1172-1180. https://doi.org/10.1016/S1473-3099(22)00149-9 [ Links ]
55. Frascella B, Richards AS, Sossen B, et al. Subclinical tuberculosis disease - a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin Infect Dis 2021;73(3):e830-e841. https://doi.org/10.1093/cid/ciaa1402 [ Links ]
56. Di Tanna GL, Khaki AR, Theron G, et al. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: Individual patient data meta-analysis. Lancet Glob Health 2019;7(2):e191-e199. https://doi.org/10.1016/S2214-109X(18)30458-3 [ Links ]
57. Naidoo P, Dunbar R, Lombard C, et al. Comparing tuberculosis diagnostic yield in smear/culture and Xpert MTB/RIF-based algorithms using a non-randomised stepped-wedge design. PLoS ONE 2016;11(3):e0150487. https://doi.org/10.1371/journal.pone.0150487 [ Links ]
58. Bohlbro AS, Hvingelby VS, Rudolf F, Wejse C, Patsche CB. Active case-finding of tuberculosis in general populations and at-risk groups: A systematic review and meta-analysis. Eur Respir J 2021;58(4):2100090. https://doi.org/10.1183/13993003.00090-2021 [ Links ]
59. Gill CM, Dolan L, Piggott LM, McLaughlin AM. New developments in tuberculosis diagnosis and treatment. Breathe 2022;18:210149. https://doi.org/10.1183/20734735.0149-2021 [ Links ]
60. Ndjeka N, Campbell JR, Meintjies G, et al. Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: A retrospective cohort study. Lancet Infect Dis 2022;22(7):1042-1051. https://doi.org/10.1016/S1473-3099(21)00811-2 [ Links ]
61. Osman M, Meehan S, von Delft A, et al. Early mortality in tuberculosis patients initially lost to follow up following diagnosis in provincial hospitals and primary health care facilities in Western Cape, South Africa. PLoS ONE 2021;16(6):e0252084. https://doi.org/10.1371/journal.pone.0252084 [ Links ]
62. Stracker N, Hanrahan C, Mmolawa L, et al. Risk factors for catastrophic costs associated with tuberculosis in rural South Africa. Int J Tuberc Lung Dis 2019;23(6):756-763. https://doi.org/10.5588/ijtld.18.0519 [ Links ]
63. Van der Walt M, Moyo S. The First National TB Prevalence Survey, South Africa 2018: Short report. NICD, 2021. https://www.nicd.ac.za/wp-content/uploads/2021/02/TB-Prevalence-survey-report_A4_SA_TPS-Short_Feb-2021.pdf (accessed 15 June 2022). [ Links ]
 Correspondence:
Correspondence:
L R Brown
laurenbrown@sun.ac.za
Accepted 28 November 2022














